Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost-Utility Analysis of Sacroiliac Joint Fusion in High-Risk Patients Undergoing Multi-Level Lumbar Fusion to the Sacrum
Authors Ackerman SJ, Deol GS, Polly DW
Received 2 June 2022
Accepted for publication 29 July 2022
Published 8 August 2022 Volume 2022:14 Pages 523—535
DOI https://doi.org/10.2147/CEOR.S377132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Stacey J Ackerman,1 Gurvinder S Deol,2 David W Polly3
1Department of Biomedical Engineering, Johns Hopkins University, San Diego, CA, USA; 2Wake Orthopedics, Raleigh, NC, USA; 3Department of Orthopedic Surgery, University of Minnesota, Minneapolis, MN, USA
Correspondence: Stacey J Ackerman, Email [email protected]
Purpose: Multi-level lumbar fusion to the sacrum (MLF) can lead to increased stress and angular motion across the sacroiliac joint (SIJ), with an incidence of post-operative SIJ pain estimated at 26– 32%. SIJ fusion (SIJF) can help obviate the need for revisions by reducing range of motion and screw stresses. We aimed to evaluate the cost-utility of MLF + SIJF compared to MLF alone among high-risk patients from a payer perspective, where high risk is defined as high body mass index and high pelvic incidence.
Methods: A Markov process decision-analysis model was developed to evaluate cumulative 5-year costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICER) of MLF + SIJF compared to MLF alone using published data; costs from Medicare claims data analyses and health state utility values (derived from EQ-5D) informed by three prospective, multicenter, clinical trials. The base case assumed a reduction in post-operative SIJ pain from 30% to 10% (relative risk reduction [RRR] of 67%). Costs and utilities were discounted 3% annually. The ICER is reported in 2020 US dollars. One-way, multi-way, and probabilistic sensitivity analyses were performed.
Results: With an assumed 30% incidence of SIJ pain after MLF alone, stabilizing with SIJF was associated with an additional 5-year cost of $2421 and a gain of 0.14 QALYs, resulting in an ICER of $17,293 per QALY gained (similar to total knee arthroplasty and more favorable than open discectomy). ICERs were most sensitive to the RRR of post-operative SIJ pain conferred by SIJF, time horizon, and probability of successful treatment with MLF alone. At a willingness-to-pay threshold of $50,000/QALY gained, MLF + SIJF has a 97.7% probability of being cost-effective in the target patient population.
Conclusion: Fusing the SIJ in high-risk patients undergoing MLF was cost-effective when the incidence of post-operative SIJ pain after MLF alone exceeds approximately 25%, providing value-based healthcare from a payer perspective.
Keywords: cost-effectiveness analysis, lumbar spinal fusion surgery, sacroiliac joint pain
Plain Language Summary
Spine fusion is a common surgery to treat back pain, ranking among the top ten operating room procedures in the United States. Fusing multiple vertebrae in the lower back to the triangular bone at the bottom of the spine (the sacrum) can lead to increased biomechanical stress across the pelvis, causing post-operative pelvic pain in about one-third of patients attributable to the sacroiliac (SI) joint. Fusing the pelvis to the sacrum can help stabilize the joint, thereby reducing the biomechanical stress and risk of SI joint pain – which can help avoid additional hospital-inpatient surgery with associated costs. This is the first study to examine – for obese patients representing about 40% of the US adult population – whether fusing the pelvis to the sacrum in patients undergoing multiple-vertebrae fusion improves health quality at an acceptable cost. Our results demonstrated that, when considering the five years after surgery, fusing the pelvis to the sacrum during multi-level spine fusion surgery appears to be cost-effective, providing value-based healthcare.
Introduction
Spinal fusion is a common surgery to treat back pain, ranking among the top ten hospital inpatient operating room procedures in the United States.1 Multi-level lumbar fusion to the sacrum (MLF) can lead to increased stress and angular motion across the sacroiliac joint (SIJ) with an incidence of post-operative SIJ pain estimated at 26.1% to 32.1% among patients without pelvic fixation.2–5 Investigators have previously reported that increased pre-operative body mass index (BMI) and higher pelvic incidence (PI) are risk factors associated with the new onset of post-operative SIJ pain after MLF.5,6
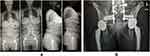
SIJ fusion (SIJF) using porous, 3D printed titanium, triangular-shaped implants placed posteriorly across the SIJ in the sacral-alar-iliac (SAI) trajectory (Figure 1) is designed to help obviate the need for revisions by reducing range of motion and screw stresses. Additional potential clinical and economic benefits of permanent stabilization of the SIJ through SIJF are reduced incidence of rod breakage, pelvic screw loosening, and set screw failures.
Yet the upfront treatment costs for the SIJF implants need to be weighed relative to the likelihood of future cost-savings and quality-of-life improvements. As such, we sought to explore under what scenarios fusing the SIJ in “high-risk” patients undergoing 2–4 level lumbar fusions could improve health quality at an acceptable cost from the payer perspective, where high risk is defined as high BMI and high PI.
Materials and Methods
Model Overview
Using established best practices, a Markov process decision-analysis model was developed in Microsoft Excel to estimate cumulative 5-year costs (2020 United States dollars [USD]) and quality-adjusted life years (QALYs) from a US payer perspective with 1-year cycle length.7–9 Both costs and QALYs were discounted 3% annually.10 The model interventions were (1) multi-level lumbar fusion surgery without pelvic fixation in which 2–4 levels are fused; and (2) multi-level lumbar fusion surgery with pelvic fixation and additional placement of 3D printed implants in a trajectory parallel to the SAI screws (iFuse Bedrock™, SI-BONE, Inc., Santa Clara, CA, USA). Of note, patients with 2 to 4-level fusions do not normally undergo pelvic fixation.
The model population was obese adults (BMI of 30–39.9) with high PI (>60°) who are undergoing MLF with versus without SIJF.6,11–14 Diagnoses for the model population included spondylolisthesis; lumbosacral intervertebral disc disorder; adult degenerative scoliosis; post-laminectomy syndrome; and flat back syndrome. The model population excludes patients with morbid obesity (BMI 40+), heavy smoking, uncontrolled diabetes (A1C > 7.5%), low T-score (severe osteoporosis), congenital neuromuscular disease, prior pelvic or SIJ fixation, and grade IV spondylolisthesis. We assumed that patients would undergo MLF with or without SIJF in the hospital inpatient setting (as opposed to outpatient surgery) due to factors that place these patients at higher risk such as obese, elderly, presence of comorbidities, 3 or more levels treated, supplemental fixation, or more than 2 hours of operative time.
Figure 2 depicts a simplified version of the model structure. The model entry point was patients who underwent either MLF + SIJF or MLF alone. After initial surgery, the post-surgical outcomes were no SIJ pain or SIJ pain, indicating successful versus unsuccessful surgical responses, respectively. Among patients with a post-surgical outcome of no SIJ pain after initial surgery, it was assumed that patients remained in the no SIJ pain health state. Over time, unsuccessful patients (mild or severe SIJ pain) could either remain in the same SIJ pain health state (with non-surgical management [NSM]), transition from the mild to severe SIJ pain health state, or – among those with severe SIJ pain – undergo SIJF revision surgery (starting Year 2). As patients transitioned among health states, they accrued the costs and health state utility values associated with the amount of time spent in those health states. The cumulative 5-year costs and QALYs of each treatment (MLF + SIJF versus MLF alone) were then used to calculate the incremental cost-effectiveness ratio (ICER), defined as the difference in total costs divided by the difference in total QALYs.
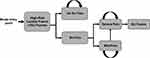 |
Figure 2 Simplified diagram of model structure. Abbreviation: SIJ, sacroiliac joint. |
Model Inputs
Data were sourced from the published literature (Tables 1–3). Medicare payments were used as a proxy for “cost”, a well-accepted methodological approach. Specifically, costs were sourced from Medicare and commercial payer health insurance claims data analyses and Medicare fee schedules.15–17 Commercial payments were converted to Medicare payments by applying a multiplier of 0.75 based on publications reporting the relationship between Medicare and commercial insurance payments for hospital-based orthopedic procedures, noting that hospital-based services accounted for 76.5% of total costs over 5 years post-operatively among Medicare patients who underwent lumbar spinal fusion.15,18,19 Costs were inflation-adjusted to 2020 USD using the compound annual growth rate (CAGR) of 0.5% per year.20
 |
Table 1 Inputs for Clinical Effectiveness Probabilities and Health State Utility Weights |
 |
Table 2 Health State Transition Probabilities After Unsuccessful Surgical Responsea |
 |
Table 3 Inputs for Costs (2020 USD) |
Health state utility values (derived from EQ-5D TTO) were informed by three prospective, multicenter, clinical trials (INSITE, SIFI, iMIA), where “success” was defined as a decrease in Visual Analog Scale (VAS) of at least 20 points.21–24 The health state utility values for mild and severe SIJ pain were 0.77 and 0.45, respectively, where 1.0 reflects full health and 0 reflects death.21–24 The health-state utility value of 0.45 for severe pain captures the utility decrement associated with an unsuccessful response, including complications and subsequent procedures.21–24
Among patients with MLF who did not undergo pelvic fixation, Finger et al reported the onset of post-operative SIJ pain in 29.4% of patients at 12-month follow-up; similarly, Unoki et al reported a post-operative incidence of SIJ pain of 32.1%, where the mean time to onset of SIJ pain was approximately 4 months (range, 1–10 months).4,5 Based on two randomized clinical trials of minimally invasive SIJF using triangular titanium implants, among patients with a history of lumbar fusion, Polly et al and Dengler et al reported relative risk reductions (RRR) in post-operative SIJ pain of 82% and 73%, respectively, at 6-month follow-up.21,25 Hence, in the base case analysis, we conservatively assumed a reduction in post-operative SIJ pain from 30% for MLF alone to 10% for MLF + SIJF (that is, an RRR of 67%).
Sensitivity Analyses
One-way, multi-way, and probabilistic sensitivity analyses (PSA) were performed to address uncertainty and assess the robustness of the model results. In the one-way and multi-way sensitivity analyses, parameters were varied based on published ranges or, when a range was not known, as greater/less than 20% of the model parameter; wider ranges were used for parameters with greater uncertainty as informed via consultation with clinical experts. For example, in light of the uncertainty in the annual transition probability from mild to severe SIJ pain after an unsuccessful index procedure, we applied a wide range of this input in the sensitivity analysis; similarly, a wide range was also applied to the annual probability of SIJF among patients with severe SIJ pain after an unsuccessful index procedure (Table 2). One-way and multi-way sensitivity analyses were used to identify the model parameters that most greatly influenced the model results.
The PSA were conducted using Monte Carlo simulation with 1000 iterations where model inputs were varied simultaneously by randomly sampling from probability distributions (Tables 1 and 3), thus reflecting 1000 hypothetical patient cohorts undergoing MLF with versus without SIJF. The PSA distributions reflected those reported in the published data sources, or when not known, were based on the widely used distributions for that parameter type; for instance, the gamma distribution for right-skewed data such as costs, and the beta distribution for variables that range from 0 to 1 such as probabilities. The PSA assumed independence of each parameter. The PSA was implemented in Excel using a customized Visual Basic for Applications (VBA) programming script and demonstrated that 1000 iterations were adequate to achieve stabilization.
Results
Base Case Results
The economic evaluation of MLF + SIJF relative to MLF alone is presented in Table 4. Over a 5-year time horizon with an assumed 30% incidence of SIJ pain after MLF alone, stabilizing with SIJF was associated with an additional cost of $2421 and a gain of 0.14 QALYs. The corresponding ICER for MLF + SIJF relative to MLF alone was $17,293 per QALY gained.
 |
Table 4 Cost-Effectiveness Results in the Base Case Analysis (2020 USD) |
Among patients initially treated with MLF + SIJF, 4.05% underwent a subsequent SIJF procedure within five years; in contrast, among patients initially treated with MLF alone, 12.15% underwent a subsequent SIJF within five years. These results are consistent with the assumed RRR of 67%.
Sensitivity Analyses
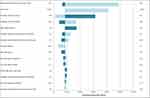
Referring to the Tornado Diagram (Figure 3), the one-way sensitivity analyses demonstrated that ICERs were most sensitive to the RRR in the incidence of post-operative SIJ pain conferred by SIJF, time horizon, and probability of successful treatment with MLF alone. The model results were fairly insensitive to health state transition probabilities that apply to both index procedures after an unsuccessful surgical response (defined as mild or severe SIJ pain); specifically, the annual transition from mild to severe SIJ pain and the annual probability of SIJF among patients with severe SIJ pain (Figure 3). Assuming 40% and 50% incidence of post-operative SIJ pain after MLF alone resulted in cost neutrality and cost-savings, respectively. The ICER was less than $50,000 per QALY (a commonly accepted willingness-to-pay threshold) provided that the incidence of post-operative SIJ pain after MLF alone was at least 22%.
The cost-effectiveness plane (CEP) (Figure 4) shows the results of the PSA simulation of 1000 hypothetical patient cohorts undergoing MLF + SIJF versus MLF alone; the black triangle represents the base case, whereas the black line shows the commonly accepted willingness-to-pay threshold of $50,000 per QALY gained. The CEP illustrates that the majority of the simulated ICERs occupy the northeast quadrant of the CEP, demonstrating the trade-off between increased costs and increased QALYs. At the commonly accepted willingness-to-pay threshold of $50,000 per QALY gained, the cost-effectiveness acceptability curve (CEAC) highlights that MLF + SIJF has a probability of being cost-effective of 97.7% in the target population (Figure 5).
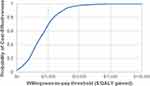 |
Figure 5 Cost-effectiveness acceptability curve. Abbreviation: QALY, quality-adjusted life-year. Note: Currency is presented as 2020 US dollars. |
In the base case analysis, we assumed a reduction in the incidence of post-operative SIJ pain from 30% for MLF alone to 10% for MLF + SIJF (that is, an RRR of 67%).4,5,21,25 Given that the ICERs were most sensitive to the RRR in the incidence of post-operative SIJ pain conferred by SIJF and, in light of the uncertainty in the actual RRR, we explored parameter combinations that are cost-effective at various WTP thresholds (Figure 6), where the RRR reflects the reduction in the new onset of SIJ pain conferred by the addition of SIJF. RRR of 0 indicates no risk reduction and RRR of 1 indicates complete elimination of post-operative SIJ pain. Parameter combinations to the upper right of a given WTP threshold indicate that the combination (of incidence of post-operative SIJ pain with MLF alone versus RRR with the addition of SIJF) is cost-effective at that threshold.
Referring to Figure 6, for example, at the $50,000/QALY WTP threshold and assuming a 25% incidence of post-operative SIJ pain with MLF alone, MLF + SIJF is cost-effective provided that the RRR of post-operative SIJ pain is approximately 50% or greater (that is, a reduction in the incidence of post-operative SIJ pain from 25% to 12.5% or less); at the $100,000/QALY WTP threshold, the RRR would need to be approximately 30% or greater. Cost-effectiveness varies with the incidence of post-operative SIJ pain with MLF alone. For instance, with the $50,000/QALY WTP threshold, if the incidence of post-operative SIJ pain is 15% with MLF alone, then the RRR would have to be 80% or greater; if the incidence with MLF alone is 10% or lower, then adding SIJF to MLF is not cost-effective at this WTP threshold.
Discussion
The present study evaluated the scenarios under which adding SIJF to MLF could be cost-effective, for patients with high BMI and high PI. In this economic study using a decision analytic modelling approach, we observed an ICER for MLF + SIJF relative to MLF alone of $17,293 per QALY gained, suggesting that while the upfront cost of MLF + SIJF exceeds that of MLF alone, cost offsets accrue over time, potentially resulting in cost-effectiveness. Investigators have similarly reported favorable ICERs of $15,000 per QALY gained and $3000 per QALY gained (2020 USD) for minimally invasive SIJF compared to NSM among patients with chronic SIJ pain.17,26,27 The base case ICER reported in our study ($17,293/QALY) is also similar to the results (2020 USD) for other orthopaedic interventions such as total knee arthroplasty for osteoarthritis ($13,000/QALY to $23,000/QALY), total hip arthroplasty for osteoarthritis ($8700/QALY), and laminectomy without fusion for lumbar spinal stenosis ($17,000/QALY).26,28–31 Whereas, our results are slightly more favorable than the ICERs (2020 USD) for open discectomy for intervertebral disc herniation ($28,000/QALY) and posterior lumbar interbody fusion for spinal stenosis ($24,000/QALY).26,32,33
Economic modeling is informative and routinely performed when considering an expanded surgical procedure, such as MLF + SIJF, where parameter values are based upon best estimates from the published literature and other sources. While no studies have been reported to date on this specific topic, a multicenter, randomized, clinical study is currently underway (SILVIA; NCT04062630).34 As such, multi-way sensitivity analyses (Figure 6) were performed to assess what level of benefit (that is, reduction in the incidence of post-operative SIJ pain conferred by the addition of SIJF) is needed for this intervention to be cost-effective.
Given that the durability of MLF + SIJF is currently under investigation, in the current model we have conservatively assumed similar durability to MLF alone.34 Ivanov et al previously reported that fusing the L5-S1 and L4-S1 increased the motion (stress) of the sacroiliac joint by 52% and 168%, respectively.2 We expect that MLF + SIJF will be more durable compared to MLF alone due to the minimal increase in motion of the lumbar spine (2–4%) and minimal increase in hip stress (5%) after fusing the SIJ.35,36 Furthermore, the benefits of minimally invasive SIJF using triangular titanium implants have been shown to be durable at 5-year follow-up based on 103 patients at 12 centers.37
Because investigators have previously reported that increased pre-operative BMI and higher PI are risk factors for the new onset of post-operative SIJ pain, in our model we conservatively defined high risk as having both high BMI and high PI.5,6 According to the Centers for Disease Control and Prevention, approximately 41.1% of US adults are obese.11 Assuming a mean PI of 50° (standard deviation of 10°) in normal populations, 15.9% of patients have high PI (>60°).6,12–14 Assuming statistical independence, 6.5% of patients undergoing lumbar fusion are both obese and have high PI, whereas 57% of patients are either obese or have high PI. Hence, the applicable high-risk population likely ranges from 6.5% to 57% of MLF patients, which merits further research to more precisely define the true population at high risk.
In our study, we compared 2–4 level fusion to the sacrum without pelvic fixation versus MLF with pelvic fixation that includes a SIJF device. Currently, the primary practice in “short” fusions is stopping at the first sacral vertebra (S1); whereas, pelvic fixation (placement of a SAI screw) is generally reserved for “longer” fusions. Based on a non-randomized study we acknowledge it is possible that pelvic fixation alone could decrease post-operative SIJ pain; specifically, among patients with an average of 7 fused segments (range, 3–13) and a mean follow-up period of 33 months (range, 24–50), Unoki et al reported an incidence of post-operative SIJ pain of 26.1% in the S1 group (fusion to S1 without pelvic fixation) (n = 23) and 4.2% in the pelvic fixation group (n = 24), suggesting that pelvic fixation with SIJ fusion could prove beneficial and warrants further investigation.3
According to best practices, CEA should be conducted using inflation-adjusted costs when the cost data come from different time periods as in this study.10 Inflation adjustment using the Medical Care component of the Consumer Price Index (CPI) is a common methodological approach.38 While the utilization rate of spinal surgeries continues to increase, Medicare's national average payments for lumbar fusion have decreased over time.20,39 For example, the per-procedure Medicare reimbursement for diagnosis-related group (DRG) 460 (Spinal Fusion Except Cervical without major complication or comorbidity [MCC]) decreased from $27,800 in 2012 to $24,807 in 2020, whereas DRG 459 (Spinal Fusion Except Cervical with MCC) decreased from $46,700 in 2012 to $42,919 in 2020, noting that approximately 98.5% of these procedures fall under DRG 460.20 In light of these trends, inflation adjustment using the CPI would be insufficient. Rather, based on Medicare DRG reimbursement from 2012 to 2017, Lopez et al reported a 0.5% CAGR, which was applied in our model as the inflation-adjustment to report costs in 2020 USD.20
Payments for DRGs 459 and 460 include a global period of 90 days post-operatively. As such, costs associated with immediate revision for patients experiencing neuropathic symptoms related to implant malposition were implicitly incorporated in the model. Likewise, based on the 5-year time periods reported in the published data sources, the model’s cost inputs implicitly incorporated subsequent revision, removal, and reoperation for MLF alone and SIJF.15–17 Therefore, costs associated with these complications were not explicitly incorporated into the model so as not to double-count the associated costs.
CEA models are generally sensitive to the time horizon, particularly when considering procedures with upfront implant costs as in the present study. Hence, a sensitivity analysis was performed by varying the time horizon from 2 to 5 years. In the model, we have assumed that, among patients with an unsuccessful response to the index procedure (30% for MLF alone, and 10% for MLF + SIJF), (1) patients transition from mild to severe SIJ pain at 10% annually; and (2) patients with severe SIJ pain undergo SIJF at 15% annually starting Year 2. As we shorten the time horizon, fewer patients with MLF alone will have transitioned to the severe pain health state (with lower utility value) and fewer patients will have undergone a subsequent SIJF (with associated costs); therefore, the ICER results become less favorable. Nevertheless, the ICER remains cost-effective with 3-year and 4-year time horizons ($40,590 and $26,825 per QALY gained, respectively); whereas, with a shorter 2-year time horizon – not unexpectedly – the results are no longer cost-effective ($82,183 per QALY gained).
While the current model was developed from the payer perspective with a 5-year time horizon, its relevance to private payer decision-making warrants further comment. Using commercial health plan data from 2006 to 2018, Fang et al found an average enrollment duration of 4 years (not necessarily continuous), whereby approximately 20% of members disenrolled each year, while approximately one-third subsequently returned to the same insurer within 5 years.40 While high turnover reduces the incentive for insurers to invest in treatments where benefits accrue over time, Fang et al suggests that commercial insurers weigh upfront treatment costs relative to the likelihood of future cost-savings by focusing on individuals’ long-term health, given that many members will reenroll with the same insurer within a fairly short time period.40
Limitations
There are several limitations to this CEA. First, studies report the development of new SIJ pain of up to 32% after lumbar spinal fusion, with persistent pain ranging from 20% to 40%.4,5,42 However, given the lack of data that specifically address decreased risk over time, constant annual transition probabilities were assumed for the probabilities that apply to both index procedures after an unsuccessful surgical response, specifically, the annual transition from mild to severe SIJ pain and the annual probability of SIJF among patients with severe SIJ pain. Additional multi-way sensitivity analyses were subsequently conducted using a decreased risk over time, which had little impact on the ICER because these transition probabilities after an unsuccessful surgical response apply to both index procedures.
A second limitation is that health insurance claims data analyses published in 2014 were used to inform costs; these studies reported nationally representative claims data, large sample size, and a 5-year time horizon.15,16 Our due diligence concluded that no suitable papers have been published more recently to inform costs, in that more recently published papers were limited due to reporting on single-site, small sample size (50–100 patients), limited time horizon (such as index hospitalization or 90 days post-operatively), surgeon fee only, or European countries (with different practice patterns and cost accounting).
While MLF clinical practice patterns (including pre-operative care, hospital inpatient procedure, and post-operative care) for the relevant patient population have largely remained similar today compared to a decade ago, this point warrants further discussion. Over the past decade, the aging population has led to additional spinal surgeries, with a high correlation between increasing age and more adverse events.41 Concurrently, infection prevention protocols and technological advances have contributed to a reduction in adverse events, such as surgical site infections, resulting in a reduction in hospital length of stay (LOS) and associated costs.1,41 Conversely, technological advances in implants as well as surgical navigation systems with intra-operative 3D imaging have likely increased the cost of care. Based on a prospective study of patients who underwent spinal surgery from 2006 to 2019, Charlotte et al concluded that, in aggregate, these various factors that influence LOS (and associated costs) appear to have had the net effect of balancing each other out, further supporting the use of these previously published health insurance claims data analyses to inform costs.41
Another limitation is that patients who underwent SIJF following index treatment failure did not incur additional time in the severe SIJ pain health state for post-surgical recovery; also, patients with severe SIJ pain did not incur costs associated with lost productivity. Despite these exclusions, our approach is conservative in that the results are biased in favor of index treatment with MLF alone, given that such patients are more likely to experience severe post-operative SIJ pain and undergo a subsequent SIJF with associated lost productivity, compared to index treatment with MLF + SIJF.
Lastly, the model incorporated a 1-year cycle length (as opposed to 6-month cycles, for example). The 1-year cycle length was selected to strike a reasonable balance between capturing relevant costs and outcomes while reducing uncertainty due to available data.10 Similarly, while we acknowledge that a 5-year time horizon would not capture longer-term outcomes, the 5-year time horizon for the CEA was selected to capture the major clinical and economic outcomes, including unsuccessful surgical responses with subsequent SIJF revision surgery.10 A longer-term time horizon would have necessitated extrapolation, thereby introducing additional assumptions and uncertainty.
Conclusion
This study is the first to document – from a payer perspective – that fusing the SIJ in high-risk patients undergoing MLF appears to be cost-effective over a 5-year time horizon, providing value-based healthcare when the practice-specific incidence of SIJ pain after MLF alone exceeds approximately 25%. Further research is currently underway to evaluate the RRR in post-operative SIJ pain conferred by SIJF.34
Abbreviations
AP, anteroposterior; BMI, body mass index; CAGR, compound annual growth rate; CEA, cost-effectiveness analysis; CPI, Consumer Price Index; CPT, Current Procedural Terminology; DRG, diagnosis-related group; EQ-5D TTO, EuroQol Group Health Questionnaire with Time Trade Off Utility Index; ICER, incremental cost-effectiveness ratio; LOS, length of stay; MCC, major complication or comorbidity; MLF, multi-level lumbar fusion to the sacrum; N/n, number; NSM, non-surgical management; PI, pelvic incidence; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life years; RRR, relative risk reduction; SAI, sacral-alar-iliac; SIJ, sacroiliac joint; SIJF, SIJ fusion; SD, standard deviation; S1, first sacral vertebra; US, United States; USD, US dollars; VAS, Visual Analog Scale; VBA, Visual Basic for Applications; WTP, willingness-to-pay.
Acknowledgment
The authors thank Michael Adena, PhD AStat, of Datalytics Pty Ltd, for his contributions to the PSA VBA programming.
Disclosure
The study reported herein was sponsored by SI-BONE, Inc. (Santa Clara, CA, USA). The clinical trials and cost data publications on which this analysis is based were sponsored by SI-BONE. Stacey J Ackerman, Gurvinder S Deol, and David W Polly are consultants to SI-BONE. Stacey J Ackerman reports personal fees from SI-BONE, during the conduct of the study; personal fees from Advise Connect Inspire, personal fees from Alphatec Spine, outside the submitted work. Gurvinder S Deol reports personal fees from NuVasive, Alphatec Spine, and Globus Medical, outside the submitted work. David W Polly reports personal fees from SI-BONE and Globus Medical, during the conduct of the study; personal fees from SI-BONE and Globus Medical, outside the submitted work. In addition, David W Polly receives royalties from SI-BONE; and Institutional research support from Medtronic and MizuhoOSI. The authors report no other conflicts of interest in this work.
References
1. Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP), National Inpatient Sample (NIS). Clinical classifications software Refined (CCSR) for ICD-10-PCS procedure codes; 2018. Available from: https://www.hcup-us.ahrq.gov/faststats/NationalProceduresServlet?year1=2018&characteristic1=0&included1=1&year2=2018&characteristic2=43&included2=1&expansionInfoState=hide&dataTablesState=hide&definitionsState=hide&exportState=hide.
2. Ivanov AA, Kiapour A, Ebraheim NA, Goelet V. Lumbar fusion leads to increases in angular motion and stress across sacroiliac joint: a finite element study. Spine. 2009;34:E162–E169. doi:10.1097/BRS.0b013e3181978ea3
3. Unoki E, Miyakoshi N, Abe E, et al. Sacropelvic fixation with S2 alar iliac screws may prevent sacroiliac joint pain after multisegment spinal fusion. Spine. 2019;44(17):E1024–E1030. doi:10.1097/BRS.0000000000003041
4. Unoki E, Miyakoshi N, Abe E, et al. Sacroiliac joint pain after multiple-segment lumbar fusion: a long-term observational study – non-fused sacrum vs. fused sacrum. Spine Surg Relat Res. 2017;1(2):90–95. doi:10.22603/ssrr.1.2016-0010
5. Finger T, Bayer S, Bertog M, et al. Impact of sacropelvic fixation on the development of postoperative sacroiliac joint pain following multilevel stabilization for degenerative spine disease. Clin Neurol Neurosurg. 2016;150:18–22. doi:10.1016/j.clineuro.2016.08.009
6. Tonosu J, Kurosawa D, Nishi T, et al. The association between sacroiliac joint-related pain following lumbar spine surgery and spinopelvic parameters: a prospective multicenter study. Eur Spine J. 2019;28(7):1603–1609. doi:10.1007/s00586-019-05952-z
7. Husereau D, Drummond M, Augustovski F, et al; CHEERS 2022 ISPOR Good Research Practices Task Force. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9. doi:10.1016/j.jval.2021.11.1351
8. Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Med Decis Making. 2012;32(5):690–700. doi:10.1177/0272989X12455463
9. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi:10.1001/jama.2016.12195
10. Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; 1996.
11. National Center for Health Statistics. Health, United States, 2019: table 026. Hyattsville, MD; 2021. Available from: https://www.cdc.gov/nchs/hus/contents2019.htm.
12. Baker JF. Sacropelvic parameters and L5 spondylolysis: computed tomography analysis. Asian Spine J. 2022;16(1):66–74. doi:10.31616/asj.2020.0442
13. Oh SK, Chung SS, Lee CS. Correlation of pelvic parameters with isthmic spondylolisthesis. Asian Spine J. 2009;3(1):21–26. doi:10.4184/asj.2009.3.1.21
14. Legaye J. The femoro-sacral posterior angle: an anatomical sagittal pelvic parameter usable with dome-shaped sacrum. Eur Spine J. 2007;16(2):219–225. doi:10.1007/s00586-006-0090-3
15. Ackerman SJ, Polly DW, Knight T, Holt T, Cummings J. Non-operative care to manage sacroiliac joint disruption and degenerative sacroiliitis is costly and requires high medical resource utilization in the Medicare population. J Neurosurg: Spine. 2014;20(4):354–363. doi:10.3171/2014.1.SPINE13188
16. Ackerman SJ, Polly DW, Knight T, Schneider K, Holt T, Cummings J. Comparison of cost of non-operative care to minimally invasive surgery for sacroiliac joint disruption and degenerative sacroiliitis in a United States commercial payer population: potential economic implications of a new minimally-invasive technology. Clinicoecon Outcomes Res. 2014;6:283–296. doi:10.2147/CEOR.S63757
17. Cher DJ, Frasco MA, Arnold RJG, Polly DW. Cost-effectiveness of minimally invasive sacroiliac joint fusion. Clinicoecon Outcomes Res. 2016;8:1–14. doi:10.2147/CEOR.S107803
18. Healy WL, Rana AJ, Iorio R. Hospital economics of primary total knee arthroplasty at a teaching hospital. Clin Orthop Relat Res. 2011;469(1):87–94. doi:10.1007/s11999-010-1486-2
19. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop Relat Res. 2011;469(2):355–361. doi:10.1007/s11999-010-1526-y
20. Lopez CD, Boddapati V, Lombardi JM, et al. Recent trends in Medicare utilization and reimbursement for lumbar spine fusion and discectomy procedures. Spine J. 2020;20(10):1586–1594. doi:10.1016/j.spinee.2020.05.558
21. Polly DW, Swofford J, Whang PG, et al.; INSITE Study Group. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10(28):1–22. doi:10.14444/3028
22. Duhon BS, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T; SIFI Study Group. Triangular titanium implants for minimally invasive sacroiliac joint fusion: 2-year follow-up from a prospective multicenter trial. Int J Spine Surg. 2016;10:13. doi:10.14444/3013
23. Sturesson B, Kools D, Pflugmacher R, Gasbarrini A, Prestamburgo D, Dengler J; iMIA Study Group. Six-month outcomes from a randomized controlled trial of minimally invasive SI joint fusion with triangular titanium implants vs. conservative management. Eur Spine J. 2017;26(3):708–719. doi:10.1007/s0058
24. Cher D. SI-BONE Internal Report (12-month EQ-5D TTO data pooled from INSITE, SIFI, iMIA); June 2019.
25. Center for the Evaluation of Value and Risk in Health. The cost-effectiveness analysis registry. Boston: Institute for Clinical Research and Health Policy Studies, Tufts Medical Center. Available from: www.cearegistry.org.
26. Blissett DB, Blissett RS, Newton Ede MPN, et al. Minimally invasive sacroiliac joint fusion with triangular titanium implants: cost‑utility analysis from NHS perspective. PharmacoEcon Open. 2021;5:197–209. doi:10.1007/s41669-020-00236-5
27. Dakin H, Gray A, Fitzpatrick R, Maclennan G, Murray D; KAT Trial Group. Rationing of total knee replacement: a cost-effectiveness analysis on a large trial data set. BMJ Open. 2012;2(1):e000332. doi:10.1136/bmjopen-2011-000332
28. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–1121. doi:10.1001/archinternmed.2009.136
29. Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total Hip arthroplasty for osteoarthritis of the Hip. JAMA. 1996;275(11):858–865. doi:10.1001/jama.1996.03530350040032
30. Parker SL, Anderson LH, Nelson T, Patel VV. Cost-effectiveness of three treatment strategies for lumbar spinal stenosis: conservative care, laminectomy, and the Superion interspinous spacer. Int J Spine Surg. 2015;9:28. doi:10.14444/2028
31. Tosteson AN, Tosteson TD, Lurie JD, et al. Comparative effectiveness evidence from the spine patient outcomes research trial: surgical versus nonoperative care for spinal stenosis, degenerative spondylolisthesis, and intervertebral disc herniation. Spine. 2011;36(24):2061–2068. doi:10.1097/BRS.0b013e318235457b
32. Fujimori T, Miwa T, Iwasaki M, Oda T. Cost-effectiveness of posterior lumbar interbody fusion in the Japanese universal health insurance system. J Orthop Sci. 2018;23(2):299–303. doi:10.1016/j.jos.2017.11.014
33. Dengler JD, Kools D, Pflugmacher R, et al. 1-year results of a randomized controlled trial of conservative management vs. minimally invasive surgical treatment for sacroiliac joint pain. Pain Physician. 2017;20(6):537–550. doi:10.36076/ppj.20.5.537
34. SI Joint Stabilization in Long Fusion to the Pelvis (SILVIA). Available from: https://clinicaltrials.gov/ct2/show/NCT04062630.
35. Lindsey DP, Kiapour A, Yerby SA, Goel VK. Sacroiliac joint fusion minimally affects adjacent lumbar segment motion: a finite element study. Int J Spine Surg. 2015;9:64. doi:10.14444/2064
36. Joukar A, Chande RD, Carpenter RD, et al. Effects on hip stress following sacroiliac joint fixation: a finite element study. JOR Spine. 2019;2:e1067. doi:10.1002/jsp2.1067
37. Whang PG, Darr E, Meyer SC, et al. Long-term prospective clinical and radiographic outcomes after minimally invasive lateral transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices Evid Res. 2019;12:411–422. doi:10.2147/MDER.S219862
38. Bureau of Labor Statistics, Division of Consumer Prices and Price Indexes. CPI-All Urban Consumers (Current Series). Available from: https://data.bls.gov/cgi-bin/surveymost?cu.
39. Sheikh SR, Thompson NR, Benzel E, et al. Can we justify it? Trends in the utilization of spinal fusions and associated reimbursement. Neurosurgery. 2020;86(2):E193–E202. doi:10.1093/neuros/nyz400
40. Fang H, Frean M, Sylwestrzak G, Ukert B. Trends in disenrollment and reenrollment within US commercial health insurance plans, 2006–2018. JAMA Netw Open. 2022;2(5):e220320. doi:10.1001/jamanetworkopen.2022.0320
41. Charlotte D, Mathew NH, Tamir A, et al. Variations in LOS and its main determinants overtime at an academic spinal care center from 2006–2019. Eur Spine J. 2022;11:1–8. doi:10.1007/s00586-021-07086-7
42. Christelis N, Simpson B, Russo M, et al. Persistent spinal pain syndrome: a proposal for failed back surgery syndrome and ICD-11. Pain Medicine. 2021;22(4):807–818. doi:10.1093/pm/pnab015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.