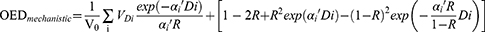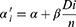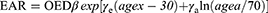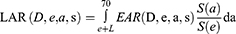Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Critical Evaluation of Secondary Cancer Risk After Breast Radiation Therapy with Hybrid Radiotherapy Techniques
Authors Zhang Q, Zeng Y, Peng Y, Yu H, Zhang S, Wu S
Received 22 July 2022
Accepted for publication 5 September 2022
Published 23 January 2023 Volume 2023:15 Pages 25—38
DOI https://doi.org/10.2147/BCTT.S383369
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Quanbin Zhang,1 Yu Zeng,2 Yingying Peng,1 Hui Yu,1 Shuxu Zhang,1 Shuyu Wu1
1Department of Radiation Oncology, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, People’s Republic of China; 2Department of Stomatology, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, People’s Republic of China
Correspondence: Shuxu Zhang; Shuyu Wu, Email [email protected]; [email protected]
Background: As hybrid radiotherapy technique can effectively balance dose distribution between targets and organs, it is necessary to evaluate the late effects related to radiotherapy. The aim of the study was to calculate and provide individual estimates of the risks for hybrid radiotherapy techniques in breast cancer patients.
Methods: Whole-breast irradiation was performed in 43 breast cancer patients by using 3D conformal, intensity-modulated and hybrid techniques. The excess absolute risk (EAR), lifetime attributable risk (LAR) and normal tissue complication probability (NTCP) were calculated to estimate risks in organs. The risk variability in contralateral breast was assessed by using the patient’s anatomic parameters.
Results: Compared with IMRT and FinF, hybrid techniques achieved satisfactory dose distribution and comparable or lower estimated risks in organs. The LAR was estimated to be up to 0.549% for contralateral lung with advantages of tangential techniques over H-VMAT. For ipsilateral lung, the LAR was estimated to be up to 9.021%, but lower in H-VMAT and FinF without significant difference. The risk of thyroid was negligible in overall estimation. For contralateral breast, the LAR was estimated to be up to 0.865% with advantages of MH-IMRT and H-VMAT over TF-IMRT. The fraction of individual variability could be explained by using anatomic parameters of minimum breast distance (MBD) and minimum target concave angle (θMTCA). NTCP for all analyzed endpoints was significantly higher in TF-IMRT relative to FinF and hybrid techniques, while TH-IMRT and H-VMAT were presenting lower toxicity risk. However, MH-IMRT presented a higher probability of toxicity in lung. For most cases, H-VMAT demonstrated a benefit for contralateral breast, heart and lung sparing.
Conclusion: The optimal treatment should be performed individually according to anatomic parameters and balances between EAR and NTCP. Individual assessment may assist in achieving optimal balances between targets and organs as well as supporting clinical decision-making processes.
Keywords: secondary cancer risk, hybrid techniques, individual assessment, breast cancer, radiotherapy
Introduction
In order to improve local control and overall survival, radiotherapy has become a consolidated adjuvant treatment for patients with early stage breast cancer after breast conserving surgery. Notwithstanding, radiotherapy techniques have advanced from three-dimensional conformal radiotherapy (3D-CRT) to intensity modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT), which can significantly reduce the incidence of pulmonary and cardiac late side effects.1,2 However, modern radiotherapy techniques are always at the cost of higher low-dose baths to achieve satisfactory dose distribution.3 It is well-known that there is no safe threshold dose of radiation, and even lower doses are significant.4 This not only increases the risk of developing secondary cancers, but also increases the long-term effects, including cardiovascular diseases.5,6 In most cases, this risk may not be negligible. It becomes more essential to make careful decisions about the choice of treatment.
However, epidemiological studies cannot accurately estimate the secondary cancer risk, because most of the available data are derived from obsolete treatment techniques from 20–30 years ago.7 Therefore, several mathematical formulas have been developed to predict the radiation dose-response relationships, to further assess late effects.8,9 With the use of some of those models, the excess absolute risk (EAR, per 10,000 person years) is used to describe the absolute risk of developing a secondary cancer between people exposed to irradiation and controls, which is also based on the organ equivalent dose (OED). Several studies on secondary cancer risk have been published from different radiotherapy techniques in breast cancer, but those estimates differ greatly between publications without considering individualization of patients.10–12 Additionally, many treatment techniques have been evaluated to reduce the dose to cardiac components and the risks of cancers in organs close to the irradiation fields.13–15 It has been found that secondary cancer risk in organs away from the treatment site decreases with increasing distance from the treatment site. In terms of individual differences in secondary cancer risk and heart disease, there are still important open questions related to the effects of current radiotherapy techniques. There is no consensus as to which treatment strategy is superior. Particularly, published data have reported on the benefit of hybrid techniques in radiation therapy for breast cancer,16–18 but more detailed and accurate data regarding radiation-induced effects by hybrid techniques on healthy organs are lacking, which can provide guidance in the choice of radiotherapy techniques in breast cancer. Furthermore, it has been found that biological evaluation, such as normal tissue complication probability (NTCP), can help to predict the biological effects on normal tissues.19,20 Compared with parameters from dose-volume-histogram (DVH), the NTCP has a more direct correlation with treatment outcomes. However, the endpoints in NTCP are very different. Thus, the optimal trade-off between EAR and NTCP is unclear, which may be tailored on a patient-specific basis.
Anatomic diversity has larger effects on the inter-patient variability in doses to contralateral breast, heart and lungs, which limits the long-term health risks from radiotherapy to patients, even for the same treatment protocol. Thus, the aim of the study was to complement and extend previous studies on the effects of anatomic diversity and to calculate secondary cancer risks among hybrid radiotherapy techniques for breast cancer. The mathematical models, such as OED, EAR and NTCP, would be used to provide critical estimation of radiation-induced cancer risks in heart, contralateral breast (CB), contralateral lung (CL), ipsilateral lung (IL), esophagus and thyroid.
Methods
Patient Cohort
A cohort of 43 patients with early stage breast cancer after breast conserving surgery was randomly enrolled for a retrospective planning study. The average age was 45.74 years ranging from 31 to 65 years. The specific characteristics of the patients are shown in Table 1. Approval for retrospective analysis of the patient data was obtained from the ethics committee of Affiliated Cancer Hospital and Institute of Guangzhou Medical University.
 |
Table 1 The Characteristics of Patients with Breast Cancer |
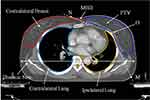
According to anatomic parameters of patients, they were enrolled in different groups. The minimum breast distance (MBD) and the minimum target concave angle (θMTCA) were used to express the anatomic conditions of the critical structures and target, as shown in Figure 1. MBD was defined as the minimum separation from contralateral breast to planning target volume (PTV), while θMTCA was defined as the minimum target concave angle of PTV. They were measured directly in the midplane axial CT slice. The reference point O was designated as the apex of the PTV concave shape at the section of its maximum curvature.
When the distance was less than 2.5 cm, it was classified as MBD1; conversely, it was classified as MBD2. If θMTCA was less than 140°, it was classified as θMTCA1; conversely, it was classified as θMTCA2. Additionally, each group contained both right-sided breast cancer (RBC) and left-sided breast cancer (LBC) to investigate the dosimetric differences between treatments of left- and right-sided breast tumors.
Treatment Planning
All patients in the supine position and ipsilateral arm abduction above the head were scanned at 3 mm slice spacing. Clinical oncologists delineated the target volumes and organs. The clinical target volume (CTV) included the whole breast. The PTV was defined as the CTV with a 5–10 mm margin to the delineated target volume to compensate for treatment setup variability and internal organ motion but did not exceed the body. The organs at risk (OARs) included the heart, contralateral breast (CB), contralateral lung (CL), ipsilateral lung (IL), esophagus and thyroid. The PTV did not include the lymph node for irradiation. Additionally, tumor-bed boosts were not considered at this time.
In total, five treatment plans were created for each patient by using five treatment modalities. The field-in-field forward plan (FinF) consisted of two parallel opposed tangential fields without wedge compensation, and added sub-fields created by a multi-leaf collimator (MLC) for dose compensation. Secondly, the tangential inverse IMRT plan (TF-IMRT) was created and modulated by using the same tangential fields from the FinF. The tangential beams were set according to the anatomic characteristics of individual patients. Thirdly, a combination of manual and inverse planning was investigated, called hybrid techniques. The tangential hybrid IMRT (TH-IMRT) was set up as a conventional tangential fields plan (cTF) where the main dose contribution was delivered by two open fields without wedge compensation, but dose homogeneity in the PTV was achieved by TF-IMRT. While the multiple fields hybrid IMRT plan (MH-IMRT) consisted of a cTF and a four fields IMRT plan (4F-IMRT), including 3 fields on the outer and inner sides of the breast respectively, such as φ-10°, φ, φ+10° and ν, ν+10°, ν+20°, where the φ and ν on both sides were set according to the tangential fields from FinF and assigned to cTF. The cTF weights were set in order to deliver 80% of the prescribed dose to the isocenter.21 The 4F-IMRT, such as φ-10°, φ+10°, ν+10° and ν+20°, were modulated using the inverse planning system in order to meet the aforementioned dosimetric constraints. For TF-IMRT and 4F-IMRT, total number of segments was set to less than 100; additionally, the minimum segment area was set to 10 cm2 and the minimum segment monitor unit was set to 10 MU. For the hybrid VMAT plan (H-VMAT), a cTF plus a two coplanar partial arcs plan (2p-VMAT) were integrated into a single treatment plan. The cTF plan and 2p-VMAT plan were hybridized by using beam weights of 80% and 20% respectively. For 2p-VMAT plan, one arc was set up in a clockwise (CW) direction from φ to ν; conversely, the second arc was performed in a counterclockwise (CCW) direction from ν to φ. The gantry spacing was 4° and collimator angle was 15° or 345°. It should be noted that the number of iterations for dose calculation does not exceed 100 during the IMRT and VMAT planning process. All radiotherapy techniques were planned and calculated on the Pinnacle3 treatment planning system (TPS, version 9.10, Philips Medical Systems, Fitchburg, WI) by using a Collapsed Cone algorithm.
Plans’ Evaluation
The total prescription doses were 50 Gy to the PTV, and they were delivered at 2 Gy per fraction. The plans were optimized to provide the dose within PTV between 95% and 107% of the prescribed dose. Dose constraints for ipsilateral lung were set as V10<30%, V20<20%, V30<10% and the average dose Dmean<15 Gy, while dose constraints for ipsilateral heart were set as V5<30% and V20<10%, but V5<15% was set for contralateral heart. The VX Gy referred to the volume receiving more than x Gy. Additionally, doses to the contralateral breast and lungs should be kept as low as possible without compromising target coverage. All plans were optimized and evaluated for optimal target coverage, conformity, homogeneity, and dose limits of OARs. The conformity index (CI), used as a measure of target volume dose distribution conformity, was defined as CI = VT,ref/VT ×VT,ref /Vref, where VT,ref was target volume covered by reference isodose, VT was target volume and Vref was volume of the reference isodose. If the CI was closer to 1, the dose conformity was better. The homogeneity index (HI), used as a measure of the evenness of dose distribution, was defined as HI = (D2–D98)/D50, where D2, D98 and D50 were the doses covering 2%, 98% and 50% of the PTV, respectively. HI = 0 was the ideal value. Analysis of the OARs included the maximum dose, mean dose and a set of appropriate define (Vx) and define (Dy) values.
Secondary Cancer Risk Assessment
Based on data of DVH, secondary cancer risks in OARs were calculated by using Schneider’s concept of organ equivalent dose (OED),22 which included the impact of repopulation, proliferation, and cell killing. The OED was calculated by full mechanistic dose-response model, as follows:
The VDi was the volume of the DVH bin receiving dose Di and V0 was the total volume of the organ. The n referred to the number of dose fractions that the organ of interest experienced, which might be different from the number of treatment fractions according to the treatment field arrangement. The model parameters were listed in Table S1.
The EAR described the absolute difference in cancer rates of persons exposed to a dose D and those not exposed to a dose beyond the natural dose exposition per 10,000 person years (PY). The EAR could be calculated as:9
EAR included population-related parameters, such as age at exposure (agex) and attained age (agea). The parameter β was the initial slope for the dose-response relationship of secondary cancer induction. γe and γa were the age-modifying factors. The model parameters were listed in Table S2. In order to remove the EAR variability due to the varying age of irradiated patient, the EAR was then recalculated based on all patients irradiated at age 30 years and who attained 70 years of age.
For secondary cancer risk, the lifetime attributable risk (LAR), which was percentage likelihood in excess of the baseline risk of secondary malignancy happening during one’s lifetime, could be estimated as an integral of excess risk and an effective measurement because it took the patient’s age at the time of treatment and predicted lifespan into account.8
L was the incubation period of solid cancer and assumed to be 5 years. The ratio S(a)/S(e) was the conditional probability of a person alive at age e to reach at least age a, which was obtained from life table of the China Office for National Statistics 2018–2019.
Normal Tissue Complication Probability
The biological indices were important indicators in choosing appropriate treatment plan. Therefore, NTCP was calculated by the parameters as reported previously for those organs with an expected risk of toxicity due to radiotherapy treatment, such as heart and ipsilateral lung. Two models, the Lyman-Kutcher-Burman (LKB) model and the seriality (Poisson-LQ) model, were used for computation for different endpoints. The Biological Evaluation Module implemented in Pinnacle was used for NTCP calculations. The models, endpoints, and parameters were summarized in Table S3.
Statistical Analysis
Statistical analysis was performed by using SPSS 19.0 (SPSS Inc., Chicago, IL). Nonparametric test was used to estimate statistical significance of differences, such as Wilcoxon’s signed rank test and Mann–Whitney test. The difference was considered statistically significant when P<0.05.
Results
A total of 215 treatment plans were calculated and evaluated for 43 breast cancer patients. All alternative techniques allowed achieving the required coverage of PTV, but H-VMAT plans showed consistently better PTV dose coverage, conformity, and homogeneity. The dose distribution in transverse for a representative patient with breast cancer in five types of treatment plans was shown in Figure S1. As shown in Figure S2, the CI and HI for PTV were optimal and superior in H-VMAT compared with other alternative plans, which were equivalent in dose distribution (P>0.05); but the treatment monitor units (MU) were much higher in TH-IMRT (P<0.05). Additionally, the dosimetric parameters of OARs were characterized in Tables S4–S8, respectively. H-VMAT had been demonstrated to reduce high-dose volumes of OARs, conversely, frequently resulting in more low-dose volumes. FinF was equivalent or even superior to the other three treatment modalities. The mean doses for individual patients obtained from alternative techniques were plotted in Figure 2, such as CB, heart and IL. For the patients in the study, the mean heart doses from right-sided breast cancer were higher in H-VMAT and lower in FinF (Figure 2A); however, most of mean heart doses were lower in FinF and H-VMAT without significant difference in left-sided breast cancer (Figure 2B). Additionally, the V5 of heart was similar among alternative techniques except MH-IMRT, while the V25 and V30 of heart were less in H-VMAT. The mean IL doses from MH-IMRT were considerably higher than those from other four treatment modalities (Figure 2C). However, when mean CB dose was in the region below 3Gy, H-VMAT frequently resulted in significantly higher dose than other four treatment modalities, but MH-IMRT and H-VMAT might help to reduce mean CB dose if it exceeded 3 Gy, as shown in Figure 2D.
The radiation-induced secondary cancer risks in organs for all alternative techniques were calculated by using parameter sets, as shown in Tables 2 and 3. Regarding the risk of CB, there was a slight advantage of MH-IMRT over FinF and H-VMAT, but a significantly higher risk in TF-IMRT. The LAR was estimated to be up to 0.865% for CB, while the risks were similar between FinF and H-VMAT without statistical significance. The LAR of CL was estimated to be up to 0.549% in H-VMAT, while it was lower in FinF (<0.1%). For IL, the risk was lower in H-VMAT and FinF without significant difference (P>0.05), while MH-IMRT might have more risk in IL (P<0.05). As IL was close to or even constituted target area to be irradiated, the LAR was estimated to be up to 95% CI:7.880%−9.021%. Such a large risk was inconsistent with the probability of radiation-induced lung cancer observed in follow-up studies of breast radiotherapy.23 It warranted some special consideration to estimate the risk for IL. The risk of thyroid was negligible in the overall estimation.
 |
Table 2 Excess Absolute Risk (EAR) for Patients Obtained from Schneider, 95% CI in Brackets |
 |
Table 3 Lifetime Attributable Risk (LAR) for Patients Obtained from Schneider, 95% CI in Brackets |
When considering the effect of anatomic parameters, the mean differences of risks were illustrated in Table 4 for organs from different groups. For all alternative techniques, there were no significant differences of risks in organs between right- and left-side, MBD1 and MBD2, θMTCA1 and θMTCA2, respectively, except for CB. It might mean that they were almost independent of distance and target concave angle, but depended on radiotherapy techniques. In CB, the mean differences of risks were up to 84.18% in MBD and 54.99% in θMTCA (P<0.05), but had no statistical significance between right and left side (P>0.05). Furthermore, the relationship between inter-individual variability of mean dose and EAR in CB and anatomic parameters, such as MBD and θMTCA, was calculated and shown in Figure 3. In all alternative techniques, mean dose and EAR in CB exponentially decreased with increasing MBD but exponentially increased with increasing θMTCA. It should be noted that mean CB dose was higher in H-VMAT under MBD larger than 2.5cm and θMTCA less than 140°. Furthermore, when MBD was less than 2.5cm, the variability of risks among alternative techniques was significantly greater than that at MBD larger than 2.5cm, especially for TF-IMRT. Similarly, the variability of risks was significantly greater at θMTCA larger than 140° than at θMTCA less than 140°. These anatomic parameters could serve as predictors of patient-specific risks of CB. Interestingly, as shown in Figure 4, if MBD was less than 2.5cm, the EARs among alternative techniques had no statistical significance between θMTCA1 and θMTCA2 (P>0.05), but the EARs were lower in θMTCA1 compared with θMTCA2 at MBD larger than 2.5cm (P<0.05). Additionally, if MBD was less than 2.5cm, H-VMAT and MH-IMRT could help to have a significant reduction in EARs without the effect of θMTCA relative to the other three techniques. In contrast, if MBD was larger than 2.5 cm, the tangential techniques were more beneficial in reducing EAR under θMTCA less than 140°, but it was comparable for all alternative techniques under θMTCA larger than 140° (P>0.05). A composite LARs of organs, except IL, were calculated and summated based on anatomic parameters for all alternative techniques, as illustrated in Figure 5. Compared to the other four alternative techniques, the composite LARs for H-VMAT were larger and increased significantly, especially under the condition of MBD larger than 2.5cm. Comparatively, the composite LARs for FinF might be the lowest.
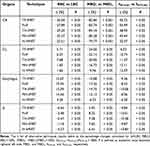 |
Table 4 The Mean Differences of Secondary Cancer Risks in Organs Based on Anatomic Parameters |
In response to the integral management of disease treatment, the aforementioned results should be balanced with NTCP estimates for early and late toxicity. The NTCP for all available endpoints of heart and lung from all alternative techniques were reported in Table 5. Compared with TF-IMRT, there was a statistically significant reduction in NTCP of heart from other alternative techniques (P<0.001). A significantly lower NTCP was observed in H-VMAT with 95% CI:1.28%−2.06%. The endpoints, such as pneumonitis, pneumonitis grade ≥2, symptomatic or radiographic pneumonitis for ≤ 6 mo, symptomatic or radiographic fibrosis for ≥ 6 mo, had covered a broad range of lung complications. Compared with alternative techniques, TH-IMRT and H-VMAT were favorable in reducing pneumonitis and symptomatic or radiographic fibrosis for ≥ 6 mo, but the differences among TF-IMRT, FinF, TH-IMRT and H-VMAT were not large and significant for pneumonitis grade ≥ 2, symptomatic or radiographic pneumonitis for ≤ 6 mo. However, MH-IMRT presented a higher probability of toxicity in lung (P>0.05).
 |
Table 5 Calculated NTCP Values (Mean Values and 95% CI, %) in Different Techniques with an Endpoint for the Heart, Ipsilateral Lung (IL) and Total Lung |
Discussion
As modern irradiation techniques, such as IMRT and VMAT, always come at the cost of higher low-dose baths, they bear an increased risk of inducing secondary cancers. Considering the excellent prognosis and long life expectancy after radiotherapy, advanced planning strategies have been actively tested and performed to reduce the related complications of radiotherapy. In order to balance the homogeneous and conformal dose distribution of PTV and the low-dose baths of OARs, the concept of hybrid intensity modulated radiotherapy was proposed by Mayo et al,16 combining 3DCRT and IMRT, which may benefit in decreasing the areas of low-dose exposure. Much research has shown the benefit of hybrid techniques for breast cancer.16–18 In our study, the hybrid techniques could improve the CI and HI compared to TF-IMRT and FinF, especially H-VMAT with significance. Additionally, the comparison of hybrid combinations has shown a possibility to reduce the doses in OARs compared with our previous study.15 Interestingly, the benefits of TH-IMRT are not as obvious as MH-IMRT and H-VMAT, attributed to much higher of the MUs, which can lead to more scatter and leakage radiation outside the irradiation field. Although H-VMAT yields more low-dose volumes in OARs, H-VMAT has advantages over TH-IMRT and MH-IMRT with regard to dose conformity, heart dose and ipsilateral lung dose, even contralateral breast dose, which are consistent with other reports.17,24 As reported, the rate of ischemic heart disease would be increased by 7.4% after adding 1 Gy to the mean heart dose, without a threshold dose.25 Moreover, at higher doses (≥30 Gy), there is a dose-response relationship for factors predicting cardiac dysfunction, while there is also a relationship between cardiac mortality and low doses (~5 Gy).26,27 As with our study, not all V25 of heart obtained from alternative techniques fulfill the dose criteria as the recommendations should be limited to less than 10%.26 Nevertheless, H-VMAT benefits in decreasing high-dose volumes of heart relative to other alternative techniques, while low doses (~5 Gy) among them are similar. H-VMAT presents a lower probability of toxicity with 1.67%, 95% CI:1.28%−2.06% of NTCP in heart. Combined with NTCP estimates, the heart is better protected by H-VMAT in left-sided breast irradiation. Although the NTCP estimates of heart are not meaningful in right-sided breast irradiation, the effects of numerous low-doses from H-VMAT on long-term cardiac toxicity cannot be ignored. 1 Gy increase in mean heart dose results in a 4% increase in the long-term risk of late heart disease from baseline.28 Thus, the mean heart dose (range in 95% CI) from H-VMAT would lead to increase of 6.36%−7.40% in late heart disease for right-sided breast irradiation, which is higher than FinF with 4.32%−5.80%. FinF is more helpful in decreasing the long-term cardiac toxicity in right-sided breast irradiation where low-dose dominates mean heart dose.
In addition to minimizing acute and late toxicity by studying dose-volume tolerances and examining the reasonable limits acceptable to ipsilateral and contralateral structures, it is important to provide an optimal estimation of the risk of secondary cancer induction. Several mathematical models have been developed to estimate secondary cancer risk, while the EAR is proportional to the simulated dose-response relationship for radiation-induced cancer, as well as the age at exposure and the attained age, which can represent a better description of the dose-risk relationship. The dose-risk relationship is linear at lower doses (lower than 2 Gy) for all solid organs. However, it is assumed that the risk of cancer induction is no longer linear but decreasing at higher doses (up to 40 Gy) due to a balance between cell killing and repopulation effects.9,29 The medium to high dose levels (in the range of radiotherapy) are of great interest in this frame. On the other hand, the current predictive power of NTCP parameters is limited. Therefore, an optimal estimation of risk may require a balance between EAR and acute and late radiation-induced toxicity. In our study, MH-IMRT shows superiority to H-VMAT in EAR and LAR of CB while increasing the NTCP of lung. In terms of risk in CB, especially total EAR and LAR in all organs, FinF is comparable or superior to H-VMAT but at the cost of an increased NTCP of heart or lung. TH-IMRT has an NTCP of lung comparable to H-VMAT but at the cost of an increased EAR and LAR, which may be due to much higher of the MUs. According to the calculations of EAR and NTCP, FinF with lower scattered radiation can be considered as a preferred alternative rather than consolidated H-VMAT. It has been found that VMAT presents an increased EAR relative to FinF.11 Compared with previous and present study, not only the risk of toxicity but also the risk of radiation-induced cancer can be minimized through selecting the appropriate techniques. As expected, in the study there was a positive impact of the adoption of advanced radiotherapy techniques.
Although the absolute incidence of secondary cancer in breast cancer treatment is low, it is not negligible despite selecting the optimal radiotherapy technique for individual patients, and the anatomic and clinical characteristics need to be taken into account. For example, doses to the heart, especially in left-sided breast cancer, vary widely, which is strongly related to the distance from heart to thoracic wall. But it was not considered in this study. In the study, the anatomic parameters, such as right and left side, minimum breast distance (MBD) and minimum target concave angle (θMTCA), were taken into account for secondary cancer risk. As expected, the EARs in CB exponentially decreased with increasing MBD and exponentially increased with increasing θMTCA, but there was no significant difference between right and left side. This may be attributed to the physical effects of radiation transport. The amount of scatter radiation to the CB, coming from the linac head and the body (especially the treated breast), would increase with decreasing distance from the target. As θMTCA increase, the distance decreases from the CB to the irradiated tangent field; conversely, the distance increases. Nevertheless, as shown in Figures 3 and 4, it collectively reflects that the MBD can serve as the first predictor of patient-specific risks of CB, followed by θMTCA. However, the variation of EAR in CB among alternative techniques is not identical under the anatomic parameters for group. The fraction of individual variability can be explained by using anatomic parameters of MBD and θMTCA. Furthermore, if the risks of all organs except IL are considered together, the composite LAR may be the lowest in FinF due to lower scattered radiation. In particular, the age at exposure is lower while the secondary cancer risk would be much higher. As is well-known, the secondary cancer risk in CB appears to be common and especially important for younger patients (<45 years) exposed to radiotherapy.30 For older patients, cardiovascular damage is a higher mortality risk than breast cancer itself.31 Thus, it suggests that the age at irradiation exposure may also have an impact on technique selection.
Though FinF technique can reduce the burden of VMAT component, it increases the planning time because the subfields are adjusted and dose is calculated repeatedly. Additionally, the planner’s experience and time pressure have a larger impact on quality of these plans. However, the hybrid technique calculates and optimizes the dose distribution by an inverse approach without relying on planner’s experience, resulting in less planning time. Even H-VMAT can reduce the individual treatment time. On the other hand, as H-VMAT has more freedom to get a coverage that approaches the requested value and to reduce doses to other organs, it is beneficial in decreasing high-dose volumes of CB relative to other alternative techniques, especially when CB is closer to the PTV. Not only secondary breast cancer risk but also breast fibrosis risk, which is defined primarily by the high-dose volume,13 can be reduced. In theory, better HI and CI are expected to be associated with lower local recurrence rates and even fewer radiation-induced complications. However, there are still no studies available to provide clear clinical impact of these parameters. Remarkably, there is a 2.9% excess mortality due to secondary lung cancer, which is higher than the expected mortality due to late heart toxicity.32 Additionally, it has been found that the secondary cancer risk after radiation treatment is mainly from ipsilateral lung in breast cancer patients treated with current radiotherapy techniques,11 although the secondary cancer risk of contralateral breast is comparable higher in the past.33 Although H-VMAT may be less safe for younger patients in terms of secondary cancer risk in all organs, it should still be considered as a valid treatment option, especially in left-sided breast cancer, because it can improve the treatment quality with reducing the subsequent risk for heart disease and radiation pneumonitis, which is well correlated with the dose.
After the previously mentioned critical evaluation, if giving higher priority to CB and lung sparing, especially at MBD less than 2.5cm or θMTCA larger than 140°, H-VMAT should be preferred among alternative techniques for younger patients with breast cancer, followed by a superior FinF. But FinF may be an optimal treatment technique at MBD larger than 2.5cm and θMTCA less than 140°. It is essential to note that FinF should be based on extensive experience of planners, otherwise it is prone to lead to non-optimal dose distribution, resulting in increased secondary cancer risk. If H-VMAT is selected as an alternative at MBD larger than 2.5cm and θMTCA less than 140°, low-dose constraints should be much stricter during the optimization of treatment planning, especially for right-sided breast cancer. The selection is not entirely consistent relative to older patients. As giving a higher priority to heart and lung sparing, H-VMAT is not recommended as a priority for older patients with right-sided breast cancer, while FinF or even TH-IMRT may be more appropriate; conversely, H-VMAT or even a superior TH-IMRT is recommended as a priority for older patients with left-sided breast cancer.
There are still some limitations to our study. Firstly, the weighting between 3D-CRT and IMRT or VMAT is uniform for all combinations of hybrid techniques, such as 80% vs 20%. Although the study has identified 80% vs 20% and 70% vs 30% as optimal weighting for hybrid techniques to achieve balanced results between PTVs and OARs, the fact cannot be ignored that individual anatomy, even patient age, is an important factor for selecting optimal weighting. Furthermore, it should be combined with the risk assessments. For example, long-term toxicity is negligible and early toxicity should be given more consideration in older patients, while it is just the opposite for younger patients. Secondly, as H-VMAT may be prone to uncertainties in intra- and inter-fractional positioning, reproducibility of patient positioning is strictly demanded during treatment planning and execution. Optimally, image-guided radiotherapy from CT acquisition should be performed at the deep-inspiration breath-hold (DIBH).24 Thirdly, due to lack of the available data from large-scale epidemiological studies in advanced radiotherapy techniques, the uncertainties and assumptions in mathematical models, the data from breast cancer patients treated with advanced radiotherapy techniques, especially young patients with long survival, will need to be collected in the following years to improve risk estimation of a population. Finally, due to whole breast irradiation without the boost and lymph node in the present study, the current work should have an extension to consider boost and lymph node irradiation to explore the balance between secondary cancer risk, NTCP, and target coverage.
Conclusion
In summary, hybrid techniques achieve satisfactory dose distribution, especially H-VMAT. The treatment plans should be evaluated not only on the basis of the conventional factors of DVH, but also on the basis of secondary cancer risk assessment as well as NTCP. Furthermore, according to the anatomic parameters, individual estimates can be used effectively for patient stratification and optimal application of radiotherapy techniques by giving a higher priority to contralateral breast, heart and/or lung sparing, which can avoid the “blindness” of technique selection. This may be of assistance in achieving an optimal balance between targets and organs as well as supporting clinical decision-making processes.
Data Sharing Statement
All relevant data are within the paper. There is no separate data set to share.
Ethical Approval and Informed Consent
Institutional Review Board (IRB) approval of the study protocol was obtained from Affiliated Cancer Hospital and Institute of Guangzhou Medical University and consent requirement had been waived by ethical board. It was conducted in compliance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects and the ethical principles of the Declaration of Helsinki. The study was conducted without individual informed consent as the study relied on retrospective data collected as part of radiotherapy treatment for breast cancer patients. In this retrospective study, no patient identifiers were used and data were anonymized. In order to maintain confidentiality, names and other personal identifiers were not included in the data collection either.
Informed Consent
The informed consent requirement was waived.
Acknowledgments
This study was supported by Guangzhou Key Medical Discipline Construction Project Fund; Key Clinical Technology of Guangzhou (Grant Number: 2019ZD17); Medical Science and Technology Foundation of Guangdong Province (Grant Number: A2021457); Medical Science and Technology Foundation of Guangzhou (Grant Number: 20211A011096); GuangDong Basic and Applied Basic Research Foundation, China (Grant Number: 2020A1515110577); Natural Science Foundation of Guangdong Province (Grant Number: 2021A1515011329); Science and Technology Plan Project of Guangzhou City-school Joint (Grant Number: 202201020121).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chen CH, Hsieh CC, Chang CS, Chen MF. A retrospective analysis of dose distribution and toxicity in patients with left breast cancer treated with adjuvant intensity-modulated radiotherapy: comparison with three-dimensional conformal radiotherapy. Cancer Manag Res. 2020;12:9173–9183. doi:10.2147/CMAR.S269893
2. Choi KH, Ahn SJ, Jeong JU, et al. Postoperative radiotherapy with intensity-modulated radiation therapy versus 3-dimensional conformal radiotherapy in early breast cancer: a randomized clinical trial of KROG 15-03. Radiother Oncol. 2021;154:179–186. doi:10.1016/j.radonc.2020.09.043
3. Fogliata A, Seppälä J, Reggiori G, et al. Dosimetric trade-offs in breast treatment with VMAT technique. Br J Radiol. 2017;90:20160701. doi:10.1259/bjr.20160701
4. Shah DJ, Sachs RK, Wilson DJ. Radiation-induced cancer: a modern view. Br J Radiol. 2012;85:e1166–73. doi:10.1259/bjr/25026140
5. Schmitz KH, Prosnitz RG, Schwartz AL, Carver JR. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer. 2012;118:2270–2276. doi:10.1002/cncr.27462
6. Cheng YJ, Nie XY, Ji CC, et al. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6:e005633. doi:10.1161/JAHA.117.005633
7. Newhauser WD, Durante M. Assessing the risk of second malignancies after modern radiotherapy. Nat Rev Cancer. 2011;11:438–448. doi:10.1038/nrc3069
8. National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase. Vol. 2. Washington (DC): National Academies Press; 2006.
9. Schneider U, Sumila M, Robotka J. Site-specific dose-response relationships for cancer induction from the combined Japanese A-bomb and Hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med Model. 2011;8:27. doi:10.1186/1742-4682-8-27
10. Lee B, Lee S, Sung J, Yoon M. Radiotherapy-induced secondary cancer risk for breast cancer: 3D conformal therapy versus IMRT versus VMAT. J Radiol Prot. 2014;34:325–331. doi:10.1088/0952-4746/34/2/325
11. Abo-Madyan Y, Aziz MH, Aly MM, et al. Second cancer risk after 3D-CRT, IMRT and VMAT for breast cancer. Radiother Oncol. 2014;110:471–476. doi:10.1016/j.radonc.2013.12.002
12. Simonetto C, Rennau H, Remmele J, et al. Exposure of remote organs and associated cancer risks from tangential and multi-field breast cancer radiotherapy. Strahlenther Onkol. 2019;195:32–42. doi:10.1007/s00066-018-1384-1
13. Hoekstra N, Habraken S, Swaak-Kragten A, Breedveld S, Pignol JP, Hoogeman M. Reducing the risk of secondary lung cancer in treatment planning of accelerated partial breast irradiation. Front Oncol. 2020;10:1445. doi:10.3389/fonc.2020.01445
14. Paganetti H, Depauw N, Johnson A, Forman RB, Lau J, Jimenez R. The risk for developing a secondary cancer after breast radiation therapy: comparison of photon and proton techniques. Radiother Oncol. 2020;149:212–218. doi:10.1016/j.radonc.2020.05.035
15. Zhang Q, Liu J, Ao N, et al. Secondary cancer risk after radiation therapy for breast cancer with different radiotherapy techniques. Sci Rep. 2020;10:1–12. doi:10.1038/s41598-019-56847-4
16. Mayo CS, Urie MM, Fitzgerald TJ. Hybrid IMRT plans-concurrently treating conventional and IMRT beams for improved breast irradiation and reduced planning time. Int J Radiat Oncol Biol Phys. 2005;61:922–932. doi:10.1016/j.ijrobp.2004.10.033
17. Chen YG, Li AC, Li WY, et al. The feasibility study of a hybrid coplanar arc technique versus hybrid intensity-modulated radiotherapy in treatment of early-stage left-sided breast cancer with simultaneous-integrated boost. J Med Phys. 2017;42:1–8. doi:10.4103/jmp.JMP_105_16
18. Farace P, Zucca S, Solla I, et al. Planning hybrid intensity modulated radiation therapy for whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2012;84:e115–22. doi:10.1016/j.ijrobp.2012.02.025
19. Seppenwoolde Y, Lebesque JV, De Jaeger K, et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2003;55:724–735. doi:10.1016/S0360-3016(02)03986-X
20. Saini AS, Das IJ, Hwang CS, Biagioli MC, Lee WE. Biological indices evaluation of various treatment techniques for left-sided breast treatment. Pract Radiat Oncol. 2019;9:e579–90. doi:10.1016/j.prro.2019.06.020
21. Balaji K, Yadav P, BalajiSubramanian S, Radha CA, Ramasubramanian V. Hybrid volumetric modulated arc therapy for chest wall irradiation: for a good plan, get the right mixture. Phys Medica. 2018;52:86–92. doi:10.1016/j.ejmp.2018.06.641
22. Schneider U, Zwahlen D, Ross D, Kaser-Hotz B. Estimation of radiation-induced cancer from three-dimensional dose distributions: concept of organ equivalent dose. Int J Radiat Oncol Biol Phys. 2005;61:1510–1515. doi:10.1016/j.ijrobp.2004.12.040
23. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106.
24. Venjakob A, Oertel M, Hering DA, Moustakis C, Haverkamp U, Eich HT. Hybrid volumetric modulated arc therapy for hypofractionated radiotherapy of breast cancer: a treatment planning study. Strahlenther Onkol. 2021;4:296–307. doi:10.1007/s00066-020-01696-8
25. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New England J Med. 2013;368:987–998. doi:10.1056/NEJMoa1209825
26. Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76:S77–85. doi:10.1016/j.ijrobp.2009.04.093
27. Chung E, Corbett JR, Moran JM, et al. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys. 2013;85:959–964. doi:10.1016/j.ijrobp.2012.08.002
28. Sardaro A, Petruzzelli MF, D’Errico MP, Grimaldi L, Pili G, Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 2012;103:133–142. doi:10.1016/j.radonc.2012.02.008
29. Gonzalez AB, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–233. doi:10.1016/j.ijrobp.2012.09.001
30. Travis LB, Ng AK, Allan JM, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J Natl Cancer Inst. 2012;104:357–370. doi:10.1093/jnci/djr533
31. Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–66. doi:10.1161/CIR.0000000000000556
32. Hoekstra N, Fleury E, Lara TRM, et al. Long-term risks of secondary cancer for various whole and partial breast irradiation techniques. Radiother Oncol. 2018;128:428–433. doi:10.1016/j.radonc.2018.05.032
33. Boice JD, Harvey EB, Blettner M, Stovall M, Flannery JT. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–785. doi:10.1056/NEJM199203193261201
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.