Back to Journals » ClinicoEconomics and Outcomes Research » Volume 14
Cost-Utility Analysis of Molecular Testing for Tuberculosis Diagnosis in Suspected Pulmonary Tuberculosis in Thailand
Authors Chitpim N, Jittikoon J, Udomsinprasert W, Mahasirimongkol S, Chaikledkaew U
Received 25 November 2021
Accepted for publication 15 January 2022
Published 2 February 2022 Volume 2022:14 Pages 61—73
DOI https://doi.org/10.2147/CEOR.S350606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Natthakan Chitpim,1 Jiraphun Jittikoon,2 Wanvisa Udomsinprasert,2 Surakameth Mahasirimongkol,3 Usa Chaikledkaew4,5
1Social, Economic and Administrative Pharmacy (SEAP) Graduate Program, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand; 2Department of Biochemistry, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand; 3Medical Genetics Center, Medical Life Sciences Institute, Department of Medical Sciences, Ministry of Public Health, Bangkok, Thailand; 4Social Administrative Pharmacy Division, Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand; 5Mahidol University Health Technology Assessment (MUHTA) Graduate Program, Mahidol University, Bangkok, Thailand
Correspondence: Usa Chaikledkaew
Social and Administrative Pharmacy Division, Department of Pharmacy, Faculty of Pharmacy, Mahidol University, 447 Sri-Ayudhaya Road, Rajathevi, Bangkok, 10400, Thailand
, Tel +66 2-644-8679 ext 5317
, Fax +66 2-644-8694
, Email [email protected]
Purpose: Given the lack of economic evaluation study of molecular testing in Thailand, this study aimed to evaluate the cost-utility of molecular testing algorithms including Xpert MTB/RIF and the loop-mediated isothermal amplification (TB-LAMP) in the general population suspected of having pulmonary TB based on a societal perspective.
Methods: A hybrid decision tree Markov model using a 1-month cycle length was used to evaluate costs and outcomes of five TB diagnostic algorithms: 1) sputum smear microscopy (SSM) with culture and drug susceptibility testing (DST), 2) Xpert MTB/RIF add-on, 3) Xpert MTB/RIF initial, 4) TB-LAMP add-on, and 5) TB-LAMP initial during a lifetime period. All costs were calculated in 2021 Baht, and results were presented as an incremental cost-effectiveness ratio (ICER) for molecular testing compared with SSM with culture. One-way sensitivity and probability analyses were used to evaluate uncertainty input parameters.
Results: TB-LAMP was less expensive overall (6565 Baht) than Xpert MTB/RIF (7010 Baht) and SSM with culture (6845 Baht). Molecular testing was projected to improve quality adjusted life year (QALY) by 0.53 to 0.94 years. In comparison to SSM with culture and DST, providing an initial TB-LAMP test was the most preferred choice. Xpert MTB/RIF Initial had the lowest ICER (197 Baht per QALY gained), followed by TB-LAMP Add-on (993 Baht per QALY gained) and Xpert MTB/RIF Add-on (3940 Baht per QALY gained). One-way sensitivity analysis uncovered that sensitivity of TB-LAMP was greater than that of other parameters.
Conclusion: Providing molecular testing including Xpert MTB/RIF and TB-LAMP as either initial or add-on test for TB diagnosis was more cost-effective than SSM with culture and DST in the general population with suspected pulmonary TB in Thailand. Our study could provide useful evidence to policymakers advocating for inclusion of molecular testing in the universal health coverage benefit package in Thailand.
Keywords: molecular testing, economic evaluation, diagnosis tuberculosis, Xpert MTB/RIF, TB-LAMP
Introduction
Tuberculosis (TB) is an infectious disease that can spread easily from person to person through the air. TB is caused by bacteria, namely Mycobacterium tuberculosis (MTB), which most often affect the lungs (80%).1 In 2020, there were 10 million TB affected people worldwide, of which 2.6% were confirmed multidrug-resistant TB (MDR-TB) patients and about 1.5 million cases had died. Only 61% of the TB patients were reported, and 86% of them successfully received treatment in 2020. The underreported TB patients and underdiagnosed TB were the main causes of the discrepancy between estimated and reported cases.2
Thailand is an upper-middle-income country classified by the World Bank and has been included on high-burden country lists for TB and TB/human immunodeficiency virus (HIV).2 In 2020, an estimated TB incidence in Thailand was about 105,000 people, in which 1.5% had MDR-TB and approximately 9.5% died from TB. The number of reported cases was 83% and the number of patients who were successfully treated was increased to 85% in 2020.2 Although TB is a curable disease, TB treatment can result in a decrease in patient’s quality of life in physical, psychological and social aspects as well as an increase in economic burden to TB patients and their families.3
Both the Sustainable Development Goals (SDGs) and the World Health Organization (WHO)’s End TB Strategy are recognized as global targets and milestones for reducing the burden of TB disease. The End TB Strategy’s 2030 aims include a 90% reduction in TB deaths and an 80% reduction in TB incidence (less than 20 cases per 100,000 population, compared to 2015.4 To achieve the goal of reducing TB incidence in Thailand, the rate should be decreased by 12.5% per year, from 171 cases in 2014 to 88 cases per 100,000 population in 2030. Nevertheless, the recent data showed that TB incidence rate was decreased by only 2.7% per year during the last decade. An important pillar to achieve Thailand Operational Plan to End Tuberculosis 2017–2021 is to increase patient access to early TB diagnosis via using molecular diagnostic tests for all presumptive TB.5
In Thailand, a high-burden TB country, the chest x-ray (CXR), sputum smear microscopy (SSM) are conventional TB screening and diagnosis. SSM can lead to misdiagnosis due to less sensitivity (46%),6 but it is a useful diagnostic tool especially in situations where there are large numbers of TB patients. In addition, the culture and drug susceptibility testing (DST) are used to confirm TB infection in case of negative smear and suspected to be MDR-TB, respectively.7 Given that the conventional screening and diagnosis take several weeks to complete (more than 4 weeks for culture and at least 6 weeks for DST), this can cause a delay in diagnosis and treatment for several weeks, ultimately resulting in increasing mortality and enhancing community transmission. According to a previous study, a delay of more than 21 weeks resulted in a 1.59-fold increase in mortality compared to a delay of less than 7.6 weeks (95% CI 1.01 to 2.48).8
To improve the performance of TB screening and diagnosis, a rapid diagnosis method, a new technology, is required. The WHO has recommended molecular testing including Xpert MTB/RIF and loop-mediated isothermal amplification (TB-LAMP) as a diagnostic tool for TB and drug-resistant TB in suspected tuberculosis patients with signs and symptoms. The Xpert MTB/RIF is an automated cartridge-based real-time polymerase chain reaction (PCR) test by detection gene sequence of MTB and rpoB gene mutation related to rifampicin resistance. The ability of Xpert MTB/RIF is to detect both of MTB and rifampicin resistance within approximately two hours in either positive or negative sputum smear. Laboratory requirement for Xpert MTB/RIF includes stable electricity, controlled room temperature, sample temperature at 2–8 degree Celsius and regular instrument calibration.9 On the other hand, TB-LAMP is the point-of-care (POC) test, which can install at peripheral setting where microscopy is already set. TB-LAMP is temperature-independent technique for amplifying deoxyribonucleic acid (DNA), which requires reading through ultraviolet light within 1 hour, but needs highly skilled technicians, since it is not automated like Xpert MTB/RIF. The facilities require less infrastructure and also sample can be stored at room temperature. It is highlighted that TB-LAMP can detect only MTB but not resistance TB; therefore, TB-LAMP cannot be used in high risk of MDR-TB settings.10 Consequently, the molecular testing requires less laboratory equipment, small room space, less technician time, and results can be obtained in as little as 1–2 days. This can aid in improving TB case detection, allowing for prompt treatment of TB patients and ultimately reducing TB morbidity and mortality.11 It has been recommended that molecular testing should be used as an initial diagnostic test for presumptive pulmonary TB and MDR-TB. Thailand, 1 of 30 high TB burden countries, has applied the above recommendation to national policy for improving early TB case detection.5
Although molecular testing is effective and very accurate for diagnosing TB, it is still costly. Recently in Thailand, SSM with culture and DST was already covered in all health benefit schemes. However, molecular testing, ie, Xpert MTB/RIF and TB-LAMP recommended by the National TB Control Programme,12 has not been reimbursed by the Universal Health Coverage (UHC) scheme, which covered 70% of Thai populations.13 Although recently published systematic reviews provided data on the cost-effectiveness of Xpert MTB/RIF derived from a variety of settings and from several viewpoints,14,15 there is, to date, no substantial evidence evaluating the cost-effectiveness of available molecular testing, ie, Xpert MTB/RIF and TB-LAMP in Thailand. This information could be applied as supporting evidence for policymakers to make decision whether molecular testing should be included into the UHC health benefit package. Accordingly, the purpose of this study was to evaluate the cost-utility of diagnosing TB using molecular testing algorithms compared with SSM with culture and DST as a conventional diagnostic approach in the Thai general population suspected of having pulmonary TB.
Materials and Methods
Target Population
The Thai general population over the age of 15 years who presented with signs and symptoms related to pulmonary TB or who had an abnormal chest X-ray diagnosed and verified by a consultant chest physician and a radiologist were included in the analysis. The suspected patients were not classified as either high-risk or drug-resistant populations, as defined in the 2018 Thailand National Tuberculosis Control Program (NTP) guideline.12
Model Structure
A hybrid decision tree Markov model-based cost-utility analysis was applied to evaluate costs and outcomes of SSM with culture as well as DST and molecular testing algorithms for TB diagnosis in suspected pulmonary TB in Thailand during a lifetime period with a 1-month cycle length using a Microsoft Excel software program. At the beginning of TB diagnosis, the cohort patients would enter to the decision tree model, and it was determined that they would get the diagnostic algorithms developed from Thai NTP.12 Once the cohorts were identified as positive or active TB, they received the appropriate treatment regimens based on their drug-sensitive profile: drug sensitive TB (DS-TB), MDR-TB, or extensive drug resistant TB (XDR-TB). On the other hand, cohorts with negative TB should not undergo therapy and be considered normal population.
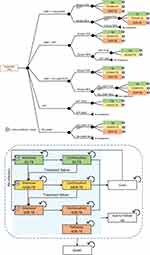
The Markov model was constructed based on the progression and treatment of TB disease, which was modified from Schnippel et al.16 The model health states justified initiating treatment for DS-TB with an intensive phase of 2 months followed by a continuation phase of 3–6 months. After 2 and 6 months of DS-TB treatment, the cohort who failed sputum culture conversion would undergo MDR-TB treatment. For 9 months, the MDR-TB cohort received a shorter MDR-regimen consisting of intensive phase month 1–4 and continuation phase month 5–9. Treatment failure in MDR-TB was examined by sputum conversion at months 4 and 9 and transferred to XDR-TB. Treatment for XDR-TB required 20 months (8 months for intensive phase and 12 months for continuation phase) based on the National TB Control Programme Guideline Thailand 2018.12 If sputum conversion was not achieved after completing the treatment, the XDR-TB patient was transferred to palliative care until death. At the end of treatment, if sputum culture was confirmed to be negative for DS-TB, MDR-TB, or XDR-TB, these cohorts were considered to be cured. However, the cohorts might be re-infected and returned to TB treatment again. In each health state of TB treatment, the cohort could discontinue treatment due to loss to follow-up (LTF) or death. Our model assumptions were as follows: 1) all patients would receive treatment immediately after diagnosis (no delay), 2) no patient would experience an adverse drug reaction (ADR) while taking anti-tuberculosis drugs, 3) the patients who lost to follow-up could not re-enter treatment, and 4) TB diagnosis would occur only once during the first episode (Figure 1).
This study was conducted based on a societal perspective, and the costs analyzed herein included direct medical and direct non-medical care costs that were both related to the disease and drug treatment costs associated with TB, laboratory tests for diagnosis, and formal care costs. All costs were reported in year 2021 values (Baht) adjusted by Thailand’s consumer price index.17 The costs and health outcomes were discounted at a rate of 3% per annum in accordance with Thai Health Technology Assessment (HTA) guidelines.18
Interventions and Comparators
This study evaluated the following TB diagnostic algorithms developed in accordance with NTP guidance of Thailand in 2018.12
SSM + Culture/DST
This algorithm was a comparator representing the conventional diagnostic approach currently used in Thailand. SSM was used to diagnose the whole target population. Positive SSM was advised to test with the first-line DST (FL-DST) and the second-line DST (SL-DST) for testing drug-resistant pattern to select the appropriate treatment regimen for the patients such as DS-TB, MDR-TB or XDR-TB treatment regimen. Although the “culture” was utilized as a confirmed diagnosis test in the negative SSM, it was not used in the positive SSM. Both FL-DST and SL-DST would then be used to categorize and assign the patients to the appropriate treatment regimen.
Xpert MTB/RIF Add-on
In smear-negative patients, the Xpert MTB/RIF interventions could be used as a secondary diagnostic or follow-on test to SSM rather than culture.
Xpert MTB/RIF Initial
Moreover, the Xpert MTB/RIF could be used as an initial diagnosis in all patients or instead of SSM. For patients receiving Xpert MTB/RIF add-on or initial, a treatment regimen that was assigned to TB patients was selected based on Xpert MTB/RIF result, which was either a DS-TB regimen or a rifampicin resistant (RR-TB) regimen.
TB-LAMP Add-on
Additionally, the TB-LAMP was selected as a follow-on test to SSM rather than culture in smear-negative patients.
TB-LAMP Initial
Alternatively, the TB-LAMP could be used as an initial diagnostic test in all patients or in place of SSM. For patients who tested positive for TB using TB-LAMP, the FL-DST and SL-DST would be utilized to determine drug resistance profile and to start the appropriate treatment regimen for patients classified as DS-TB, MDR-TB, and XDR-TB.
Model Parameters
The probability of various variables including sensitivity, specificity of a TB diagnosis, transition probabilities between health states, costs, and health utilities were used as input parameters in the model (Table 1). This study analyzed data derived from both systematic reviews of randomized controlled trial (RCT) and meta-analysis published in Thailand or other countries.
 |  |  |  |
Table 1 Model Parameters |
Transitional Probabilities
Probability of treatment outcomes in terms of cure, mortality, and loss to follow-up were calculated using data from global TB report 2021 by the WHO.2 The probability of reinfection after complete treatment was calculated from Chuchottaworn et al.19 Furthermore, the probability related to TB natural parameters including the proportion of suspected TB patients with either abnormal CXR or clinical symptoms, the proportion of DS-TB, MDR-TB, or XDR-TB in each intervention was retrieved from the National Tuberculosis Information Program, Division of tuberculosis, Ministry of Public Health, Thailand.20,21 Sensitivity and specificity of diagnostic tests were obtained from the WHO and meta-analysis studies.9,22 All probabilities were calculated in unit of a month when they were linked to a cycle length at 1 month.
Costs
Direct medical and direct non-medical costs were both calculated from literature reviews and previous studies that gathered data on TB in Thailand. Direct medical costs included costs related to disease and drug treatment costs consisting of cost of anti-tuberculosis drugs: first-line drug regimen being 2HRZE/4HR, MDR-TB shorter regimen being 4Km Mfx Pto Cfz Z E H/5 Mfx Cfz Z E, and XDR-TB regimen being 8Cm 12Lzd 20Cfz 20Mfx 6Bdq,23 in addition to laboratory costs for TB screening and diagnosis (CXR, SSM, Culture, DST, Xpert MTB/RIF, TB-LAMP) and costs of monitoring (OPD charge).23–25 Direct non-medical costs including transportation costs, costs of food, hotel costs, and formal care costs were retrieved from Wongrot et al.26
Utilities
The health utility was used to calculate the quality-adjusted life years (QALYs). Data on the utilities of DS-TB, MDR-TB, and cure were obtained from Kittikraisak et al.3 The utility of XDR-TB was considered to be equivalent to that of the MDR-TB health state. The health utility of patients who were classified as LTF in either DS-TB, MDR-TB, or XDR-TB health states was believed to be equivalent to the treatment received before to their loss. In addition to this, the utility of palliative care was half that of XDR-TB throughout treatment.
Uncertainty Analysis
For uncertainty analysis in treatment costs and effective parameters, which had an influence on the results of cost-effectiveness analysis, one-way sensitivity analysis and probability sensitivity analysis (PSA) were undertaken based on economic evaluation in health care practice.27 One-way sensitivity analysis was used to determine the impact on the predicted costs and outcomes of changing the value of a specified variable. The transitional probability was changed by 95% confidence interval (CI) or 10% in the absence of 95% CI, cost parameters 25%, and discounting rate 3%. The parameters that had the greatest impact on the results were presented as a tornado diagram. We analysed the PSA using Monte Carlo simulation for 5000 iterations at random, and the parameter values underwent their distributions. The impact of uncertainty on the result of an economic evaluation was represented as a cost-effectiveness plane and a cost-effectiveness acceptability curve.
Result Presentation
This study measured outcomes in the terms of total costs, life-year gained (LYs), and QALYs, which are computed by multiplying LYs by a quality of life score (utility score). The incremental cost-effectiveness ratio (ICER) was used to describe the cost-effectiveness of healthcare interventions in cost-effectiveness analysis. The ICER is defined as the ratio of the change in costs between a conventional algorithm and molecular testing algorithms divided by the difference in effectiveness outcomes for each intervention, with a cost-effectiveness threshold of 160,000 Baht per QALY gained.28
Results
Cost-Utility Analysis
This base case analysis included patients over the age of 15 years having either symptoms related to PTB or abnormal CXR, and cost-effectiveness results of each algorithm are summarized in Table 2. Without molecular testing, the total cost of SSM + Culture/DST was 6845 Baht. The total cost of Xpert MTB/RIF Add-on increased to 8924 Baht; however, molecular testing could decrease the cost of TB-LAMB Initial to 6565 Baht (4% decrease). Overall, the total cost of molecular testing algorithm uncovered that Xpert MTB/RIF Add-on (8924 Baht) and TB-LAMP Add-on (7626 Baht) were more expensive than Xpert MTB/RIF Initial (7010 Baht) and TB-LAMP Initial (6565 Baht). The molecular testing algorithms had more favorable outcomes than those without molecular testing, thereby possibly increasing LY by 0.64 to 0.85 years and QALY by 0.53 to 0.94 years. In parallel with this, the cost-effectiveness analysis revealed that TB-LAMP Initial was dominant, indicating that TB-LAMP was less expensive and more beneficial for TB diagnosis than SSM + Culture/DST. Additionally, Xpert MTB/RIF Initial had the lowest ICER (197 Baht per QALY gained), followed by TB-LAMP Add-on (993 Baht per QALY gained) and Xpert MTB/RIF Add-on (3940 Baht per QALY gained).
 |
Table 2 Cost-Effectiveness Results of Smear + Culture/DST Compared with Molecular Testing Algorithms for TB Diagnosis in Thailand (Baht, 2021) |
Uncertainty Analysis
One-way sensitivity analysis represented as tornado diagram is illustrated in Figure 2. Result of TB-LAMP Initial showed that sensitivity of TB-LAMP was more sensitive than other parameters. Furthermore, discounting rate of costs at 3%, unit cost of TB-LAMP, discounting rate of outcome, and specificity of SSM were all found to be influenced the results.
 |
Figure 2 Tornado diagram comparing the provision of TB-LAMP Initial with SSM + Culture/DST. |
Figure 3A demonstrates the cost-effectiveness plane of molecular testing alternatives compared with SSM + Culture/DST, in which the green line represented the WTP threshold at 160,000 Baht per QALY gained in Thailand. The finding indicated that almost all simulations of TB-LAMP either initial or add-on were below the threshold. Apart from those analyses, the PSA was further executed and presented as the cost-effectiveness acceptability curve (Figure 3B). The analysis showed that of all TB diagnostic test algorithms, TB-LAMP Initial had the highest probability of being cost-effective (83.4%) at the WTP 160,000 Baht per QALY-gained.
 |
Figure 3 (A) Cost-effectiveness plane. (B) Cost-effectiveness acceptability curve. |
Discussion
This study is the first to evaluate the cost-utility of molecular testing algorithms including Xpert MTB/RIF and TB-LAMP compared with SSM with culture and DST in the Thai general population suspected of having pulmonary TB in Thailand. Currently, the SSM with culture and DST which have been widely used as a conventional approach, but typically takes several weeks to months to complete, thereby preventing patients from receiving therapy,12,15 has been already reimbursed in Thailand’s health benefit packages, but not Xpert MTB/RIF and TB-LAMP which have been recommended by the National TB Control Programme.
The results of this study suggested that the molecular testing including Xpert MTB/RIF and TB-LAMP used as either an initial or add-on test was more cost-effective than SSM with culture and DST at the WTP of 160,000 Baht per QALY gained. Interestingly enough, providing an initial TB-LAMP test was a dominant option yielding lower cost and higher effectiveness compared with a conventional approach.28 Furthermore, the results derived from one-way sensitivity analysis unveiled that the sensitivity of TB-LAMP used as an initial test was greater than other parameters. Additionally, the finding derived from PSA suggested that of all molecular testing alternatives, providing an initial TB-LAMP test would be the most cost-effective at various levels of WTP.
Our finding was consistent with several previous cost-utility analysis studies of Xpert MTB/RIF11,29–37 suggesting that Xpert MTB/RIF was more cost-effective than SSM with sputum culture. Similarly, a study by Khumsri et al15 conducted in Thailand revealed that Xpert MTB/RIF was more cost-effective than SSM.
Nonetheless, our result differed from previous studies conducted in Malawi38 and South Africa,39 indicating that the cost-effectiveness of Xpert MTB/RIF was similar to light-emitting diodes (LED) microscopy and sputum smear. For possible reasons for conflicting results, there were a variety of methodological issues were found in our study compared to other studies. First, all data and parameters in our study were retrieved from the WHO and the National Tuberculosis Information Program, which could realistically represent the TB patients in Thailand.2,21 Second, we included all possible molecular testing algorithms including Xpert MTB/RIF and TB-LAMP currently available and ready to be implemented in Thailand for TB diagnosis. These TB diagnostic algorithms were suggested and approved by TB clinical experts and stakeholders related to TB in Thailand. Third, we used SSM with culture and DST as a comparator, which was a standard practice recommended by the National TB Control Programme.12 Last, most previous studies used a decision analysis model to simulate TB infection across the TB diagnosis algorithms, whereas we applied a hybrid decision tree and Markov model to imitate that TB could infect and re-infect individuals throughout their lives.19,40 Therefore, costs and health outcomes associated with TB diagnosis and treatment in our study could be captured realistically.
It was noted that the results of this study served as supporting evidence for the Subcommittee for the Development of the UHC benefit package under the National Health Security Office (NHSO) in determining whether molecular testing should be included in the benefit package. As a result, Xpert MTB/RIF has been covered by the UHC health insurance scheme as the rapid molecular assay for detecting TB in general population or high-risk population, ie, prisoner, household closed contact who is abnormal chest X-ray (CXR) since 2020.13
Although our study uncovered that an initial TB-LAMP was a dominant option and more cost-effective than Xpert MTB/RIF when compared with SSM with culture and DST, the implementation of TB-LAMP in healthcare settings requires additional considerations. According to the WHO policy recommendations, the use of TB-LAMP for diagnosis of pulmonary TB still has some limitations. First, the TB-LAMP operation requires highly skilled technicians, because it is not fully automated like Xpert MTB/RIF. Subsequently, it should be concerned whether it would be economically worth setting TB-LAMP in a diagnostic service and training of the laboratory personnel. Second, the most notable limitation is the fact that TB-LAMP should not be utilized in countries with a high risk of MDR-TB, since TB-LAMP is incapable of detecting drug resistance at the time of detection and requires confirmation with DST. Third, it cannot be recommended in substitution of the Xpert MTB/RIF, because Xpert MTB/RIF has been reported to detect both TB and rifampicin resistant TB. Forth, Xpert MTB/RIF machines have already been set up at various hospitals, particularly at peripheral hospitals where TB-LAMP cannot replace.10 Last, Xpert MTB/RIF machines also have been used to diagnose non-tuberculous mycobacteria pulmonary and coronavirus infectious disease, which are likely to be useful in a pandemic situation. In light of the aforementioned reasons along with our findings, the Xpert MTB/RIF may be a viable alternative for rapid diagnostic test in countries with a high TB burden like Thailand.
Despite the important findings presented herein, there were several limitations that needed to be taken into considerations as follows. First, the model assumed that all patients received treatment, while the actual TB situation in Thailand in 2019 indicated that only 84% of the patients received treatment. For that reason, our analysis would overestimate the cost and benefit of TB diagnosis.2 Second, given that our model assumed that all patients had no adverse drug reactions (ADRs) such as hepatic dysfunction, fatigue, and vomiting, whereas in reality, most patients had symptomatic ADRs, this might result in an underestimate of cost and benefit during TB therapy.41 Third, our analysis employed static models (decision tree model and Markov model) to evaluate outcomes that do not take into account for infectious disease or TB that can be transmitted between individuals. In that context, it has been suggested that by substituting a dynamic model for the static model, we can capture additional perspectives including transmission layer, thus increasing the reliability of the results.42 Forth, our study gathered all of the parameters via a literature review; however, certain factors, such as sensitivity of the TB-LAMP parameter, were not performed in the Thailand setting. Nonetheless, an uncertainty analysis was performed to demonstrate the robustness of results. For those reasons, these restrictions should be modified for future research.
Conclusion
This study provided supporting evidence suggesting that molecular testing for TB diagnosis including Xpert MTB/RIF and TB-LAMP was more cost-effective than SSM with culture and DST in the general population with suspected pulmonary TB in Thailand. Our research may provide valuable information for policymakers advocating for the inclusion of molecular testing as a means of rapid diagnosis in Thailand’s UHC health benefit package, in accordance with the WHO and Thailand’s operational strategy to eradicate TB.
Ethics Approval
Our study used data related to quality of life as well as direct medical and non-medical costs from published studies. The Institutional Review Boards (IRB) of Mahidol University approved this study (COA. No. MU-DT-PY-IRB 2019/02.0701) through the expedited review procedure. The need for informed consent for this study was waived by the ethics committees, as all data were obtained from published studies. All procedures performed in the study were in compliance with international guidelines for human research protection such as Declaration of Helsinki, the Belmont Report.
Acknowledgments
The authors would like to appreciate the Division of Tuberculosis, Department of Disease Control ministry of Public Health Thailand for support, providing epidemiological TB data, and giving guidance about data. This study was funded by the Health System Research Institute (HSRI), Thailand. The funder had no involvement with analysis, results, conclusion, or preparation of manuscript. The first author acknowledges the Thailand Science Research and Innovation under the Ministry of Higher Education, Science, Research and Innovation for providing the Royal Golden Jubilee Ph.D. scholarship for NC and UC (grant no. PHD/0104/2559).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Centers for Disease Control and Prevention. Core Curriculum on Tuberculosis: What the Clinician Should Know. Division of Tuberculosis Elimination; 2013.
2. World Health Organization. Global tuberculosis report 2021. Geneva: World Health Organization; 2021.
3. Kittikraisak W, Kingkaew P, Teerawattananon Y, et al. Health related quality of life among patients with tuberculosis and HIV in Thailand. PLoS One. 2012;7(1):e29775. doi:10.1371/journal.pone.0029775
4. World Health Organization. The End TB Strategy. Geneva: World Health Organization; 2015.
5. Thailand operational plan to end tuberculosis 2017–2021. Division of Tuberculosis Department of Disease Control Ministry of Public Health Thailand; 2018. Available from: https://tbthailand.org/download/Manual/Thailand%20Operational%20Plan%20To%20End%20%20TB_2017_2021.pdf.
6. Chadha VK, Anjinappa SM, Rade K, et al. Sensitivity and specificity of screening tools and smear microscopy in active tuberculosis case finding. Indian J Tuberc. 2019;66(1):99–104. doi:10.1016/j.ijtb.2018.05.015
7. World Health Organization. National tuberculosis program guideline, Thailand. Division of Tuberculosis Department of Disease Control Ministry of Public Health Thailand; 2018.
8. Virenfeldt J, Rudolf F, Camara C, et al. Treatment delay affects clinical severity of tuberculosis: a longitudinal cohort study. BMJ Open. 2014;4(6):e004818. doi:10.1136/bmjopen-2014-004818
9. World Health Organization. Policy Update: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children. Geneva: World Health Organization; 2017.
10. World Health Organization. The Use of Loop-Mediated Isothermal Amplification (TB-LAMP) for the Diagnosis of Pulmonary Tuberculosis. Geneva: World Health Organization; 2016.
11. Menzies NA, Cohen T, Lin HH, et al. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med. 2012;9(11):e1001347. doi:10.1371/journal.pmed.1001347
12. World Health Organization. National tuberculosis control programme guideline Thailand 2018. Division of Tuberculosis Department of Disease Control Ministry of Public Health Thailand; 2018.
13. NHSO annual report year 2020. Thailand: National Health Security Office, Ministry of Public Health; 2020. Available from: https://www.nhso.go.th/.
14. Hao X, Lou H, Bai J, et al. Cost-effectiveness analysis of Xpert in detecting Mycobacterium tuberculosis: a systematic review. Int J Infect Dis. 2020;95:98–105. doi:10.1016/j.ijid.2020.03.078
15. Khumsri J, Hanvoravongchai P, Hiransuthikul N, et al. Cost-effectiveness analysis of Xpert MTB/RIF for multi-outcomes of patients with presumptive pulmonary tuberculosis in Thailand. Value Health Reg Issues. 2020;21:264–271. doi:10.1016/j.vhri.2019.09.010
16. Schnippel K, Firnhaber C, Page-Shipp L, et al. Impact of adverse drug reactions on the incremental cost-effectiveness of bedaquiline for drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2018;22(8):918–925. doi:10.5588/ijtld.17.0869
17. Trade Policy and Strategy Office. Report for consumer price index of Thailand YEAR 2021 BASE YEAR 2019. Thailand: Ministry of Commerce; August 5, 2021. Available from: http://www.price.moc.go.th/price/cpi/index_new_all.asp.
18. Guideline Development Working Group, the Medical Association of Thailand. Guideline for health technology assessment in Thailand updated edition: 2019; 2021.
19. Chuchottaworn C, Roongruangpitayakul C. Drug susceptibility test and treatment outcome of recurrent pulmonary tuberculosis in Thailand. J Med Assoc Thai. 2017;100:733.
20. Division of Tuberculosis Department of Disease Control Ministry of Public Health Thailand. Drug resistance surveillance (DRS) 5th. supranational Thailand reference laboratory. Available from: https://srlthailand.org/DRS5TH/Enrollmentreport.php#.
21. National Tuberculosis Information Program (NTIP). Division of tuberculosis department of disease control ministry of public health Thailand; July 29, 2021. Available from: http://tbcmthailand.ddc.moph.go.th/UIForm/Tableau/TEST_tbcm.php.
22. Shete PB, Farr K, Strnad L, et al. Diagnostic accuracy of TB-LAMP for pulmonary tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):268. doi:10.1186/s12879-019-3881-y
23. Drug And Medical Supply Information Center Ministry of Public Health Thailand. The median drug prize. Available from: http://dmsic.moph.go.th/index/drugsearch/3.
24. Thai Laboratory List (TLL). The comptroller general’s department, Thailand. Available from: https://mbdb.cgd.go.th/wel/searcheqpandmed.jsp#.
25. Riewpaiboon A. Standard Cost Lists for Health Technology Assessment. Bangkok: Tana Press Co., Ltd.; 2011.
26. Wongrot P. Economic burden of out-of-pocket payment for healthcare expenditures of tuberculosis patients in Thailand [dissertation]. Mahidol University; 2020.
27. Drummond M, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford University Press; 2001.
28. Thavorncharoensap M, Teerawattananon Y, Natanant S, et al. Estimating the willingness to pay for a quality-adjusted life year in Thailand: does the context of health gain matter? Clinicoecon Outcomes Res. 2013;5:29–36. doi:10.2147/ceor.S38062
29. Adelman M, Mcfarland D, Tsegaye M, et al. Cost-effectiveness of the World Health Organization-recommended algorithms for tuberculosis case finding at Ethiopian Human Immunodeficiency Virus (HIV) Clinics. Open Forum Infect Dis. 2016;3(suppl_1):549. doi:10.1093/ofid/ofw172.412
30. Choi HW, Miele K, Dowdy D, et al. Cost-effectiveness of Xpert® MTB/RIF for diagnosing pulmonary tuberculosis in the United States. Int J Tuberc Lung Dis. 2013;17(10):1328–1335. doi:10.5588/ijtld.13.0095
31. Langley I, Lin -H-H, Egwaga S, et al. Assessment of the patient, health system, and population effects of Xpert MTB/RIF and alternative diagnostics for tuberculosis in Tanzania: an integrated modelling approach. Lancet Glob Health. 2014;2(10):e581–e591. doi:10.1016/S2214-109X(14)70291-8
32. Pinto M, Steffen RE, Cobelens F, et al. Cost-effectiveness of the Xpert® MTB/RIF assay for tuberculosis diagnosis in Brazil. Int J Tuberc Lung Dis. 2016;20(5):611–618. doi:10.5588/ijtld.15.0455
33. Shah L, Rojas M, Mori O, et al. Cost-effectiveness of active case-finding of household contacts of pulmonary tuberculosis patients in a low HIV, tuberculosis-endemic urban area of Lima, Peru. Epidemiol Infect. 2017;145(6):1107–1117. doi:10.1017/s0950268816003186
34. Tesfaye A, Fiseha D, Assefa D, et al. Modeling the patient and health system impacts of alternative xpert® MTB/RIF algorithms for the diagnosis of pulmonary tuberculosis in Addis Ababa, Ethiopia. BMC Infect Dis. 2017;17(1):318. doi:10.1186/s12879-017-2417-6
35. Wikman-Jorgensen PE, Llenas-García J, Pérez-Porcuna TM, et al. Microscopic observation drug-susceptibility assay vs. Xpert® MTB/RIF for the diagnosis of tuberculosis in a rural African setting: a cost–utility analysis. Trop Med Int Health. 2017;22(6):734–743. doi:10.1111/tmi.12879
36. Winetsky DE, Negoescu DM, DeMarchis EH, et al. Screening and rapid molecular diagnosis of tuberculosis in prisons in Russia and Eastern Europe: a cost-effectiveness analysis. PLoS Med. 2012;9(11):e1001348. doi:10.1371/journal.pmed.1001348
37. You JHS, Lui G, Kam KM, et al. Cost-effectiveness analysis of the Xpert MTB/RIF assay for rapid diagnosis of suspected tuberculosis in an intermediate burden area. J Infect. 2015;70(4):409–414. doi:10.1016/j.jinf.2014.12.015
38. Zwerling AA, Sahu M, Ngwira LG, et al. Screening for tuberculosis among adults newly diagnosed with HIV in Sub-Saharan Africa: a cost-effectiveness analysis. J Acquir Immune Defic Syndr. 2015;70(1):83–90. doi:10.1097/QAI.0000000000000712
39. Vassall A, Siapka M, Foster N, et al. Cost-effectiveness of Xpert MTB/RIF for tuberculosis diagnosis in South Africa: a real-world cost analysis and economic evaluation. Lancet Glob Health. 2017;5(7):e710–e719. doi:10.1016/S2214-109X(17)30205-X
40. Cudahy PGT, Wilson D, Cohen T. Risk factors for recurrent tuberculosis after successful treatment in a high burden setting: a cohort study. BMC Infect Dis. 2020;20(1):789. doi:10.1186/s12879-020-05515-4
41. Apichaya T, Rapin P. Symptom experience of adverse drug reaction among male and female patients with newly diagnosed pulmonary tuberculosis in Thailand. Belitung Nurs J. 2021;7(3). doi:10.33546/bnj.1337
42. Lugnér AK, Mylius SD, Wallinga J. Dynamic versus static models in cost-effectiveness analyses of anti-viral drug therapy to mitigate an influenza pandemic. Health Econ. 2010;19(5):518–531. doi:10.1002/hec.1485
43. World Health Organization. Systematic Screening for Active Tuberculosis: An Operation Guide. Geneva: World Health Organization; 2015.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.