Back to Journals » Infection and Drug Resistance » Volume 16
COVID-19 and Severe Acute Respiratory Infections: Monitoring Trends in 421 German Hospitals During the First Four Pandemic Waves
Authors Leiner J , Hohenstein S , Pellissier V, König S, Winklmair C, Nachtigall I, Bollmann A, Kuhlen R
Received 22 December 2022
Accepted for publication 12 April 2023
Published 8 May 2023 Volume 2023:16 Pages 2775—2781
DOI https://doi.org/10.2147/IDR.S402313
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Johannes Leiner,1,2 Sven Hohenstein,2 Vincent Pellissier,2 Sebastian König,1,2 Claudia Winklmair,3 Irit Nachtigall,4 Andreas Bollmann,1,2,5 Ralf Kuhlen3,5,6 On behalf of the scientific advisory board of the Initiative of Quality Medicine (IQM)
1Heart Centre Leipzig at University of Leipzig, Department of Electrophysiology, Leipzig, Germany; 2Real World Evidence and Health Technology Assessment at Helios Health Institute, Berlin, Germany; 3Initiative of Quality Medicine, Berlin, Germany; 4Department of Infectious Diseases and Infection Prevention, HELIOS Hospital Emil-von-Behring, Berlin, Germany and Charité - Universitaetsmedizin Berlin, Institute of Hygiene and Environmental Medicine, Berlin, Germany; 5Helios Health Institute, Berlin, Germany; 6Helios Health, Berlin, Germany
Correspondence: Ralf Kuhlen, Initiative Qualitaetsmedizin e.V, Alt-Moabit 104, Berlin, 10559, Germany, Tel +49 30 7262 152 - 0, Email [email protected]
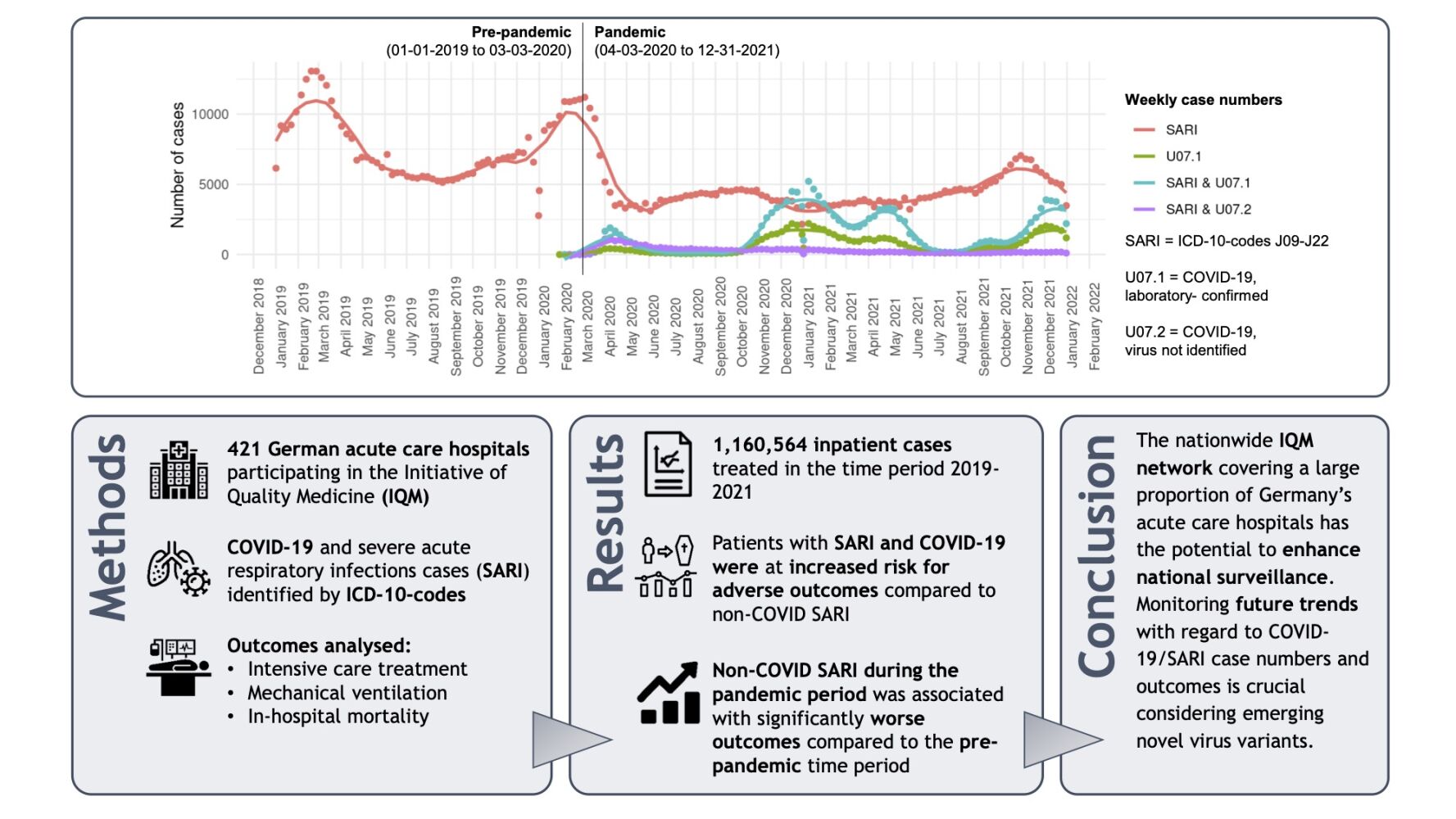
Introduction: Reliable surveillance systems to monitor trends of COVID-19 case numbers and the associated healthcare burden play a central role in efficient pandemic management. In Germany, the federal government agency Robert-Koch-Institute uses an ICD-code-based inpatient surveillance system, ICOSARI, to assess temporal trends of severe acute respiratory infection (SARI) and COVID-19 hospitalization numbers. In a similar approach, we present a large-scale analysis covering four pandemic waves derived from the Initiative of Quality Medicine (IQM), a German-wide network of acute care hospitals.
Methods: Routine data from 421 hospitals for the years 2019– 2021 with a “pre-pandemic” period (01– 01-2019 to 03– 03-2020) and a “pandemic” period (04– 03-2020 to 31– 12-2021) was analysed. SARI cases were defined by ICD-codes J09-J22 and COVID-19 by ICD-codes U07.1 and U07.2. The following outcomes were analysed: intensive care treatment, mechanical ventilation, in-hospital mortality.
Results: Over 1.1 million cases of SARI and COVID-19 were identified. Patients with COVID-19 and additional codes for SARI were at higher risk for adverse outcomes when compared to non-COVID SARI and COVID-19 without any coding for SARI. During the pandemic period, non-COVID SARI cases were associated with 28%, 23% and 27% higher odds for intensive care treatment, mechanical ventilation and in-hospital mortality, respectively, compared to pre-pandemic SARI.
Conclusion: The nationwide IQM network could serve as an excellent data source to enhance COVID-19 and SARI surveillance in view of the ongoing pandemic. Future developments of COVID-19/SARI case numbers and associated outcomes should be closely monitored to identify specific trends, especially considering novel virus variants.
Keywords: initiative of quality medicine, Germany, COVID-19, SARI, inpatient, hospital network
Graphical Abstract:
Introduction
Close monitoring of temporal trends with respect to, eg, case numbers, intensive care (ICU) capacities and mortality in the inpatient sector represents an integral part of pandemic control, as the ongoing COVID-19 pandemic still poses a major burden for health-care systems worldwide. The importance of leveraging existing national surveillance systems for respiratory diseases has been emphasized by the World Health Organization (WHO) and European Centre for Disease Prevention and Control (ECDC)1–3 as it is crucial to additionally monitor trends of other circulating pathogens (eg, influenza). With regard to the inpatient sector, the ECDC recommends surveillance for severe acute respiratory infections (SARI) to meet this need.3 A SARI surveillance system (ICOSARI) based on ICD-10-codes (International Statistical Classification of Diseases and Related Health Problems, Version 10) using routine data of the Helios hospital network was established in Germany in 2017.4 ICOSARI data of 71 German hospitals is continuously analysed and reported by the federal government agency Robert-Koch-Institute (RKI) as part of the national pandemic monitoring program.5
Within the German health-care system, inpatient care is provided by around 1900 hospitals run by the state, local authorities, non-profit organisations and private providers.6 Quality assurance is regulated by law and overseen by the Federal Joint Committee (G-BA), which in turn is supported by the independent Institute for Quality and Efficiency in Health Care (IQWiG) and the independent Institute for Quality and Transparency in the Health Care System (IQTIG).6 In addition to statutory quality assurance, more than 500 hospitals in Germany and Switzerland are voluntarily organised in the “Initiative of Quality Medicine” (IQM, Berlin, Germany).7 Utilizing a large-scale administrative dataset derived from the IQM, we conducted an extended analysis of SARI and COVID-19 cases covering four pandemic waves to report on patient characteristics and respective outcomes and compare pre-pandemic with pandemic time periods.
Methods
Within the IQM, member hospitals provide anonymized routine data semi-annually to a central data evaluation centre (3M Health Information Systems, Berlin, Germany) where quality indicators are calculated and made publicly available.8,9 For this analysis, administrative data of 421 German hospitals between 2019 and 2021 (full inpatient treatment only) was retrospectively analysed. The periods 01–01-2019 to 03–03-2020 and 04–03-2020 to 31–12-2021 were defined as “pre-pandemic” and “pandemic”, respectively. To account for potential differences with regard to the dominating SARS-CoV-2 variant, we defined time periods for Wildtype, Alpha and Delta dominance (according to data by RKI)10 and performed subgroup analyses (Table S1a–c).
Present COVID-19 was identified by ICD-10-codes U07.1 (PCR-confirmed SARS-CoV-2 infection) and U07.2 (suspected COVID-19, virus not identified) as main or secondary diagnosis. Additionally, we identified SARI cases by ICD-10-codes J09-J22 in accordance with common methods of SARI surveillance in Germany.4 Four groups were defined consecutively: SARI without any concomitant coding for COVID-19 (hereafter referred to as “SARI”), proven COVID-19 (U07.1) and SARI with concomitant COVID-19 codes (SARI&U07.1; SARI&U07.2). The following outcomes and treatments were analysed utilizing the Operation and Procedure Classification System (OPS): ICU treatment (OPS-codes 8–980/d/f, 8–70x, 8–711/2/3/4/8, 8–721.1/2/3, 8–97a/b), mechanical ventilation (duration of ventilation >0 h), in-hospital mortality.
For the description of patient characteristics, we employed logistic regression for binary variables and linear regression for numeric variables. For the comparison of outcomes in the different cohorts, we used logistic regression with a logit link function.
The analysis was carried out according to the principles outlined in the Declaration of Helsinki. Given the retrospective evaluation of anonymized data, patient informed consent has not been obtained and ethics committee approval was determined not to be required in accordance with German law [Professional Code for Physicians (Saxony) §15]. Within the IQM network, data security is ensured by local data protection authorities of all member hospitals.
Results
A total of 1,160,564 cases fulfilling the above-mentioned definition of SARI or COVID-19 (or both) in the period 2019–2021 were included in the analysis. Temporal trends and weekly case numbers are depicted in the graphical abstract. The four COVID-19 waves present themselves in peaks of U07.1 and SARI&U07.1 cases. Case numbers of SARI&U07.1 were higher in comparison to U07.1 suggesting a considerable proportion of severe disease courses among hospitalized patients. A considerable number of SARI&U07.2 cases was only present during the first wave. There was no peak of SARI cases in the winter period 2020/2021, but a small peak in fall/winter of 2021 where case numbers remained significantly lower as compared to the winter periods of 2018/2019 and 2019/2020 due to enhanced hygiene measures adopted by the German federal government during the fourth wave.11
Case numbers of SARI decreased markedly in 2020 and 2021 (Table 1). Mean age was significantly lower in the U07.1 group and significantly higher in SARI&U07.1 and SARI&U07.2 groups compared with SARI. Male gender was more frequent only in the U07.1 group. Of note, COVID-19 patients hospitalized during the Wildtype period were older in comparison to Alpha and Delta (Table S1a-c). Compared to SARI, U07.1 cases had lower odds for all outcomes of interest, whereas significantly higher odds were observed for SARI&U07.1, but not SARI&U07.2 (Table 1). This applied to the total cohort as well as the three sub-cohorts considering the dominating virus variant. In-hospital mortality rates for U07.1/SARI&U07.1 were highest during the Wildtype period, followed by Delta (Table S1a-c).
 |
Table 1 Case Numbers, Patient Characteristics and Outcomes in SARI and COVID-19 Patients |
Comparing SARI cases without any additional coding for COVID-19 in the pre-pandemic and pandemic periods, we found a slightly different age and gender distribution. Interestingly, SARI (excluding COVID-19) during the pandemic period was associated with significantly worse outcomes compared to pre-pandemic times (Table S2).
Discussion
In this analysis of claims data derived from 421 German acute care hospitals, which represents to the best of our knowledge the largest German dataset of COVID-19 and SARI inpatients yet analysed, we highlight the following main findings: COVID-19 patients with concomitant SARI compared to SARI not caused by SARS-CoV-2 were at a markedly increased risk for adverse outcomes independent of the dominating virus variant. Comparing the pandemic to the pre-pandemic period, non-COVID SARI was associated with significantly worse outcomes during the pandemic, for example with a 27% higher in-hospital mortality risk. Mean age was higher in the “pandemic” group, albeit the observed difference was only small with questionable clinical relevance (63.9 vs 65.2 years; P < 0.01). Patients’ restraint to enter the healthcare system during the pandemic which consecutively would lead to delayed treatment and increased case severity or, on the other hand, limited treatment, and ICU capacities due to the high burden of COVID-19 could have possibly influenced outcomes of non-COVID SARI patients. This contrasts observations made for the first year of the pandemic where a reduced in-hospital mortality risk was reported for patients with respiratory diseases after excluding SARS-CoV-2 cases.12 It may also be assumed, that SARI patients indeed suffered a SARS-CoV-2 infection in their domestic environment but were hospitalized at a time when the virus could no longer be detected by PCR. In this context, a possible selection bias must be mentioned as a limitation, as unequal time periods with clearly different monthly case numbers were analysed (Table S2). The reduced overall SARI case numbers during the pandemic period might have led to a larger relative share of more severe cases which required hospitalization. However, this trend requires close future monitoring.
As mentioned before, real-world comprehensive SARI surveillance is of uttermost importance in the light of the ongoing pandemic and is established in several European countries including Germany.13–16 Routine data fully based on ICD-codes should be considered a reliable data source to serve this purpose as has also been stated by authors of a previous study focusing on emergency department attendees in Germany.17 Monitoring timely trends of COVID-19 and SARI cases can also facilitate the assessment of SARS-CoV-2-related disease severity, which was just recently proposed by members of this author group.18 This is especially important with regard to possible future virus variants.
As a limitation it must be noted that the IQM administrative dataset contains no information with regard to patients’ vaccination status and this influencing variable could therefore not be considered separately in this analysis.
Conclusion
Quickly available standardized routine data of the IQM network comprising COVID-19, SARI and ICU cases provide an excellent basis for monitoring trends and enhance nationwide surveillance and pandemic management. This data source comprising a large amount of acute care hospitals has the potential to supplement the existing ICOSARI network in Germany due to the extended coverage.
Abbreviations
CI, Confidence interval; COVID-19, Coronavirus disease 2019; ECDC, European Centre for Disease Prevention and Control; ICD-10, International Statistical Classification of Diseases and Related Health Problems Version 10; ICOSARI, ICD-code-based SARI surveillance system in Germany; ICU, Intensive care unit; IQM, Initiative of Quality Medicine; OPS, Operation and Procedure Classification System; OR, Odds Ratio; PCR, Polymerase chain reaction; RKI, Robert-Koch-Institute; SARI, Severe acute respiratory infection(s); SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Data Sharing Statement
The data that support the findings of this study are not publicly available as they contain information that could compromise the privacy of research participants but are available from the IQM network ([email protected]) upon reasonable request.
Ethics Approval and Consent to Participate
The analysis was carried out according to the principles outlined in the Declaration of Helsinki. Given the retrospective evaluation of anonymized data, patient informed consent has not been obtained and ethics committee approval was determined not to be required in accordance with German law [Professional Code for Physicians (Saxony) §15].
Consent for Publication
All authors gave final approval for publication.
Acknowledgments
Members of the scientific advisory board of the Initiative of Quality Medicine in alphabetic order:
- Prof. Dr. Boris Augurzky, Head of Health Competence Area, Rheinisch-Westfälisches Institut für Wirtschaftsforschung e. V.
- Christian Dreissigacker, Managing Director (Chairman), BG Klinikum Unfallkrankenhaus Berlin
- Prof. Dr. Maria Eberlein-Gonska, Head of the Quality and Medical Risk Management Division, Universitätsklinikum Carl Gustav Carus Dresden
- Dr. Heidemarie Haeske-Seeberg, Head of Quality Management and Clinical Risk Management, Sana Kliniken AG
- Prof. Dr. Udo X. Kaisers, Chairman of the Board and Chief Medical Director, Universitätsklinikum Ulm
- Jürgen Klauber, Managing Director, Wissenschaftliches Institut der AOK (WIdO)
- Prof. Dr. Ralf Kuhlen, Helios Health and Helios Health Institute, Berlin
- Prof. Dr. Jörg Martin, Managing Director and Spokesperson of the Management Board, RKH- Regionale Kliniken Holding GmbH
- Dr. Ulrike Nimptsch, Health Care Management, Technische Universität Berlin
- Prof. Dr. Heidi Petry, Head of Center for Clinical Nursing Research, UniversitätsSpital Zürich
- Ralf Rambach, Member of the Board (Treasurer), Deutsche Leukämie- & Lymphom-Hilfe e.V.
- Dr. Jens Schick, Board of Directors, Sana Kliniken AG
- Daniel Schmithausen, Sales Lead Analytics, 3M Deutschland GmbH, Health Information Systems,
- Prof. Dr. med. Jochen Schmitt, Chair of Social Medicine and Health Services Research Medical Faculty, Universitätsklinikum Carl Gustav Carus Dresden
- Prof. Dr. Peter C. Scriba, Former Medical Director of the Klinikum Innenstadt, Klinikum der Universität München (LMU)
- Prof. Dr. Stephan Timm, Chief Physician of the Clinic for Surgery and Medical Director, Malteser Norddeutschland gGmbH - St. Franziskus-Hospital
- Andreas Westerfellhaus, Former State Secretary, Bundesministerium für Gesundheit
- Prof. Dr. Josef Zacher, Medical Advisor | Liaison Officer Medical Quality, Helios Health GmbH
Author Contributions
JL is the first author of this manuscript. RK is senior author of this manuscript and the corresponding author. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
We declare no conflicts of interest associated with this publication.
References
1. World Health Organization. Operational considerations for COVID-19 surveillance using GISRS: interim guidance; 2020. Available from: https://apps.who.int/iris/handle/10665/331589.
2. European Centre for Disease Prevention and Control. Strategies for the surveillance of COVID-19. Available from: https://www.ecdc.europa.eu/en/publications-data/strategies-surveillance-covid-19.
3. European Centre for Disease Prevention and Control. COVID-19 surveillance guidance - Transition from COVID-19 emergency surveillance to routine surveillance of respiratory pathogens. Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-surveillance-guidance.
4. Buda S, Tolksdorf K, Schuler E, Kuhlen R, Haas W. Establishing an ICD-10 code based SARI-surveillance in Germany – description of the system and first results from five recent influenza seasons. BMC Public Health. 2017;17:1. doi:10.1186/s12889-017-4515-1
5. Robert-Koch-Institut. Wochenberichte zu COVID-19 (Weekly reports on COVID-19). Available from: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenberichte_Tab.html;jsessionid=8286D2EA87630E86B19A3F1014BD10C8.internet051?nn=13490888.
6. Federal Ministry of Health Department L 8. Public relations, publications: das deutsche gesundheitssystem - deutsche version (The German healthcare system - German version) 2022; 2022. Available from: https://www.bundesgesundheitsministerium.de/fileadmin/user_upload/Das-deutsche-Gesundheitssystem_bf.pdf.
7. Initiative for Quality Medicine (IQM). Members; 2023. Available from: https://www.initiative-qualitaetsmedizin.de/en/members.
8. Initiative for Quality Medicine (IQM). Quality Methodology; 2023. Available from; https://www.initiative-qualitaetsmedizin.de/en/quality-methodology.
9. Nimptsch U, Mansky T. Krankheitsspezifische Versorgungsmerkmale in Deutschland: Analyse anhand der Bundesauswertung der German Inpatient Quality Indicators (G-IQI) [Disease-specific patterns of hospital care in Germany analyzed via the German Inpatient Quality Indicators (G-IQI)]. Dtsch Med Wochenschr. 2012;137(28–29):1449–1457. German. doi:10.1055/s-0032-1305086
10. Robert-Koch-Institut. Anzahl und Anteile von VOC und VOI in Deutschland (Numbers and shares of VOC and VOI in Germany). Available from: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/VOC_VOI_Tabelle.html.
11. [Bundesregierung: videoschaltkonferenz der Bundeskanzlerin mit den Regierungschefinnen und Regierungschefs der Länder am 18. November 2021]. Federal Government: video conference of the Federal Chancellor with the heads of government of the states on November 18, 2021. Available from: https://www.bundesregierung.de/resource/blob/974430/1982598/defbdff47daf5f177586a5d34e8677e8/2021-11-18-mpk-data.pdf?download=1.
12. König S, Pellissier V, Hohenstein S, et al. A comparative analysis of in-hospital mortality per disease groups in Germany before and During the COVID-19 pandemic from 2016 to 2020. JAMA Network Open. 2022;5(2):e2148649–e2148649. doi:10.1001/jamanetworkopen.2021.48649
13. Klavs I, Serdt M, Učakar V, et al. Enhanced national surveillance of severe acute respiratory infections (SARI) within COVID-19 surveillance, Slovenia, weeks 13 to 37 2021. Eurosurveillance. 2021;26(42). doi:10.2807/1560-7917.ES.2021.26.42.2100937
14. Grgič Vitek M, Klavs I, Učakar V, et al. Vaccine effectiveness against severe acute respiratory infections (SARI) COVID-19 hospitalisations estimated from real-world surveillance data, Slovenia, October 2021. Eurosurveillance. 2022;27:1.
15. UK Health Security Agency. Weekly national Influenza and COVID-19 surveillance report Week 1 report (up to week 52 data) 6 January 2022. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1045027/Weekly_Flu_and_COVID-19_report_w1.pdf.
16. European Centre for Disease Prevention and Control. Survey on the implementation of integrated surveillance of respiratory viruses with pandemic potential. Available from: https://www.ecdc.europa.eu/en/publications-data/survey-implementation-integrated-surveillance-respiratory-viruses-pandemic.
17. Boender TS, Cai W, Schranz M, et al. Using routine emergency department data for syndromic surveillance of acute respiratory illness, Germany, week 10 2017 until week 10 2021. Eurosurveillance. 2022;27:27. doi:10.2807/1560-7917.ES.2022.27.27.2100865
18. Leiner J, Pellissier V, Hohenstein S, et al. Characteristics and outcomes of COVID-19 patients during B.1.1.529 (Omicron) dominance compared to B.1.617.2 (Delta) in 89 German hospitals. BMC Infect Dis. 2022;22:1. doi:10.1186/s12879-022-07781-w
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
