Back to Journals » Drug Design, Development and Therapy » Volume 15
CpG Oligodeoxynucleotide Developed to Activate Primate Immune Responses Promotes Antitumoral Effects in Combination with a Neoantigen-Based mRNA Cancer Vaccine
Authors Li Q, Ren J, Liu W, Jiang G , Hu R
Received 21 June 2021
Accepted for publication 1 September 2021
Published 18 September 2021 Volume 2021:15 Pages 3953—3963
DOI https://doi.org/10.2147/DDDT.S325790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Qin Li,1,* Jie Ren,2,* Wei Liu,3 Guoqin Jiang,2 Rongkuan Hu1
1GenePharma Co., Ltd., Suzhou, 215125, Jiangsu, People’s Republic of China; 2Department of General Surgery, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, 215004, People’s Republic of China; 3Department of Pathology, The First Affiliated Hospital of Soochow University, Suzhou, 215006, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guoqin Jiang
Department of General Surgery, The Second Affiliated Hospital, Soochow University, 1055 Sanxiang Road, Suzhou, 215004, Jiangsu, People’s Republic of China
Tel +86-512-67784797
Email [email protected]
Rongkuan Hu
GenePharma Co., Ltd., 199 Dongping Street, Suzhou, 215125, Jiangsu, People’s Republic of China
Tel +86-512-86668828
Email [email protected]
Purpose: The purpose of our research was to identify and evaluate synthetic phosphorothioate-modified CPG oligodeoxynucleotides (CPG-ODNs) activating innate and adaptive immune responses. Furthermore, combined treatment with CpG and an mRNA cancer vaccine was evaluated in melanoma models as a therapeutic approach.
Methods: A molecular assay was used to screen new CpG molecules; mouse modeling and pathological analysis were used to confirm the antitumor effect of CpG alone or in combination with an mRNA vaccine. Finally, safety was assessed by monitoring blood biochemistry.
Results: We first screened and identified a new CpG-B class ODN (CpG2018B) that effectively stimulated type II interferons in both mouse plasmacytoid dendritic cells (pDCs) and human peripheral blood mononuclear cells (PBMCs). In addition, CpG2018B promoted cytokine production mainly via toll-like receptor 9 (TLR9) pathways. We further demonstrated that intratumoral (IT) injection of CpG2018B inhibited melanoma growth in syngeneic models and could turn “cold” tumors into “hot” tumors. Then, CpG2018B and an mRNA-based neoantigen cancer vaccine were encapsulated in lipid nanoparticles (LNPs) and intratumorally injected into melanoma mouse models. Interestingly, vaccination with CpG or the mRNA vaccine alone could inhibit tumor growth, while combination of CpG with the mRNA vaccine enhanced the antitumor effect. Finally, we described the long-term safety and tolerability of CpG2018B and mRNA therapy in mice model.
Conclusion: We identified a novel CpG-B class ODN to promote the immune response, and CpG combined with mRNA cancer vaccines is an attractive candidate approach for immunostimulatory sequence (ISS)-based therapeutic strategies.
Keywords: CpG, IFN-γ, TLR9, mRNA vaccine, neoantigen
Introduction
Toll-like receptors (TLRs) constitute a set of receptors that recognize pathogen-associated molecular patterns from microorganisms and danger-associated molecular patterns of a host and induce protective immune responses against infections.1,2 More than ten TLRs have been identified both in mammalian species and in nonmammalian species. Briefly, TLR3 is activated by double-stranded RNA (dsRNA),3 while TLR7 and TLR8 are often activated by nucleosides and viral single-stranded RNA (ssRNA).4 TLR9 recognizes the unmethylated CpG present in bacteria or synthetic ODNs with an unmethylated CpG motif.5 The other TLRs are expressed on the cell surface. They are located on the cell or plasma membrane and recognize cell constituents, such as lipoproteins, flagellin and lipopeptides.
Among the TLRs, TLR9 can induce a Th1-type immune response by recognizing bacterial and synthetic DNA containing unmethylated CpG motifs.6 The immune activities are multifactorial and depend on the ODN backbone, sequence and number of CpG motifs. CPG oligodeoxynucleotides (ODNs), also known as immunostimulatory sequences (ISSs), have recently received widespread attention after approval by the FDA as vaccine adjuvants and have been reported to be strong candidates for therapeutic immune potentiation.7 CPGsare composed of phosphorothioate-modified residues and require at least one or two CpG nucleotides. Based on the sequence, structure and immune profiles induced, CPGshave been divided into several classes including classes A, B and C. Those in class B strongly activate B cell function and induce plasmacytoid dendritic cells (pDCs) to release IFN-γ and TNF-α. Those in class A containing a CpG within a self-complementary (palindrome) region strongly induce IFN-A secretion but poorly promote B cell proliferation. CpG-C requires at least one CG motif and contains at least two or more CpG dinucleotides. CpGs are excellent candidates for ISS-based therapies.8,9
In recent decades, more than 100 clinical trials have mainly focused on immune-oncology or adjuvants based on these types of CpGs, including CpG7909 (Pfizer), SD101 (Dynavax), MGN1703 (Mologen), CMP-001 (Checkmate) and IMO-2125 (Idera). In 2017, a CpG in combination with vaccines designed to prevent hepatitis B virus (HBV) infection was approved by the US FDA, which was the first commercialized CpG treatment (HEPLISAV).7 Moreover, several CpG combination therapies are still being tested in Phase II/III trials, including CpG plus rituximab and CpG combined with an anti-PD-1 or anti-OX40 antibody (NCT02521870, NCT03410901, etc.). Wang et al showed that intratumoral injection of CpG relieves resistance to PD-1 blockade treatment;10 combination of SD101 with an anti-OX40 antibody can trigger a T cell immune response, which in turn remarkably cures multiple types of cancer and prevents spontaneous genetically driven cancers.11
Very recently, the FDA-authorized mRNA COVID-19 vaccines BNT162b2 (Pfizer/BioNTech) and MRNA-1273 (Moderna) have shown high efficacies in clinical trials. mRNA therapeutics represent a promising alternative to conventional vaccine approaches because of their rapid development, low manufacturing cost and easy administration. The first study on the use of in vitro-transcribed (IVT) mRNA in vivo was reported in the 1990s.12,13 However, this promising result did not lead to substantial investment in mRNA therapeutics owing to mRNA instability, innate immunogenicity and inefficient delivery. The use of chemically modified mRNAs, including mRNAs modified with pseudouridine (Ψ),N1-Methylpseudouridine(m1Ψ), 2-thiouridine (s2U) or 5-methylcytidine (m5C), has beneficial safety and stability features, leading to several mRNA-based clinical trials (NCT03313778, NCT03323398, etc.). In addition, lipid nanoparticles (LNPs) have been identified as an efficient delivery vehicle for systemic mRNA administration. Unlike DNA plasmid and viral gene delivery-based vaccines, mRNA does not transit to the nucleus and does not integrate into the chromosomes, therefore avoiding insertional mutagenesis and oncogenesis. mRNA vaccines provide transient, half-life-dependent protein expression and can restore “undruggable” targets, such as transcription factors and nonmembrane proteins. In addition, mRNA-based neoantigens that can mobilize immunity against cancer mutations have been reported.14,15 Intranodal vaccination with a personalized mRNA vaccine has been shown to induce antitumor immunity. Furthermore, intratumoral adoptive transfer of IL-12 mRNA-engineered T cells was found to enhance antitumor efficacy and show few side effects.16 A single intratumoral dose of IL-12 mRNA led to tumor regression in multiple syngeneic mouse models.17 Intratumoral administration of IL-23, IL-36γ and OX40L mRNAs encapsulated in LNPs resulted in a complete response in treated tumors.18 Therefore, the mRNA vaccine field is developing extremely rapidly, and sets of preclinical and clinical data have accumulated over recent years.
Here, we identified a new class B CpG (CpG2018B) that stimulates IFN-γ secretion and gene expression in both mouse pDCs and human PBMCs. Then, the antitumor effect of CpG2018B in a melanoma mouse model was observed, which converted “cold tumors” into “hot tumors” by stimulating CD4 and CD8-positive T cells. Then, CpG combined with an mRNA-based neoantigen encapsulated in lipid nanoparticles was injected to prevent tumor growth. Finally, we described the long-term safety and tolerability of CpG and mRNA therapy in mouse models. These results indicate a new therapeutic approach using CpG and an mRNA vaccine for cancer treatment.
Materials and Methods
Materials
RPMI-1640 medium, fetal bovine serum (FBS), and collagen I were purchased from Sigma-Aldrich (St. Louis, USA). CpGs and mRNA vaccines were synthesized by GenePharma (Suzhou, China) (Table 1). All ELISA kits including those for IFN-γ, IL-6, and TNF-α were purchased from Abcam (MA, USA).
 |
Table 1 Sequence of CpG Oligos, Full-Strands are Phosphorothioate (PS) Modified |
Primary Cell Isolation and Cell Culture
pDCs were isolated from 6- to 8-week-old female C57BL/6J mice (Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) or C57BL/6-TLR9-/- mice (Shanghai Model organisms, Shanghai, China) using an EasySepTM Mouse Plasmacytoid DC isolation kit (STEMCELL Technologies, MA, USA). Human peripheral blood mononuclear cells (PBMCs) were isolated using a SepMate kit following the manufacturer’s protocol of STEMCELL Technologies. PBMCs were cultured in RPMI-1640 medium (Sigma) supplemented with 10% FBS (Sigma) at 37°C in 5% CO2. B16-F10 cell lines were obtained from ATCC. Tumor cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS at 37°C in 5% CO2.
Clinical Blood Samples
Five healthy volunteers without diagnosed metastatic tumors were enrolled in 2018 (median age, 43 years). A 10 mL blood sample was collected from each person at the Second Affiliated Hospital of Soochow University. All of the blood samples were obtained after each person provided informed consent, and the study was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University.
In vitro IFN-γ and Other Cytokine Production Assay
pDCs or PBMCs (106 cells) were isolated from the mouse spleen and human whole blood using separate StemCell isolation kits. Cell stimulations were performed in 96-well plates, and pDCs or PBMCs were stimulated for 24 to 48 hours with control 1040ODN or CpGs prior to supernatant harvest. The supernatants were centrifuged and filtered for IFN-γ, TNF-α and IL-6 measurement using ELISA kits following the manufacturer’s protocol (Abcam, MA, USA).
Western Blotting and Immunohistochemistry
Mouse pDCs were stimulated with CpGs for 48 hours and lysed using SDS buffer (1% SDS). The total protein concentration was measured with a BCA assay kit (Thermo Fisher Scientific). Equal amounts of protein samples were subjected to 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, USA). The membranes were blotted with primary antibodies against p-ERK, P-P38, p-JNK and actin at 4°C overnight (1:1000 each). After incubation with a secondary antibody (1:5000), the blots were developed and detected as previously described.19
Immunohistochemistry (IHC) was performed according to the protocol described in our previous study.19 Briefly, paraffin-embedded samples were deparaffinized and incubated with rabbit anti-CD4, anti-CD8 or anti-TLR9 antibodies (1:200) for 1 h at 37 °C. After washing with PBS, the slides were incubated with a goat anti-rabbit secondary antibody (1:2500) and treated with a 3-diaminobenzidine solution for 20 minutes.
Quantitative Real Time PCR (qRT-PCR)
Total RNA was isolated from cells or tissues using TRIzol (Thermo Fisher). One microgram of RNA was reverse transcribed using the SuperScript Reverse Transcriptase Kit (Thermo Fisher). qRT-PCR was performed using the Power SYBR Green Master Kit (Applied Biosystems). The primers used are described in Table 2. The relative expression of each gene was calculated with the delta-delta CT method and normalized to the expression of the reference gene β-actin.
 |
Table 2 Prime Sequences of qPCR |
mRNA Production and LNP Formulation
A neoantigen sequence for the B16-F10 melanoma model in C57BL/6 mice was identified and reported previously.14 The M30 peptide sequence (DWENVSPELNSTDQP) was optimized into an mRNA sequence (5ʹ-atgGACTGGGAGAATGTAAGTCCAGAACTTAATTCTACAGATCAACCCtaa-3ʹ) and then synthesized by IVT using T7 polymerase, where UTP was substituted with 1-methylpseudo UTP. The DNA template incorporated the 5ʹ UTR, and the 3ʹUTR included a poly-A tail, as reported previously.20 The mRNA transcript was further capped with cap 0 and cap 1 by using the Vaccinia Capping Kit (NEB). LNP formulations were prepared using ionizable lipids (DLIN-MC3), DSPC, cholesterol and PEG at a molar ratio of 50:10:38.5:1.5. The lipid mixture was combined with mRNA at a volume ratio of 3:1 using a microfluidic mixer (Precision Nanosystems, Vancouver, BC) as previously described.20 The nanoparticle size was approximately 71±11 nm with a polydispersity index (PDI) of 0.031 ± 0.017, and the encapsulation efficiency was 92.5% ± 5.75%. The final product was filtered through a 0.22 µm filter and stored in presterilized vials, which were frozen until used.
Animal Studies
B16-F10 tumor cells (5 x 105) were injected subcutaneously at sites in the abdomen of C57BL/6J mice (6–8 weeks old, female, Vital River Laboratory Animal Technology Co., Ltd., Beijing, China). When the tumor size reached 50–100 mm3, the mice were injected intratumorally with CpG (2.5 mg/kg) and/or the mRNA vaccine encapsulated in LNPs (0.5 mg/kg) twice per week. Tumor size was monitored every 2–3 days and used to calculate volume (length x width2)/2. The mice were sacrificed when the tumor size reached 3000 mm3. The tumors were photographed and stained with hematoxylin and eosin (H&E). The animal and primary cell studies were approved by the Ethics Committee for Animal Experimentation of GenePharma Co., Ltd. and conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Clinical Biochemistry Panel
All C57BL/6J mice were sacrificed, and blood samples were collected in serum separator tubes and shipped to the CAM-SU Genomic Resource Center of Soochow University for sample analysis. Clinical biochemistry parameters were measured in a blinded manner with a standard chemistry analyzer (HITACHI 7100) as described by the CAM-SU Genomic Resource Center.
Statistical Analysis
All results are shown as the mean and SD. Significance tests were performed using GraphPad Prism 6.0 (GraphPad Software Inc., CA, USA). The statistical significance of intergroup differences was calculated with ANOVA or Student’s t-test. A p value < 0.05 was considered significant.
Results
CpG2018B Promotes IFN-γ Secretion in Both Mouse pDCs and Human PBMCs
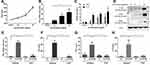
Several studies have shown that synthetic CpGs with a CG motif have a major impact on immune activation, but it is unclear whether different CpG sequences and chemical modifications produce different immune effects. To evaluate this possibility, we tested a panel of CpG ODNs with phosphorothioate backbone modifications and detected IFN-γ secretion by both mouse pDCs and human PBMCs (Table 1). The immunostimulatory activity of an ODN without a CG motif (1040ODN) was set as a negative control, while CpG-1018, which was approved by the FDA (Dynavax), and two other clinically used CpGs, CpG 7909 and SD-101, were set as positive controls. Among the tested ODNs, CpG2018A and CpG2018B showed generally similar patterns of immunostimulatory activity in mouse pDCs. Of note, CpG2018B, which was unique in this panel because it contained two CG-rich regions, showed preferential induction of IFN-γ synthesis (Figure 1A). Furthermore, 5 sets of human PBMCs were isolated from 5 healthy volunteers by centrifugation with a Ficoll density gradient and incubated with CpGs for 48 hours. Consistent with the mouse findings, CpG2018B exhibited marked immunomodulatory activity in human PBMCs (Figure 1B–F).
CpG2018B Stimulates IFN-γ and Other Cytokine Secretion via TLR9
TLR9 signaling is known to induce the activation of a variety of innate and adaptive immune cells. To determine the molecular mechanism of CpG2018B, we first detected IFN-γ expression in mouse pDCs incubated with different concentrations of CpGs. We found that IFN-γ secretion and mRNA expression stimulated by CpG2018B occurred in a dose-dependent manner (Figure 2A and B). In addition, CpG2018B also promoted the expression of two other cytokines, TNF-α and IL-6 (Figure 2C). Furthermore, the phosphorylation of p38 (Thr180/Tyr182), ERK (p-ERK, Thr202/Tyr204) and JNK (p-JNK, Thr183/Tyr185) was also increased (Figure 2D). These results indicate that CpG2018B promotes cytokine expression and activates MAPK signaling.
To further investigate the potential mechanism of CpG2018B, pDCs from TLR9 knockout mice (Shanghai Model Organisms, Shanghai, China) were treated with CpG2018B (1 µg/mL). As shown in Figure 2E and F, the IFN-γ expression in pDCs stimulated by CpG2018B was abrogated by TLR9 knockout. Consistently, TNF-α and IL-6 levels were also decreased in TLR9 knockout pDCs (Figure 2G and H). These data suggest that CpG2018B promotes IFN-γ, TNF-α and IL-6 secretion mainly via TLR9.
In situ Vaccination with CpG2018B Induces Cytokine Expression and Tumor Regression in Melanoma Mouse Models
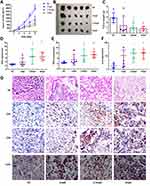
Considering that CpGs stimulate IFN-γ secretion and may inhibit tumor cell proliferation in vivo, we then explored whether CpG2018B has these effects. First, we established syngeneic mouse models (B16-F10) of melanoma and then injected CpG2018B into the tumor nodule. When evaluating the effect of intratumoral injection of CpGs (2.5 mg/kg, twice per week), tumor growth was found to be significantly suppressed by CpG2018B (Figure 3A–C). Furthermore, cytokine expression in tumor samples, including that of IFN-γ, TNF-α and IL-6, was detected by qPCR. The results showed that CpG2018B significantly stimulated the expression of these genes in tumor samples (Figure 3D–F).
We then analyzed intratumoral T cells for their expression of functional markers and observed that CpG2018B could activate CD4 and CD8-positive T cell expression in the tumor microenvironment (TME) with accumulated TLR9 levels (Figures 3G and S1). In addition, H&E staining indicated that there were few tumor cells in the CpG treatment groups (Figure 3G). These results suggest that CpG treatment may turn “cold” tumors into “hot” tumors.
CpG2018B Combined with a Lipid Nanoparticle (LNP)-Formulated mRNA Vaccine Enables Efficient Immunotherapy Against Melanoma
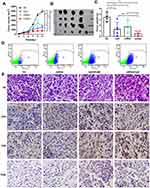
In vitro-transcribed (IVT) mRNA has recently come into focus as a potential new type of drug that acts without alter genetic information. These synthetic mRNAs can be engineered to express proteins by structurally resembling natural mRNAs. mRNA vaccination is a promising strategy for cancer prevention and has entered into clinical development.12,21 In particular, mRNA vaccines targeting tumor-specific mutations (neoantigens) are ideal agents for cancer immunotherapy. As reported previously, Sebastian et al mapped nonsynonymous mutations in B16F10 tumors by next-generation sequencing, and vaccination with mRNA vaccines encoding these neoantigens produced tumor control in mice.14 There is growing evidence to suggest that adjuvant effects associated with stimulation of type I and type II interferons may support adaptive immune responses.22,23 Considering these facts, we hypothesized that CpG2018B could augment mRNA vaccine treatment and help to induce robust antitumor immune responses. To test this hypothesis, we implanted C57B/6J mice with B16-F10 cells as a melanoma model. mRNA targeting a tumor-specific antigen (M30) was incorporated into lipid nanoparticles (LNPs) as described in the Methods. Then, CpG and LNP-formulated mRNA vaccines were injected into syngeneic xenograft models twice per week. The mice were monitored for tumor growth and sacrificed when the tumor size reached 3000 mm3. As shown in Figure 4A–C, tumors in 1040ODN-treated mice grew progressively, while CpG or mRNA vaccine administration alone caused mild regression of tumors. As predicted, the combination of CpG2018B and LNP-mRNA resulted in almost complete regression in the melanoma model.
A previous study indicated that CpG function may be dependent on CD8+ T cells;10 therefore, primary tumor cells were isolated, and CD8-positive T cells were quantified by fluorescence-activated cell sorting (FACS). Consistent with the tumor regression data, there were a relatively large amount of CD8-positive T cells infiltrating the tumor samples (Figure 4D). H&E staining indicated pathological improvement after mRNA vaccine, CpG or combination treatment (Figure 4E). Moreover, after injection of CpG and the mRNA vaccine, there was upregulation of CD4 and CD8-positive T cells (Figures 4E and S2), suggesting that CpG treatment could promote tumor-infiltrating and effector T cell populations.
Safety of Combined Therapy with CpG and the mRNA Vaccine
To evaluate the safety and tolerability of mRNA vaccine and CpG combined therapy in syngeneic mouse models, clinical observation and body weight monitoring were performed throughout the study; clinical observations were performed at least 3 times per week. No mRNA vaccine- or CpG-related clinical findings were identified throughout the entirety of the study. Body weight was similar among all groups at all time points (data not shown). After sacrifice, the liver, heart, spleen, lungs, and kidneys were weighed, and no significant toxicities were observed.
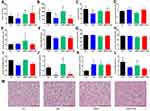
Furthermore, clinical biochemistry evaluations were performed for all mice at the end of the study (Figure 5A–L). Across all clinical biochemistry parameters, no toxicologically relevant findings were detected except a slightly elevated creatinine level. Importantly, no increases in markers of liver toxicity (ALT and AST) were observed in LNP-mRNA-treated mice even though Dlin-MC3-DMA targeted the liver. Consistently, histopathological examination of mice treated with CpG and/or the mRNA vaccine was performed at the end of the study to further evaluate safety. No gross pathological findings related to the mRNA vaccine or CpG were observed in the liver (Figure 5M). This result for mice treated with efficacious doses of the mRNA vaccine and/or CpG is suggestive of a beneficial effect on liver pathology.
Discussion
Several studies have demonstrated that synthetic CpGs are superb vaccine adjuvants in both mice and humans. In this study, we were interested in the identification of an optimal B class CpG for use as an immune activator in both mice and humans. Initially, we performed in vitro screening of IFN-γ induction by CpG molecules. Many chemical modifications were added, including phosphorothioate (PS), 2ʹOME and cholesterol. As a result, we found that CpG2018B with a PS backbone produced the strongest promotion of IFN-γ in mouse pDCs and 5 human PBMC samples. In contrast, other modifications also stimulated IFN-γ secretion but not as strongly as PS, which was consistent with a previous study. An interesting result was that CpG2018B showed better activity in stimulating IFN-γ secretion in both mice and humans than did the FDA-approved molecule ISS-1018 ODN (Dynavax). For all 5 human PBMC samples, their responses to CpGs varied widely.
Furthermore, our data support the following key mechanistic events mediating the immune-activating effect of CpG2018B. In mice, CpG2018B induced dose-dependent induction of IFN-γ, along with increased expression of TNFα and IL-6 in pDCs. Numerous reports have indicated that CpG stimulates TLR9 and activates the MAPK pathway. Consistently, we observed promotion of MAPK substrate phosphorylation and cytokine expression (Figure 2D). Furthermore, knockdown of TLR9 reduced the production of the cytokines IFN-γ, TNFα and IL-6 (Figure 2E–H). Interestingly, knockdown of TLR9 did not change the MAPK pathway (data not shown). One explanation is that CpG activates TLR9 via other pathways, such as TRIF (IKKα and IKKβ) rather than the MAPK pathway.24 Thus, CpG2018B regulates IFN-γ, TNFα and IL-6 induction mainly mediated by TLR9, but the downstream mechanism needs to be further investigated.
Considering that CpG therapy is currently being tested in patients with melanoma, we hypothesized that the therapeutic effect of locally injected CpG could be beneficial in this disease. We found that intratumoral injection of CpG alone could suppress tumor growth in syngeneic mouse models through enhanced immune activity. These data indicated that CpG could turn “cold” tumors into “hot” tumors by stimulating CD4 and CD8-positive T cell populations (Figure 3). Furthermore, as shown in Figure 3, there was increased TLR9 expression in the CpG treatment groups, along with stimulated CD19-positive B cells (Figure S3). These results may support the hypothesis that CpG stimulates TLR9 expression in CD19-positive B cells and thus leads to systemic antitumor adaptive immunity.25
Very recently, tumor antigen- or cytokine-based mRNA vaccines have been tested in tumor patients as a single agent or in combination with PD-1 blockade (mRNA-4157, BNT111, etc.).15,16,18 Here, we transcribed the mRNA sequence of a previously identified mutant MHC class II epitope in vitro (Table 1).14 Then, this mutant mRNA sequence was encapsulated into LNPs, as described in the Methods. In our study, intratumoral injection of CpG and/or the mRNA-based tumor vaccine into a melanoma mouse model resulted in reduced tumor growth. The results from our study demonstrate the potency of this treatment combining CpG with a neoantigen mRNA vaccine in human melanoma.
Furthermore, the safety of the mRNA vaccine was detected by evaluating blood biochemistry. In our protocol, mRNA was transcribed by incorporating N1-methylpseudo-UTP and cap 1 analogs (CleanCap, GAG). These chemical modifications may provide the advantage of avoiding the toxicities and immunogenicity that occur with lipid nanoparticle administration. Our results demonstrated that ALT and AST levels were not significantly increased, even though cationic lipids target the liver. Additionally, pathological analysis of the liver did not find apoptosis or necrosis. There were an increased CREA level and decreased bilirubin level in the combination treatment group, which need to be further analyzed.
In summary, our results identified a new TLR9-dependent B class CpG molecule that promotes a primary immune response in both mice and humans. Furthermore, the combination of this CpG molecule with an mRNA cancer vaccine prevented melanoma growth, suggesting a new approach for cancer treatment.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please contact corresponding author Dr. Hu if you want to request the dataset.
Ethics Approval and Consent to Participate
Study approval was obtained from the independent ethics committee of the Second Affiliated Hospital of Soochow University (Suzhou, China). The privacy of the patients involved was protected.
Consent for Publication
Study participants provided consent for the publication of the data and any associated images.
Acknowledgments
We would like to thank the R&D team at GenePharma.
Funding
This study was supported by funding from the Suzhou Technology Development Program (ZXL2018171) and Jiangsu Science and Technology Project (BZ2018066) to Rongkuan Hu and from The National Natural Science Foundation of China (81873730) and Jiangsu Women and Children Health Key Discipline Program (FXK201758) to Guoqin Jiang.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Cutting edge: diminished T cell TLR expression and function modulates the immune response in human filarial infection. J Immunol. 2006;176(7):3885–3889. doi:10.4049/jimmunol.176.7.3885
2. Alvarez Y, Valera I, Municio C, et al. Eicosanoids in the innate immune response: TLR and non-TLR routes. Mediators Inflamm. 2010;2010:1–14. doi:10.1155/2010/201929
3. Suh HS, Zhao ML, Choi N, Belbin TJ, Brosnan CF, Lee SC. TLR3 and TLR4 are innate antiviral immune receptors in human microglia: role of IRF3 in modulating antiviral and inflammatory response in the CNS. Virology. 2009;392(2):246–259. doi:10.1016/j.virol.2009.07.001
4. Lai CY, Liu YL, Yu GY, et al. TLR7/8 agonists activate a mild immune response in rabbits through TLR8 but not TLR7. Vaccine. 2014;32(43):5593–5599. doi:10.1016/j.vaccine.2014.07.104
5. Byadgi O, Puteri D, Lee JW, et al. The effect of TLR9 agonist CpG oligodeoxynucleotides on the intestinal immune response of cobia (Rachycentron canadum). J Immunol Res. 2014;2014:273284. doi:10.1155/2014/273284
6. Williamson RD, McCarthy FP, Kenny LC, McCarthy CM. Activation of a TLR9 mediated innate immune response in preeclampsia. Sci Rep. 2019;9(1):5920. doi:10.1038/s41598-019-42551-w
7. Hyer RN, Janssen RS. Immunogenicity and safety of a 2-dose hepatitis B vaccine, HBsAg/CpG 1018, in persons with diabetes mellitus aged 60–70 years. Vaccine. 2019;37(39):5854–5861. doi:10.1016/j.vaccine.2019.08.005
8. Martinson JA, Tenorio AR, Montoya CJ, et al. Impact of class A, B and C CpG-oligodeoxynucleotides on in vitro activation of innate immune cells in human immunodeficiency virus-1 infected individuals. Immunology. 2007;120(4):526–535. doi:10.1111/j.1365-2567.2007.02530.x
9. Yamamoto Y, Sugimura R, Watanabe T, et al. Class A CpG oligonucleotide priming rescues mice from septic shock via activation of platelet-activating factor acetylhydrolase. Front Immunol. 2017;8:1049. doi:10.3389/fimmu.2017.01049
10. Wang S, Campos J, Gallotta M, et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8 + T cells. Proc Natl Acad Sci USA. 2016;113(46):E7240–E7249. doi:10.1073/pnas.1608555113
11. Sagiv-Barfi I, Czerwinski DK, Levy S, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10(426):eaan4488. doi:10.1126/scitranslmed.aan4488
12. Sahin U, Kariko K, Tureci O. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi:10.1038/nrd4278
13. Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86(16):6077–6081. doi:10.1073/pnas.86.16.6077
14. Kreiter S, Vormehr M, van de Roemer N, et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. doi:10.1038/nature14426
15. Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi:10.1038/nature23003
16. Etxeberria I, Bolanos E, Quetglas JI, et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8(+) T cells. Cancer Cell. 2019;36(6):613–629 e7. doi:10.1016/j.ccell.2019.10.006
17. Hewitt SL, Bailey D, Zielinski J, et al. Intratumoral IL12 mRNA Therapy promotes TH1 transformation of the tumor microenvironment. Clin Cancer Res. 2020;26(23):6284–6298. doi:10.1158/1078-0432.CCR-20-0472
18. Hewitt SL, Bai A, Bailey D, et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36gamma, and OX40L mRNAs. Sci Transl Med. 2019;11(477):eaat9143. doi:10.1126/scitranslmed.aat9143
19. Huang H, Wang Y, Li Q, Fei X, Ma H, Hu R. miR-140-3p functions as a tumor suppressor in squamous cell lung cancer by regulating BRD9. Cancer Lett. 2019;446:81–89. doi:10.1016/j.canlet.2019.01.007
20. Richner JM, Himansu S, Dowd KA, et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;169(1):176. doi:10.1016/j.cell.2017.03.016
21. Kulkarni JA, Cullis PR, van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28(3):146–157. doi:10.1089/nat.2018.0721
22. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - A new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi:10.1038/nrd.2017.243
23. Richner JM, Himansu S, Dowd KA, et al. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168(6):1114–1125 e10. doi:10.1016/j.cell.2017.02.017
24. Volpi C, Fallarino F, Pallotta MT, et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun. 2013;4:1852. doi:10.1038/ncomms2874
25. Bai L, Chen W, Chen J, et al. Heterogeneity of Toll-like receptor 9 signaling in B cell malignancies and its potential therapeutic application. J Transl Med. 2017;15(1):51. doi:10.1186/s12967-017-1152-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.