Back to Journals » Infection and Drug Resistance » Volume 17
COVID-19-Associated Rhinocerebral Mucormycosis, an Incidental Finding or a Matter of Concern – Mixed-Method Systematic Review
Authors Andreescu M, Moldovan C , Lespezeanu DA, Mocanu AI, Schipor MA, Mocanu H
Received 18 October 2023
Accepted for publication 9 January 2024
Published 31 January 2024 Volume 2024:17 Pages 387—402
DOI https://doi.org/10.2147/IDR.S445458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Mihaela Andreescu,1,2,* Cosmin Moldovan,3,4,* Delia-Andreea Lespezeanu,5,6,* Adela-Ioana Mocanu,5,7 Mihai-Adrian Schipor,8 Horia Mocanu9,10,*
1Department of Hematology, Faculty of Medicine, “Titu Maiorescu” University, Bucharest, 031593, Romania; 2Department of Hematology, Colentina Clinical Hospital, Bucharest, 01171, Romania; 3Department of Medical Surgical Disciplines, Faculty of Medicine, “Titu Maiorescu” University, Bucharest, 031593, Romania; 4Department of General Surgery, Witting Clinical Hospital, Bucharest, 010243, Romania; 5Doctoral School, Faculty of Medicine, “Titu Maiorescu” University, Bucharest, 031593, Romania; 6“Ion Pavel” Diabetes Center, National Institute of Diabetes, Nutrition and Metabolic Diseases “Prof.Dr.N.C. Paulescu”, Bucharest, 030167, Romania; 7Department of ENT&HNS, Polimed Medical Center, Bucharest, 040067, Romania; 8Institute of Space Technology and Space Applications, University of the Bundeswehr, München, 85579, Germany; 9Department of ENT&HNS, Faculty of Medicine, “Titu Maiorescu” University, Bucharest, 031593, Romania; 10Department of ENT&HNS, Găești City Hospital, Găești, Dâmbovița, 135200, Romania
*These authors contributed equally to this work
Correspondence: Adela-Ioana Mocanu, Polimed Medical Center, Bloc 67, Calea Văcărești 280, Bucharest, 040063, Romania, Tel +40723400435, Email [email protected] Mihaela Andreescu, Faculty of Medicine, “Titu Maiorescu” University, Str. Gheorghe Petrașcu 67A, Bucharest, 031593, Romania, Tel +40744559730, Email [email protected]
Abstract: With the advent of COVID-19, the number of patients diagnosed with mucormycosis has increased, especially in developing countries. The reason behind this increase is that COVID-19 causes hypoxia that promotes the growth of fungus. To identify the association between mucormycosis and COVID-19, in critically ill or immunocompromised COVID-19 patients. The literature included in the review was researched from October 1, 2021, to November 1, 2022, by using the Google Scholar database as the search engine. Of the 20 articles included, there were 4 case reports, 2 case series, 10 narrative reviews, and 4 quantitative studies. Mucormycetes growth is caused by several factors, including hyperglycemia owing to previously existing diabetes or excessive use of steroids, increased ferritin levels owing to the inflammatory cascade initiated by COVID-19, and immunosuppression caused by the use of steroids or other immunosuppressive therapy. Reduced white-cell count and activity in COVID-19 leads to increased germination of fungal spores hence developing a catastrophic picture of rhinocerebral mucormycosis. Considering that the hematological patient is frequently treated with cortisone, immunosuppressed due to the underlying condition, but also through the administered therapy, the association with a possible diabetes makes this patient susceptible to developing rhinocerebral mucormycosis during COVID-19 infection. Despite being severe, the association between mucormycosis and COVID-19 is specific and treatable. Development of mucormycosis in hematological patients suffering from severe COVID-19 disease is dangerous, yet not compulsory and can be prevented. Using a common steroid-dose protocol with hyperbaric oxygen and necessary preventive measure reveals the disease as a superadded infection. Hypoxia, poor glycemic control and overuse of steroids or immunosuppressive drugs cause it.
Keywords: COVID-19-associated mucormycosis, immunocompromised, critical, COVID-19, mucormycosis, fungal infection
Introduction
COVID-19, a new coronavirus disease, was first introduced in capital of Hubei, China, on December 21, 2019. The virus spread rapidly all around the globe, affecting millions of people, and was labeled a pandemic by the World Health Organization on March 11, 2020.1 As of January 2023, almost 674,411,804 people have been affected by this virus, with 6,724,248 mortalities globally.2 This disease’s most common presenting clinical features are fever, cough, shortness of breath, and pneumonia. In contrast, some extrapulmonary features of COVID-19 include changes in taste or olfactory sensations, dermatological manifestations including urticaria, and neurological features including dizziness, cerebrovascular accidents, and altered consciousness.3 Like MERS-CoV, COVID-19 causes lower respiratory tract infections, leading to ground-glass opacities causing Acute Respiratory Distress Syndrome. SARS-CoV-2 leads to the initiation of a severe inflammatory cascade leading to diffuse alveolar damage. A decrease in T cell count (CD4+ and CD8+) makes the patients immuno-compromised, increasing their susceptibility to infections especially fungal infections.4 Patients with malignant etiologies often exhibit unfavorable outcomes in COVID-19 infections. Due to the depleted humoral response by cytotoxic therapies and malignant conditions, such patients demonstrate prolonged shedding of SARS-CoV-2. Underlying malignant etiologies and cytotoxic treatment therapies im-pair the immune system which diminishes its ability to fight infections.
The incidence of fungal infections is greater in patients critically ill due to COVID-19 disease and requiring mechanical ventilation or patients who have remained hospitalized for more than 50 days. It is, therefore, essential to observe patients with COVID-19 for fungal infections, especially during the terminal stages of life.5 According to a study by Chen et al6 in China, almost 5 cultures out of 99 extracted from COVID-19 -positive patients were found positive for fungal growth, including 1 case of Aspergillus flavus, 1 case of Candida glabrata and 3 cases of Candida albicans. Similarly, in one study by a German scientist, Invasive Pulmonary Aspergillosis was found in 26.3% of the critically ill COVID-19 patients suffering from moderate to severe Acute Respiratory Distress Syndrome.7 Another study in the Netherlands revealed the occurrence of Invasive Pulmonary Aspergillosis in 19.4% of COVID-19 patients admitted to ICU.8 Of all the fungal co-infections occurring in critically ill COVID-19 patients, the percentage of novel cases of Mucormycosis was approximately 1.7 million.9 Mucormycosis, also known as Zygomycosis, is considered a dreadful infection caused by fungi called mucormycetes.10
Several classes of fungi can cause mucormycosis, including Rhizopus, Rhizomucor, Apophysomyces, Mucor, Lichtheimia, and Syncephalastrum.10 Of all these types of fungi, Rhizopus is considered the most prevalent kind of fungus responsible for most of the mucormycosis cases (60%) and Rhino-orbital-cerebral (90%).11 Due to excessive growth in soil, these fungi are transmitted by contact with fungal spores in the environment. Depending upon the organs affected by the fungus, clinical manifestations of patients frequently vary.12 With the advent of COVID-19, the number of patients diagnosed with mucormycosis has increased, especially in developing countries. The reason behind this increase is that COVID-19 causes hypoxia that promotes the growth of fungus. Moreover, hyperglycemia owing to previously existing diabetes or chronic steroid intake increased ferritin levels owing to inflammatory cascade initiated by COVID-19 and immunosuppression caused by the use of steroids for treatment of COVID-19 allow the growth of micromycetes in the human body. Additionally, decreased white-cell count, as well as activity in COVID-19, leads to increased germination of fungal spores hence developing a catastrophic picture of rhinocerebral mucormycosis.12
Clinical manifestations of mucormycosis are variable and can lead to mortality. Patients with rhinocerebral mucormycosis can present with orbitomaxillary cellulitis, lesions in the nasal cavity or palate, halitosis, nasal congestion, and some may present dull pain. This can complicate ophthalmoplegia, loss of vision, cavernous sinus thrombosis, epidural or subdural abscess, and several other intracranial complications.13 It is therefore important to identify whether rhinocerebral mucormycosis is an incidental finding in COVID-19 patients or an association that should be considered a matter of concern in all critically ill COVID-19 patients. The possible association of COVID-19 and mucormycosis in the hematological patient is of utmost importance to clinicians and represents the main reason for this review.
Research Objectives and Questions
To identify the association of mucormycosis and COVID-19 in patients who are critically ill or immunocompromised due to COVID-19 infection. Based on these objectives, the study raises the following critical question: What is the relationship between mucormycosis and COVID-19 and which are the risk factors, most common species involved, associated pathologies, therapeutic protocols and outcomes for this type of pathology, as described in literature worldwide?
Methodology
Search Strategy
The literature included in the review was researched from October 1, 2021, to November 1, 2022, by using the Google Scholar database as the search engine. The search strategy for the review article was constructed using COVID-19, Mucormycosis, COVID-19 Associated Mucormycosis (CAM), and Immuno-compromised and Critical as keywords. The reference lists of screened articles were searched to identify relevant articles. Duplicates were removed from the articles recruited by literature search. Studies retrieved by search strategies were further screened by title, and the shortlisted studies were screened first via abstract. Studies found irrelevant by abstract were excluded. After title and abstract screening, all articles fulfilling the inclusion criteria were enrolled in the study and included a number of 502 cases reported in various previous case series and systematic reviews. The complete search strategy, including the number of articles retrieved by the database, is shown in Figure 1.
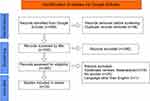 |
Figure 1 Showing the data screening and extraction with PRISMA. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.14 |
Inclusion and Exclusion Criteria
Studies discussing the development of mucormycosis in patients who were COVID-19 positive were included in this review. In contrast, systematic reviews and meta analysis, case reports, articles published in languages other than English, and articles with limited access were excluded. Two independent reviewers selected studies, and both, in collaboration with each other, agreed upon the final selection of articles.
Methodological Quality Assessment
The quality assessment of included review articles was done by utilizing CASP checklist (Critical Appraisal Skills Program Checklist)15 whilst methodological quality assessment of included case reports was done by the JBI (Joanna Briggs Institute) Critical Appraisal checklist.16 The CASP checklist graded each item as a yes, no, or unclear response. The checklist comprises 10 questions that assess the articles’ quality according to the research principles. The studies having CASP scores of less than 6 are considered poor-quality articles.
Recruitment and Screening
A total of 596 papers were found using the search approach, and after duplicates were eliminated, 540 articles underwent title and abstract screening. Two hundred and eighty studies were excluded after title screening, and 260 were tested for eligibility via text screening. Of these 260 articles, 219 were excluded on the basis of selection criteria. Resultantly, 20 articles were considered eligible for the review. The CASP checklist was fulfilled for the included quantitative studies and reviews, whereas JBI critical appraisal checklist was applied to case reports and case series. The critical appraisal scoring of most of the included studies was greater than 8. The JBI Critical appraisal checklist of included case reports and case series is provided in Table 1. In contrast, the CASP checklist for included quantitative studies and reviews is provided in Tables 2 and 3, while the Critical Appraisal score with study titles of study is given in Table 4.
 |
Table 1 Critical Appraisal Skills Program (CASP) Checklist of Included Case Reports |
 |
Table 2 Critical Appraisal Skills Program (CASP) Checklist of Included Quantitative Studies |
 |
Table 3 Critical Appraisal Checklist for Included Review Articles |
 |
Table 4 Critical Appraisal Score with Titles of Included Articles |
Results
Of the 20 articles included in this systematic review, there were 2 case series, 10 narrative reviews, and 4 quantitative studies. The summary studies included in this review and their central concept are summarized in Table 5. At the same time, the findings of cases discussed in case reports and case series are pro-vided in Table 6.
 |
Table 5 Summary of Included Studies |
 |
Table 6 List of Findings of Cases Discussed in Case Reports and Case Series |
COVID-19 Associated Mucormycosis; an Incidental Finding or a Matter of Concern
Although COVID-19 has been primarily a pulmonary affliction, showing modifications of various biological parameters such as high levels of inflammatory cytokines, Neutrophil-to-lymphocyte ratio (NLR) and Platelets-to-lymphocyte (PLR) Ratio, similar to other pneumological pathologies such as Bronchiectasis,38,39 the prevalence of fungus infections has grown with the release of COVID-19.17 After the patients had recovered for a few months, numerous researchers discovered fungal co-infections in COVID-19 patients. COVID-19-associated Mucormycosis (CAM) has been documented in a number of nations, including Australia, France, Iran, India, Brazil, and the United States. About 0.3% of the COVID-19 co-infections that have been detected are related with mucormycosis.23
Some studies have not been included in our detailed analysis due to the scope of the present review; however, we would like to acknowledge their effort in describing COVID-19 associated Mucormycosis. For instance, Baghel et al reported that out of 124 patients with invasive fungal sinusitis and COVID-19, 92.2% presented with mucor infection, 16.9% aspergillus and 12.9% presented with both.40 Similarly, Arora et al reported that in their study regarding co-infection of COVID-19 and mucormycosis, all patients (n=24) presented with paranasal involvement, 7 with palatal involvement, 13 with intra-orbital and 3 with intracerebral involvement.41 In a case presentation, complementary MRI and CT scan of left maxillary sinus revealed invasive fungal sinusitis limited to that area and did not show any associated complications with the orbit or brain.42
Causes and Characteristics of Mucormycosis
Mucormycosis is a fungal infection caused by saprophytic fungi, including several species of Mucorales, ie, Rhizopus, Mucor and Lichtheimia, and Rhizomucor. Mucor, Rhizopus, and Lichtheimia account for almost three-fourth of the cases diagnosed with COVID-19 -associated Mucormycosis.17,34 Mucormycosis is an opportunistic infection that may affect the lungs, skin, gastrointestinal system, central nervous system, and cerebral areas; hence, the patient may present with various clinical symptoms depending upon the organ affected. The disease can present in disseminated form, and literature shows that most of the patients presenting with the disseminated disease are those who either have not received treatment for the disease or have any previous systemic illness.23,26,27
Although the distribution of fungal species that cause mucormycosis varies with a geographical region, Rhizopus arrhizus is considered the most common causative agent among all the fungal species. In contrast, a few cases suffering from Mucor and Chrysosporium lucknowense are also reported. The severe form of mucormycosis forms black eschar due to which it is also known as Black Fungus by some researchers. It should be noted that Mucormycosis is not spread from person to person via direct contact. Only the entry of spores of fungus from the environment by the respiratory tract or direct entry of fungal spores in the wound may lead to mucormycosis via germination of angio-invasive hypha (20). A COSMIC study presented the most common features of CAROCM (COVID-19 associated rhino-orbito-cerebral mucormycosis), including facial or orbital pain, edema, nasal obstruction, loss of vision, ptosis, proptosis, facial edema, and nasal discharge with the occurrence of 23%, 9%, 19%, and 21%, respectively. Patients suffering from pulmonary mucormycosis usually have symptoms overlapping with COVID-19, including pyrexia, dyspnea, and cough.23
Patients with CAROCM usually present with features of sinusal mucormycosis before the extension to orbits and cranial cavities. CAROCM should be suspected in patients presenting with clinical features described in the COSMIC study with the concurrent occurrence of necrotic ulceration involving the nasal cavity, orbital apex, and cavernous sinus leading to orbital apex disease or may cause cavernous sinus thrombosis. Involvement of the ear in mucormycosis can lead to features of otitis externa, emphasizing that standard antibacterial therapy is not sufficient for the ontological symptomatology of patients. The occurrence of pulmonary and disseminated disease in COVID-19 patients has resulted in increased mortality and decreased survival.23
Diagnosis of Mucormycosis
The high mortality of mucormycosis is essential for early confirmation of disease and initiation of early treatment leading to improvement in the patient’s survival. Diagnosis of mucormycosis can be confirmed by direct microscopy in which microscopic slide preparation is mounted with KOH, using fungal culture technique, a biopsy of the lesion, radiological modalities including CT-scan of osteomeatal complex, and MRI with or without contrast and spectrometric analysis.28 Radiological examination is not appropriate for identifying florid sinusitis and bone erosion. REBOVasC checklist, which is essential in reporting imaging, is used in Mucormycosis. REBOVasC checklist is de-signed to focus on radiological findings of bones, rhino sinus, extra sinus, orbital, vascular, and CNS areas for the spread of infections23 (Table 7).
 |
Table 7 Overview of Literature on Rhino-Sino-Cerebral CAM |
Pathogenesis of COVID-19-Associated Mucormycosis (CAM)
CAM may result from various pathological mechanisms related to immunological and inflammatory phenomena.29,30 According to a proposed theory of COVID-Associated Mucormycosis that infection with COVID-19 causes lymphopenia that may result in excessive reduction of TCD4+ and CD8+ T-cells leading to increased chances of opportunistic fungal infections.
Additionally, increased levels of serum proinflammatory cytokines in patients suffering from severe COVID-19 disease predispose patients to fungal infections. Preexistent damaged lungs due to COVID-19 promote invasive fungus growth in patients’ respiratory tract.23
Moreover, increased serum ferritin levels due to excess inflammatory cytokines in circulation, including Interleukin-6 (IL-6), lead to macrophage activation resulting in increased availability of excessive iron within the cells. Increased cellular iron leads to the destruction of endothelial tissue and inflammation, causing endothelium.30 The role of hepcidin in promoting fungal growth is also inevitable as it mimics the action of the COVID-19 virus that leads to the induction of ferritin regardless of the underlying inflammatory reaction.23,29 The immunosuppression modulated by COVID-19 causes endothelial and alveolar damage opening the gates for fungal invasion. Damage to pancreatic tissue may cause an acute diabetes-like state leading to increased serum glucose levels. Elevated ferritin levels and body temperature also produce a favorable environment for fungus growth.
Another theory states that dysregulation in the expression of ACE-2 in multiple organs that can lead to suppression of immune system and the development of a microenvironment that facilitates the growth of COVID-19. The upregulation of a heat shock protein, 78-kDa glucose-regulated protein (GRP78), is also observed in COVID-19 patients owing to increased glucose and ferritin levels induced by the use of corticosteroids in COVID-19 infection. The cell surface GRP78, which is translocated from the endoplasmic reticulum under cellular stress and in various pathological conditions, can bind a variety of ligands and serve as the entry site for viruses like Dengue, Ebola, Zika, Japanese Encephalitis, MERS-CoV and others.45–48 Studies on this receptor’s capacity to act as an alternative entry point for SARS-CoV-2 have also been published.48–50 Moreover, it promotes the pathogenicity and virulence of Mucorales which binds to cell surface GRP78 receptors causing invasive mucormycosis.35,47,51 Diabetes with or without ketoacidosis is a common co-morbidity which could further enhance GRP78 expression in COVID-19 patients and the consequent incidence of mucormycosis.45 The overuse of Vitamin C for the treatment of COVID-19 can lead to blood acidosis which leads to over-expression of GRP78 in primary human dermal microvascular endothelial cells (HDMEC).52 Also, vitamin C promotes intestinal iron absorption, iron being a promoter of Mucorales growth.45
Treatment
Mucormycosis in COVID-19 patients who are ill critically or immunosuppressed persons is now considered a common association, yet the treatment of Mucormycosis in COVID-19 patients is not described by any guidelines.18 Mucormycosis is considered a medical and surgical emergency owing to excessive mortality associated with the disease if not treated timely or turned into a disseminated form.24 Management of mucormycosis usually comprises a medical and surgical aspect.36 Medical management of mucormycosis is based upon empiric antifungal treatment using Amphotericin B, Voriconazole, Posaconazole, or Isavuconazole. In contrast, the surgical treatment comprises surgical debridment of the pathological lesion associated with the disease.19,23,31 Managing Mucormycosis in COVID-19 patients is challenging as a patient suffering from severe COVID-19 is already on immunosuppressive therapy, especially steroids. So, an additional aim in managing mucormycosis in COVID-19 patients is to reverse the immunosuppressive state, especially by withdrawing immuno-suppressive medications to treat COVID-1927,28 (Table 7).
Moreover, immunosuppression caused by hyperglycemia, acidosis, hypoxia, and dyselectrolytemia should also be corrected in mucormycosis. A Granulocyte colony-stimulating factor in mucormycosis is also suggested in patients having severe leucopenia in COVID-19 patients.27 Liposomal Amphotericin B is a first-line antifungal in patients suffering from CNS mucormycosis. Studies recommend using intravenous liposomal amphotericin B at 10 mg/kg/dose. However, the dose should be reduced in case of renal dysfunction as the renal route excretes the drug. In patients suffering from COVID-19, most patients suffer from MOD (Multiple Organ Dysfunction) including injury to kidney and other vital organs like lung, heart and brain. Hence, the dose modification of amphotericin B should be consulted with nephrologists.19–21,36 The use of Amphotericin B in doses less than 5mg/kg/day is ineffective in treating mucormycosis involving CNS. However, the dose is therapeutic without CNS involvement.27
Several other antifungal medications, including Isavuconazole and Posaconazole, can also be used in the treatment of mucormycosis in intravenous doses of 200 mg t.d.s. on days 1–2, 1, and 200 mg per day from day 3 and 300 mg b.d. on day 1 and 300 mg per day from day 2, respectively. Posaconazole orally in doses of 200mg q.i.d. is recommended as an alternative to intravenous amphotericin B in patients suffering from multiple organ dysfunction due to COVID-19.27 Amphotericin B is also used in the treatment of RCOM, but owing to its excessive adverse effects, its use is limited to 1 to 2 initial days of treatment, after which the patient is put on liposomal amphotericin B. Treatment with any of the antifungal in CAM should be continued till the reversal of immunocompromised state initiated by COVID-19. Aside from medical treatment, surgical treatment for mucormycosis is mandatory as the antifungal medication cannot penetrate the necrotic tissue, and debridment of extensive necrotic tissue aids the microbiological diagnosis.36
Hypoxia in COVID-19 patients is also a leading cause of excessive fungal growth. The therapeutic targeting of hypoxia via hyperbaric oxygen therapy improves hypoxia and acidosis. This reverses the immunosuppressive state of COVID-19 patients and halts the multiplication of fungi in the human body.27
Prevention
CAM prevention is an essential element in reducing the incidence of mucormycosis in COVID-19 patients. Studies have suggested using low-dose steroid protocol in patients suffering from COVID-19 and getting ICU- and non-ICU-based care in hospitals.27,37 According to Mulakavalupil et al37 controlled dose steroid protocol, methylprednisolone was used at a dose of 1mg/kg/day for only 3 days in merely those patients suffering from hypoxia, which was evident by oxygen saturation less than 93% or PaO2/FiO2 ratio less than 300. The maximum daily permissible dose of methylprednisolone for every patient was 40mg b.d. Levels of C-Reactive protein (CRP) were measured daily to adjust the dose of Methylprednisolone. The dose was reduced to a maximum of 40mg/day on the fourth day of treatment if the levels of CRP fell below 50mg/L, but if the CRP levels remained above 50mg/L on Day 4, the maximum 40 mg dose of methylprednisolone twice a day was recommended for more 2 days which was later reduced to once daily dosage. The dose was reduced to 30mg of prednisolone on day 6 regardless of the patient’s clinical status, and the dose was further weaned off in the next 5 days of treatment. Intravenous, 200mg of hydrocortisone was given daily by infusion in only patients suffering from septic shock. Intra-venous insulin infusion was used to maintain glycemic control in patients with a target glucose level of 140 to 180mg/dl, which was further shifted to a subcutaneous insulin regime according to the sliding scale. The use of immunosuppressive drugs, ie, Tocilizumab, was used in addition to steroids only in patients suffering from cytokine storm, CRP >50mg/L, IL-6 >100pg/mL or who remained hypoxic despite steroid therapy for more than 24 hours.
None of the patients put on controlled steroid-dose protocol got mucormycosis suggesting it is an important preventive model for CAM.37 Additionally, the use of sterilized water in humidifiers, halting the use of excessive antibiotics, taking a good medical history to be aware of the risk of opportunistic infection for every patient, and good glycemic control is essential to prevent CAM in patients suffering from COVID-19 as it is not a compulsory association yet may develop concurrently.27
Discussion
This review summarizes the occurrence of mucormycosis in patients suffering from COVID-19. A significant body of evidence has emerged regarding the incidence of Mucormycosis in COVID-19 patients. Mucormycosis is a fatal infectious disease with associated high mortality rates.25 It is most commonly found in patients suffering from diabetes mellitus or having immune-compromised status either due to on-going immunosuppressive therapy or hypoxia by respiratory disorders leading to excessive growth of fungi in the respiratory tract of patients, especially sinuses extending to orbits and CNS leading to RCOM, Cavernous sinus thrombosis, and death.53 This review finds that the coexistence of mucormycosis is greater in patients suffering from COVID-19 along with underlying comorbidities compared to those of COVID-19 patients with no preexistent medical illness. Diabetes mellitus is considered an essential pre-disposing factor in COVID-19 as it leads to the stimulation of a pro-inflammatory state leading to suppression of antiviral immunity. The patients suffering from diabetic ketoacidosis are more prone to develop mucormycosis when mucorales, the causative factor of mucormycosis, use excessive free serum iron in its pathogenesis.54 The study by Aggarwal et al states that dysglycemia resulting from diabetes which is a common comorbidity in COVID-19 patients, and indiscriminate steroid use has resulted in a surge of rhino-orbital mucormycosis in India during the second wave of the pandemic. They report on a series of 13 cases of rhino-orbital mucormycosis in COVID-19 with a male preponderance, two cases of loss of vision, and four of intracranial extension of disease. Twelve of these patients had received steroids and 12 had preexisting or newly diagnosed diabetes, both steroid use and diabetes being the most common identified risk factors.55 Most of the patients, in our review of case reports and case series, were males. This finding is parallel to other studies in which male COVID-19 patients suffering from CAM were greater than females. Pal et al report that the majority of the patients was male (78%) and had diabetes mellitus (85%) and glucocorticoid use (85%)43 similar to Nagalli et al with a prevalence of 77.1% for Diabetes mellitus co-morbidity, followed by hypertension (29.5%) and renal disease (14.3%). 55.2% of the patients had also received dexamethasone for COVID-19 infection44 – also see Table 7. Rhino-orbital as well as rhino-orbital-cerebral are considered the most prevalent forms of CAM in which fungi invade the nasal mucosa and walls of orbit, leading to symptoms like facial pain, visual defects, and proptosis and ophthalmoplegia.56 The study by Pal et al shows in a systematic review from 2021 that rhino-orbital mucormycosis was the most common (42%), followed by rhino-orbito-cerebral mucormycosis (24%) and pulmonary mucormycosis in 10 patients (10%). The mortality rate was 34% and the use of adjunct surgery in 81% of patients was associated with better clinical outcomes.43 Patients suffering from CAM should receive medical and surgical treatment timely to prevent associated mortality.57
Our review has a few limitations. As the review is systematic, an association between COVID-19 and Mucormycosis could not be developed. Several predisposing factors occur that may lead to super-added mucormycosis in COVID-19 patients. However, as most of the articles included in our review are case series and review articles, establishing any histopathological or microbiological diagnosis based on the findings is difficult as the results are subjected to heterogeneity, and the findings may underrepresent the disease burden. The association between mucormycosis and COVID-19 has been presented in several studies from countries with large populations and extensive number of COVID-19 patients, such as India or Pakistan, but it is still a novel topic for East-European countries such as Romania where this particular association has been scares or at least rarely reported. We can clearly observe large discrepancies between reported cases in India (over 50%) and similar articles from the Netherlands or Spain with only 1–2% (Table 7).
Conclusion
Since the introduction of the pandemic of COVID-19 virus, researchers have been busy identifying the more novel and long-term complications of COVID-19. Mucormycosis, initially thought as incidental finding in COVID-19 patients, have become a significant concern. The results of the current review have shown that association of Mucormycosis with COVID-19 is dangerous yet not compulsory and can be prevented. Patients with COVID-19 are prone to developing mucormycosis owing to the immunosuppressive states developed by hypoxia, poor glycemic control, and overuse of steroids or immunosuppressive drugs. The use of low steroid-dose protocol with hyperbaric oxygen and necessary preventive measure reveals that mucormycosis is a superadded infection over COVID-19 and not a mandatory association.
Disclosure
The authors report no conflicts of interest in this work. This research received no external funding.
References
1. Ciotti M, Ciccozzi M, Terrinoni A, et al. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365–388. doi:10.1080/10408363.2020.1783198
2. WHO coronavirus (COVID-19) dashboard [Internet]. World Health Organization; 2023. Available from: https://covid19.who.int/.
3. Vetter P, Vu DL, L’Huillier AG, et al. Clinical features of COVID-19. BMJ. 2020;2020;369.
4. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5
6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
7. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528–534. doi:10.1111/myc.13096
8. Bentvelsen RG, van Arkel AL, Rijpstra TA, et al. Fungal infection during COVID-19: does aspergillus mean secondary invasive aspergillosis? Am J Respir Crit Care Med. 2020;202(6):903–904. doi:10.1164/rccm.202006-2241LE
9. Jeong W, Keighley C, Wolfe R, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25(1):26–34. doi:10.1016/j.cmi.2018.07.011
10. Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15 Suppl.5:2–9. doi:10.1111/j.1469-0691.2009.02972.x
11. Yasmin F, Najeeb H, Naeem A, et al. COVID-19 associated mucormycosis: a systematic review from diagnostic challenges to management. Diseases. 2021;9(4):65. doi:10.3390/diseases9040065
12. Singh AK, Singh R, Joshi SR, et al. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146. doi:10.1016/j.dsx.2021.05.019
13. Kontoyiannis DP, Lewis RE. Agents of Mucormycosis and Entomophthoramycosis. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases.
14. Liberati A, Altman DG and Tetzlaff J, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ, 339(jul21 1), b2700–b2700. 10.1136/bmj.b2700
15. Checklist CA, How CS. Critical appraisal skills program; 2018. Available from: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf.
16. Critical appraisal tools [Internet]. JBI; 2023. Available from: https://jbi.global/critical-appraisal-tools.
17. Crone CG, Helweg-Larsen J, Steensen M, et al. Pulmonary mucormycosis in the aftermath of critical COVID-19 in an immunocompromised patient: mind the diagnostic gap. J Medi Mycol. 2022;32(1):101228. doi:10.1016/j.mycmed.2021.101228
18. Buil JB, van Zanten AR, Bentvelsen RG, et al. Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Eurosurveillance. 2021;26(23):2100510. doi:10.2807/1560-7917.ES.2021.26.23.2100510
19. Malek I, Sayadi J, Lahiani R, et al. Acute bilateral blindness in a young COVID-19 patient with rhino-orbito-cerebral mucormycosis. J Ophthalmic Inflamm Infect. 2021;11:1–4. doi:10.1186/s12348-021-00272-0
20. Arana C, Cuevas Ramírez RE, Xipell M, et al. Mucormycosis associated with COVID‐19 in two kidney transplant patients. Transplant Infect Dis. 2021;23(4):e13652. doi:10.1111/tid.13652
21. Palou EY, Ramos MA, Cherenfant E, et al. COVID-19 associated rhino-orbital mucormycosis complicated by gangrenous and bone necrosis—a case report from Honduras. Vaccines. 2021;9(8):826. doi:10.3390/vaccines9080826
22. Riad A, Shabaan AA, Issa J, et al. COVID-19-Associated Mucormycosis (CAM): case-series and global analysis of mortality risk factors. J Fungi. 2021;7(10):837. doi:10.3390/jof7100837
23. Aranjani JM, Manuel A, Abdul Razack HI, et al. COVID-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl Trop Dis. 2021;15(11):e0009921. doi:10.1371/journal.pntd.0009921
24. Pandiar D, Kumar NS, Anand R, et al. Does COVID 19 generate a milieu for propagation of mucormycosis? Med Hypotheses. 2021;152:110613. doi:10.1016/j.mehy.2021.110613
25. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1):26. doi:10.3390/jof5010026
26. Chao CM, Lai CC, Yu WL. COVID-19 associated mucormycosis–an emerging threat. J Microbiol Immunol Infect. 2022;55(2):183–190. doi:10.1016/j.jmii.2021.12.007
27. Rudrabhatla PK, Reghukumar A, Thomas SV. Mucormycosis in COVID-19 patients: predisposing factors, prevention and management. Acta Neurol Belg. 2022;2022;1–8.
28. Malhotra HS, Gupta P, Mehrotra D, et al. COVID-19 associated mucormycosis: staging and management recommendations (Report of a multi-disciplinary expert committee). J Oral Biol Craniofac Res. 2021;11(4):569–580. doi:10.1016/j.jobcr.2021.08.001
29. Narayanan S, Chua JV, Baddley JW. Coronavirus disease 2019–associated mucormycosis: risk factors and mechanisms of disease. Clinl Infect Dis. 2022;74(7):1279–1283. doi:10.1093/cid/ciab726
30. Muthu V, Rudramurthy SM, Chakrabarti A, et al. Epidemiology and pathophysiology of COVID-19 -associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186(6):739–754. doi:10.1007/s11046-021-00584-8
31. Rudramurthy SM, Hoenigl M, Meis JF, et al. ECMM and ISHAM. ECMM/ISHAM recommendations for clinical management of COVID‐19 associated mucormycosis in low‐and middle‐income countries. Mycoses. 2021;64(9):1028–1037. doi:10.1111/myc.13335
32. Sarda R, Swain S, Ray A, et al. COVID-19 -associated mucormycosis: an epidemic within a pandemic. QJM. 2021;114(6):355–356. doi:10.1093/qjmed/hcab165
33. Asdaq SM, Rajan A, Damodaran A, et al. Identifying mucormycosis severity in Indian COVID-19 patients: a nano-based diagnosis and the necessity for critical therapeutic intervention. Antibiotics. 2021;10(11):1308. doi:10.3390/antibiotics10111308
34. Seidel D, Simon M, Sprute R, et al. Results from a national survey on COVID‐19‐associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses. 2022;65(1):103–109. doi:10.1111/myc.13379
35. Sharma S, Grover M, Bhargava S, et al. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngology Otol. 2021;135(5):442–447. doi:10.1017/S0022215121000992
36. Bayram N, Ozsaygılı C, Sav H, et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65(4):515–525. doi:10.1007/s10384-021-00845-5
37. Mulakavalupil B, Vaity C, Joshi S, et al. Absence of case of mucormycosis (March 2020–May 2021) under strict protocol driven management care in a COVID-19 specific tertiary care intensive care unit. Diabetes Metab Syndr. 2021;15(4):102169. doi:10.1016/j.dsx.2021.06.006
38. Leșan A, Man MA, Nemeș RM, et al. Serum interleukin 4 and 6 levels measured using the ELISA method in patients with acquired bronchiectasis compared to healthy subjects. An anti-inflammatory and pro-inflammatory relation. Rev Chim. 2019;70(7):2410–2414. doi:10.37358/RC.19.7.7351
39. Moțoc NS, Martinovici P, Mahler Boca B, et al. Neutrophil-to-lymphocyte ratio (NLR) and Platelets-to-lymphocyte (PLR) ratio in patients with exacerbation of bronchiectasis. Rev Chim Bucharest. 2019;70(11):3889–3892. doi:10.37358/RC.19.11.7665
40. Baghel SS, Keshri AK, Mishra P, et al. The spectrum of invasive fungal sinusitis in COVID-19 patients: experience from a tertiary care referral center in Northern India. J Fungi. 2022;8(3):223. doi:10.3390/jof8030223
41. Arora RD, Nagarkar NM, Krishna Sasanka KSBS, et al. Epidemic in pandemic: fungal sinusitis in COVID-19. J Family Med Prim Care. 2022;11(2):807–811. doi:10.4103/jfmpc.jfmpc_1352_21
42. Samir A, Abdel-Gawad MS, Elabd AM, et al. Early CT and MRI signs of invasive fungal sinusitis complicating COVID-19 infection: case report. Egypt J Otolaryngol. 2022;38(1):17. doi:10.1186/s43163-022-00206-0
43. Pal R, Singh B, Bhadada SK, et al. COVID‐19‐associated mucormycosis: an updated systematic review of literature. Mycoses. 2021;64(12):1452–1459. PMID: 34133798; PMCID: PMC8447126. doi:10.1111/myc.13338
44. Nagalli S, Kikkeri NS. Mucormycosis in COVID-19: a systematic review of literature. Infez Med. 2021;29(4):504–512. PMID: 35146358; PMCID: PMC8805463. doi:10.53854/liim-2904-2
45. Chakrabarti SS, Kaur U, Aggarwal SK, et al. The pathogenetic dilemma of post-COVID-19 mucormycosis in India. Aging Dis. 2022;13(1):24–28. PMID: 35111359; PMCID:PMC8782544. doi:10.14336/AD.2021.0811
46. Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009;11:2299–2306. doi:10.1089/ars.2009.2568
47. Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: a cell’s response to stress. Life Sci. 2019;226:156–163. doi:10.1016/j.lfs.2019.04.022
48. Elfiky AA, Baghdady AM, Ali SA, Ahmed MI. GRP78 targeting: hitting two birds with a stone. Life Sci. 2020;260:118317. doi:10.1016/j.lfs.2020.118317
49. Sabirli R, Koseler A, Goren T, Turkcuer I, Kurt O. High GRP78 levels in Covid-19 infection: a case-control study. Life Sci. 2021;265:118781. doi:10.1016/j.lfs.2020.118781
50. Carlos AJ, Ha DP, Yeh D-W, et al. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J Biol Chem. 2021;296:100759. doi:10.1016/j.jbc.2021.100759
51. Liu M, Spellberg B, Phan QT, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–1924. doi:10.1172/JCI42164
52. Visioli F, Wang Y, Alam GN, et al. Glucose-Regulated Protein 78 (Grp78) confers chemoresistance to tumor endothelial cells under acidic stress. PLoS One. 2014;9:e101053. doi:10.1371/journal.pone.0101053
53. Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: analysis of cases from 18 countries (preprint) Lancet Microbe 2022;3(7):e543–e552. doi:10.1016/S2666-5247(21)00237-8
54. Corzo-León DE, Chora-Hernández LD, Rodríguez-Zulueta AP, et al. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56(1):29–43. doi:10.1093/mmy/myx017
55. Aggarwal SK, Kaur U, Talda D, et al. Case report: rhino-orbital mucormycosis related to COVID-19: a case series exploring risk factors. Am J Trop Med Hyg. 2021;106(2):566–570. PMID: 34902834; PMCID: PMC8832906. doi:10.4269/ajtmh.21-0777
56. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clinl Infect Dis. 2005;41(5):634–653. doi:10.1086/432579
57. Uğurlu ŞK, Selim S, Kopar A, et al. Rhino-orbital mucormycosis: clinical findings and treatment outcomes of four cases. Turk J Ophthalmol. 2015;45(4):169. doi:10.4274/tjo.82474
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
