Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Cost-Effectiveness of Umeclidinium/Vilanterol versus Salmeterol/Fluticasone in Elderly Patients with Chronic Obstructive Pulmonary Diseases in China
Authors Lan Y, Yang N, Wang Y, Yang Y, Xu M, He Q
Received 26 November 2021
Accepted for publication 12 March 2022
Published 22 March 2022 Volume 2022:17 Pages 609—619
DOI https://doi.org/10.2147/COPD.S350218
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Ying Lan,1 Nan Yang,2 Yirong Wang,1 Yujie Yang,1 Min Xu,1 Qin He1
1Department of Pharmacy, The Third People’s Hospital of Chengdu, Chengdu, People’s Republic of China; 2West China School of Pharmacy, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Ying Lan, Email [email protected]
Background: Fixed dose dual bronchodilators such as long-acting muscarinic antagonists (LAMAs) plus long-acting β 2-agonists (LABAs) are a new and important inhaled preparation for COPD treatment in China. Among these, umeclidinium/vilanterol (UMEC/VIL) is increasingly being used in China, especially among the elderly.
Purpose: This study aimed to assess the cost-effectiveness of maintenance treatment with UMEC/VIL compared with salmeterol/fluticasone (FSC) as one of the main therapeutic drugs for moderate to very severe COPD in China.
Methods: A Markov model was developed to estimate the costs and outcomes from a societal perspective in a 10-year time horizon. Patients with moderate-to-very severe COPD were treated with UMEC/VIL (62.5/25μg) or FSC (50/500ug). Data concerning clinical efficacy, costs, utilities, transition probability, exacerbation rate, and mortality were obtained from the published literature and official government datasets. The costs were presented in US dollars based on 2021 prices. The indicators of total costs, life years (LYs), quality-adjusted life-years (QALYs), and mortality were used as the model output. Costs and outcomes were discounted at a 5% annual rate. Incremental cost-effectiveness ratios were calculated considering the threshold recommended by WHO. One-way and probabilistic sensitivity analyses were conducted to assess the stability of results.
Results: Compared with FSC, treatment with UMEC/VIL could save $1947.18, with a gain of 0.12 life-years and 0.05 QALYs. Further, 28.0% patients treated with UMEC/VIL and 29.2% patients treated with FSC were predicted to die after 10 years. Incremental cost effectiveness analysis showed that UMEC/VIL was dominant to FSC. Sensitivity analyses confirmed that the results were robust.
Conclusion: UMEC/VIL is a cost-effective treatment option compared with FSC among patients with moderate-to-very severe COPD.
Keywords: umeclidinium, vilanterol, salmeterol, fluticasone, cost-effectiveness, COPD, Markov
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a disease with high morbidity and mortality worldwide; it is also one of the three major causes of death worldwide.1 As a country with a large population, the disease burden of COPD is even more arduous on China. An epidemiological survey covering 33 provincial administrative units across the country conducted in 2013 showed that the prevalence of COPD among people individuals aged over 40 years in China was 7.3%.2 Another national survey study from 2012 to 2015 showed that the prevalence of COPD in individuals over 40 years is 13.7%, and that in individuals over 60 years can reach more than 50% of the total prevalence.3 In a study conducted on the mortality and prevalence of various diseases in China from 1990 to 2017, the mortality rate of COPD patients decreased from 99 individuals per 100,000 in 1990 to 68 individuals per 100,000, but COPD rose to the third highest cause of death.4 Although the results of cost measurement from original studies greatly differed, research by Zhu et al found that the direct cost of COPD ranged from 499 to 1930 USD per person per year, of which direct medical expenses ranged from 72 to 3565 USD per capita per year, accounting for 33.33% to 118.09% of the local average annual income, indirect costs were per capita per year ranged from 20 to 783 USD; the lifetime indirect cost reached 3414 USD per capita.5 Because of the continued exposure to COPD risk factors, an aging population, and longer lifespans of patients under disease control, the prevalence and disease burden of COPD is expected to increase in the next few decades.
Bronchodilators are the basic first-line treatment for COPD in China, and inhaled preparations are the first choice for treatment. According to the survey data on the market share of inhalation preparations for respiratory diseases in China in 2019, salmeterol fluticasone (FSC), tiotropium bromide (TIO), and formoterol budesonide were in a leading position.6 In recent years, both international and Chinese guidelines1,7 have recommended long-acting cholinergic receptor antagonists (LAMA) combined with long-acting β2-receptor agonists (LABA) as an important therapeutic drug for COPD treatment. Among these, umeclidinium/vilanterol (UMEC/VIL) is the first fixed-dose LAMA/LABA combination approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). This combination entered the Chinese markets in August 2018, mainly for the treatment of moderate and severe COPD. Studies have shown that UMEC/VIL and FSC had similar efficacy and safety indicators for the treatment of COPD patients with exacerbation, serious adverse reaction incidence, and mortality risk, whereas the former was significantly better in improving FEV1.8 In terms of economic evaluation, Wilson et al9 conducted a cost-utility analysis of UMEC/VIL and TIO for the treatment of moderate-to-very severe COPD from a third-party payer perspective, and the results showed that UMEC/VIL was dominant. Rajagopalan et al10 compared the cost-effectiveness of various inhaled preparations for the treatment of moderate-to-severe COPD patients from a US payer perspective, and the results showed that UMEC/VIL was dominant to TIO/Olodaterol, TIO, and FSC; besides, it was also more cost-effective compared with budesonide/formoterol, aclidinium, glycopyrrolate/formoterol, and formoterol/ vilanterol, but inferior to Indacaterol/glycopyrrolate. However, these results of economic research from other countries had poor external validity in China. Therefore, our research focuses on the economic evaluation of UMEC/VIL treatment among elderly COPD patients in China.
Materials and Methods
Model Structure
A Markov model was developed and reported in accordance with the Guidelines for Pharmaceutical Economic Evaluation of China (2020 edition),11 established by the software of Treeage Pro Healthcare 2021. According to the 2021 Global Initiative for Chronic Obstructive Lung Disease report and the COPD guideline of China, COPD was classified into four states based on the level of airflow limitation: mild (S1, post-bronchodilator FEV1 ≥ 80% of predicted), moderate (S2, 50% ≤ FEV1 ≤ 80% of predicted), severe (S3, 30% ≤ FEV1 ≤ 50% of predicted) and very severe (S4, FEV1 > 30% of predicted). As dual bronchodilators were commonly used to treat moderate-to-very severe COPD, mild COPD status was not considered in this model. Therefore, the Markov model consisted of three health states and death (Figure 1). At the start of model simulation, 83.8% of patients were assumed to have a moderate disease; 14.9%, severe disease; and 2.0%, very severe disease. The initial state ratios were calculated based on the data from the results of an epidemiological survey conducted by Fang et al12 during 2014–2015 including 66,752 individuals in China. During each Markov cycle, patients in each treatment group were assigned a probability of transitioning from one disease state to another, wherein the disease state should be progressive and irreversible and death can occur in any disease state. Within a year, the patients may experience any one of the following three events: 1) non-severe exacerbation (an exacerbation requiring outpatient treatment), 2) severe exacerbation (an exacerbation requiring hospitalization), 3) no exacerbation (event-free).
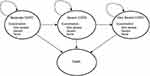 |
Figure 1 State transition diagram of Markov model. |
Comparators
The main intervention measures of this study were UMEC/VIL (62.5/25 µg, 1 inhalation administered once daily) and FSC (50/500 µg, 1 inhalation administered twice daily), and the medication adherence of patients was assumed to be good. Other maintenance measures of the two groups were consistent.
Cycle Length and Time Horizon
The cycle length of the model was 1 year. According to the latest China statistical yearbook data, the average life expectancy of Chinese residents was 76.34 years old. Patients were modelled from the age at treatment initiation (65 years) and the life-time horizon was set to 10-years.
Perspective
The analysis was conducted from a societal perspective.
Model Inputs
Clinical parameter and model inputs were as shown in Table 1.
 |
Table 1 Annual Transition Probabilities for Markov Model |
Transition Probability
Transition probabilities between moderate, severe, and very severe COPD were acquired from previous studies.9,13,14 The annual probability of transitioning from moderate to severe COPD and severe to very severe COPD was 3.1% and 3% in the UMCE/VIL group,9,13 and 2.93% and 9.56% in the FSC group,14 respectively (Table 1).
Exacerbation Risk
Exacerbations were assumed to occur at similar rates between each health state, but at different rates between treatments. Chen et al15 reported the annual non-severe and severe exacerbation rate after treatment with ICS/LABA to be 41.86% and 20.93% respectively, in a retrospective cohort study in China. As FSC was the main ICS/LABA inhalation preparation for COPD treatment in China, we assumed that the exacerbation rates in the FSC group were equivalent to those reported by Chen et al.15 Because of the lack of data on the exacerbation rate among Chinese patients treated with UMCE/VIL, calculations were performed using relative risk. The relative risk of exacerbation between UMCE/VIL and FSC group was derived from two placebo-controlled studies.16,17 After calculation, the relative risk of exacerbation against placebo in UMCE/VIL and FSC groups were 0.65 and 0.75 (Table 1). In the absence of evidence confirming that exacerbations increased the patients’ risk of death, our study assumed that exacerbations had no effect on mortality.
Mortality Risk
Mortality was calculated based on the results of a large clinical trial (TIOSPIR trial) conducted in COPD patients, which was based on age and COPD severity and was not treatment-specific (Table 2).18,19 Mortality was not impacted by the incidence of exacerbations as that would be double counting the risk.
 |
Table 2 COPD-Related Mortality Risk by Age and Severity Stage |
Utility Weights
The annual utility weights were associated with disease severity and exacerbation events, and the initial utilities with each treatment were assumed to be the same. A study on the quality of life of patients with COPD conducted in four cities in China using the EQ-5D scale showed that the utility values for patients with moderate, severe, and very severe COPD were 0.773, 0.724, and 0.675, respectively.20 Exacerbation led to the decline of utility value, and the decline varied with the severity of exacerbation. The estimated annual utility decrements were 0.01 for having a non-severe exacerbation and 0.042 for having a severe exacerbation with the same value in each health state and each treatment group.21 The health utility in the case of death was 0.
Cost Measures
As per the China Guidelines for Pharmacoeconomics Evaluations,11 for societal perspective, direct medical costs, direct non-medical costs, and indirect costs were included in the cost calculations. The direct medical costs included costs of drugs, outpatient visit, oxygen and other therapy, outpatient treatment for non-severe exacerbation, and hospitalization for severe exacerbation. The direct non-medical costs included transportation costs, health care and nutrition costs. Further, indirect costs included the productivity loss of patients and their caregivers. The costs incurred in the case of adverse drug reactions and pneumonia under each treatment were not considered in the model (Table 3). All costs were presented in US dollars based on 2021 prices (USD:RMB= 1:6.4061, November 4, 2021). When derived from former studies, the prices were adjusted to the values in 2021 with the rate of 3.5% (Table 3).
 |
Table 3 Resource Use and Annual Costs (USD 2021) for Markov Model |
The price of UMCE/VIL was consistent across the country, whereas FSC (50/500µg) had the same price in most provinces except some remote areas. The prices of 23-valent pneumococcal polysaccharide vaccines slightly differed between different manufacturers, and the average value was used in this model. The drug prices were derived from https://www.scyxzbcg.cn/std/login.html (accessed on 2021-8-31). Because the costs of long-term use of theophylline, expectorants, oral glucocorticoid and oxygen therapy in patients were hard to estimate by unit cost, literature data22 was used (Table 3).
Outpatient follow-up costs mainly included the cost of consultation and lung function examination. The price standards varied depending on provinces and cities in China. Consultation fees (in general outpatient clinics, associate chief physician outpatient clinics, chief physician outpatient clinics) differed from $0.78 to 12.49 per visit, whereas the costs of pulmonary function tests (including lung ventilation function, lung diffusion function, airway resistance, and residual air volume inspection fees) were between $26.54 and 48.08 per test. Therefore, the average price was considered. The treatment costs for non-severe exacerbation and severe exacerbation were calculated from the results reported by Chen et al23 in their survey conducted in 2011 (Table 4).
 |
Table 4 Cost-Effectiveness Analysis of Two Regimens |
Discounting
The costs and benefits were both discounted annually at a rate of 5%.
Model Calculations
The model reported total costs, total costs for exacerbation, total life years (LYs) gained, and total quality-adjusted life-years (QALYs) gained. The cost-effectiveness of UMCE/VIL vs FSC was determined by calculating the incremental total cost per unit effectiveness gained (ICER). To avoid overestimating the incremental value, half-cycle corrections to cost and utility were performed. Given the lack of a standard threshold value in China, the ICER threshold was based on the recommendations of World Health Organization (WHO 2011). If ICER < gross domestic product (GDP) per capita, the incremental cost was worthwhile; if GDP per capita <ICER < three times GDP per capita, the incremental cost was acceptable; otherwise, the incremental cost was not worthwhile.11 According to official data from the National Bureau of Statistics (http://www.stats.gov.cn/), the per capita GDP of China in 2020 was $11,239.29. Our study set three-time per capita GDP as the threshold value for willingness-to-pay (WTP). Accordingly, if ICER was lower than $33,717.86/QALY, the treatment was considered cost-effective.
Sensitivity Analyses
Deterministic (One-Way) Sensitivity Analysis
The effect of varying parameters on model outcomes was examined in one-way sensitivity analyses (OWSA) and a tornado diagram was drawn, which was adjusted by±20% for cost and ±10% for transition probability and utility. The discounting rate varied between 0% and 8% per annum. The time horizon was changed to 5 years and 15 years.
Probabilistic Sensitivity Analysis (PSA)
For the PSA (second-order Monte Carlo simulation), in which all parameters in the model varied simultaneously, distributions were stochastically sampled for 1000 iterations. Cost parameters were based on Gamma distribution and utility value, and transition probability parameters were based on Beta distribution and uniform distribution for discount rate. Scatter plots were developed to represent uncertainty, and cost-effectiveness acceptability curves were created (WTP = 33,717.86).
Results
Base Case Analysis
Over a 10-year time horizon, the probability of staying in the states of moderate COPD, severe COPD, or very severe COPD, or the probability of death in the UMEC/VIL group were 45.3%, 21.7%, 0.05%, and 28.0%, respectively, whereas these values in the FSC group were 46.1%, 13.7%, 11.0%, and 29.2%. (Figures 2 and 3).
 |
Figure 2 Cohort analysis model of UMEC/VIL group. |
 |
Figure 3 Cohort analysis model of FSC group. |
As shown in Table 4, the total treatment costs of using UMEC/VIL to treat patients with moderate-to-very severe COPD was $1947.18 lower than that of using FSC. UMEC/VIL showed similar total LYs and QALYs to those reported by FSC, with a gain of 0.12 LYs and 0.05 QALYs. Incremental cost effectiveness analysis showed that UMEC/VIL was dominant to FSC.
Sensitivity Analyses
Deterministic (One-Way) Sensitivity Analysis
One-way sensitivity analyses showed that UMEC/VIL treatment remained to be the dominant treatment over FSC within the range of uncertainty applied to all variables in the model. In the ICER tornado analysis (WTP = 33,717.86) the 10 variables that had the greatest impact on ICER were utility value in severe COPD and very severe COPD, drug cost of FSC and UMEC/VIL, discounting rate, annual transition probability of moderate COPD to severe COPD with FSC and UMEC/VIL, annual transition probability of severe COPD to very severe COPD with FSC, treatment cost for severe exacerbation in moderate COPD, annual severe exacerbation rate with UMEC/VIL (Figure 4). The results showed the dominance of UMEC/VIL over FSC over 5- and 15-year time horizons.
 |
Figure 4 Tornado diagram (ICER, UMEC/VIL vs FSC). |
Probabilistic Sensitivity Analysis
Probabilistic sensitivity analysis for the comparison of UMEC/VIL and FSC was performed using a second-order Monte Carlo simulation. Simulations were conducted 1000 times, with incremental utility as abscissa and incremental cost as ordinate. Scatter plots of the 1000 simulations were presented for patients undergoing UMEC/VIL treatment and compared with those for patients undergoing FSC at the implicit WTP threshold (approximately $33,717.86). The results showed that 91.7% simulations with green color were cost-effective (Figure 5). Cost-effectiveness acceptability curve showed that UMEC/VIL was more likely to be economically advantageous than FSC (Figure 6).
 |
Figure 5 Scatter plot of incremental cost-effective of UMEC/VIL vs FSC. |
 |
Figure 6 Cost-effectiveness acceptability curve of UMEC/VIL vs FSC. |
Discussion
This analysis estimated the cost-effectiveness of UMEC/VIL compared with FSC among elderly patients with moderate-to-very severe COPD under a Chinese in China. In the base case analysis, UMEC/VIL was found to be dominant over FSC. Patients treated with UMEC/VIL experienced slightly higher LYs and QALYs. Further, sensitivity analyses at current implicit WTP thresholds demonstrated that all cost-effectiveness findings were robust. Given the lack of Chinese data, the conclusions need to be further confirmed by large sample, multi-center, real world studies.
Because FSC is most frequently used for COPD patients in China and has a recognized effect, FSC was selected as the control drug, and only FSC (50 µg/500 µg) was included in the model because of the limit of literature on dose gradient. However, the three specifications (50 µg/100 µg, 50 µg/250 µg, and 50 µg/500 µg) of FSC in China had different prices. Therefore, assuming that only the parameters of drug costs were adjusted, when FSC (50 µg/250 µg) was used in moderate COPD and FSC (50 µg/500 µg) was used in severe and very severe COPD patients, the results of the Markov model were stable and UMEC/VIL was still dominant.
As far as possible, the transition probabilities of this study were selected from epidemiological investigation and clinical trial data of China. When the data could not be obtained directly, relative risk was used. Initially, the model was designed such that the exacerbation rate changed with age, but no reliable parameters were obtained. Therefore, the model was simplified. We first used annual exacerbation rate to estimate the exacerbation risk, which can underestimate the risk. Because the patients may have multiple exacerbations per year, especially in the case of severe or very severe COPD. Accordingly, relative risk data for treatment-specific exacerbations versus placebo were subsequently obtained from two researches, and there might be clinical heterogeneity between the two.
Research showed that there was no significant difference of the incidence of adverse reactions and serious adverse reactions between the two groups, and the incidence of severe adverse reactions was low.24 One study found that ICS/LABA may increase the risk of pneumonia,25 but other studies had shown that there was no significant difference between the two groups.8 Hence, the costs of treatment for adverse drug reactions and pneumonia were not considered. Costs of other drugs, oxygen therapy, exacerbation treatment, direct non-medical costs, and indirect costs were difficult to estimate by unit price and quantity; accordingly, these costs were obtained by past survey researches. The description of direct costs and direct medical expenses was obtained from the research by Zhu et al5 which was a systematic review, and the data of direct costs and direct medical expenses were from different original researches. Therefore, it was possible that the direct medical expenses were greater than direct costs. As the disease progresses, hospitalization expenses, oxygen therapy, and indirect costs gradually increase. Among them, home care costs may gradually become an important component of treatment costs. In addition, the income of residents and disposable medical expenses were quite different among urban, rural, coastal, and remote areas of China; therefore, the representativeness of costing results may be biased. For future studies, we suggest that cost analysis of Chinese COPD patients should give more importance to indirect costs, as it is an important component of the total cost and cannot be ignored.
In the clinical reality, besides cost-effectiveness, factors such as symptoms, frequency of exacerbations, blood eosinophil count, and asthma-like or other specific features will also affect the formulation of COPD treatment strategy. Further, if the COPD patients have complications, the drug cost and utility settings may deviate from the model. Accordingly, in the future, it may be necessary to design a model especially for COPD patients with complications.
Conclusion
The results from this model suggest that UMEC/VIL treatment would be dominant and less costly compared with FSC treatment in elderly patients with moderate-to-very severe COPD. UMEC/VIL had a small benefit in the quality of life and mortality compared with FSC.
Acknowledgments
This study was funded by Sichuan Provincial Health Commission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest related to this study.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020 [Updated 2021]. Available from: https://goldcopd.org/2021-gold-reports/.
2. Yin P, Wang H, Vos T, et al. A subnational analysis for mortality and prevalence of chronic obstructive pulmonary disease in China 1990–2013: findings from Global Burden of Disease Study. Chest. 2016;150(6):1269–1280. doi:10.1016/j.chest.2016.08.1474
3. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
4. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
5. Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi:10.2147/COPD.S161555
6. Chen Z, Li WY, Ni XF, et al. Research and development status Quo of respiratory inhalation preparations: a systematic review. China Pharm. 2021;32(14):1671–1677. Chinese. doi:10.6039/j.issn.1001-0408
7. Chronic Obstructive Pulmonary Disease Group, Respiratory Diseases Branch of Chinese Medical Association Chronic Obstructive Pulmonary Disease Working Committee of the Respiratory Physician Branch of the Chinese Medical Doctor Association. Guidelines for the diagnosis and treatment of chronic obstructive pulmonary disease (revised edition in 2021). Chin J Tuberc Respir Dis. 2021;44(3):170–204. Chinese. doi:10.3760/cma.j.cn112147-20210109-00031
8. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2(2):CD012066. doi:10.1002/14651858.CD012066.pub2
9. Wilson MR, Patel JG, Coleman A, et al. Cost-effectiveness analysis of umeclidinium/vilanterol for the management of patients with moderate to very severe COPD using an economic model. Int J Chron Obstruct Pulmon Dis. 2017;12:997–1008. doi:10.2147/COPD.S124420
10. Rajagopalan K, Bloudek L, Marvel J, et al. Cost-effectiveness of twice-daily indacaterol/ glycopyrrolate inhalation powder for the treatment of moderate to severe COPD in the US. Int J Chron Obstruct Pulmon Dis. 2018;13:3867–3877. doi:10.2147/COPD.S177097
11. Liu GG, Hu SL, Wu JH, et al. China Guidelines for Pharmacoeconomic Evaluations.
12. Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430. doi:10.1016/S2213-2600(18)30103-6
13. Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–637. doi:10.2165/00019053-200523060-00008
14. Earnshaw SR, Wilson MR, Dalal AA, et al. Cost-effectiveness of fluticasone propionate/salmeterol (500/50 mg) in the treatment of COPD. Respir Med. 2009;103(1):12–21. doi:10.1016/j.rmed.2008.10.005
15. Chen R, Gao Y, Wang H, et al. Association between adherence to maintenance medication in patients with COPD and acute exacerbation occurrence and cost in China: a retrospective cohort database study. Int J Chron Obstruct Pulmon Dis. 2020;15:963–971. doi:10.2147/COPD.S234349
16. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
17. Maqsood U, Ho TN, Palmer K, et al. Once daily long-acting beta2-agonists and long-acting muscarinic antagonists in a combined inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019;2(2):CD012930. doi:10.1002/14651858.CD012930.pub2
18. Wise RA, Anzueto A, Cotton D, et al. Investigators, Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. doi:10.1056/NEJMoa1303342
19. Selya-Hammer C, Gonzalez-Rojas GN, Baldwin M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. 2016;10(5):391–401. doi:10.1177/1753465816657272
20. Wu M, Zhao Q, Chen Y, et al. Quality of life and its association with direct medical costs for COPD in urban China. Health Qual Life Outcomes. 2015;13(1):57. doi:10.1186/s12955-015-0241-5
21. Rutten-van Mölken MP, Hoogendoorn M, Lamers LM. Holistic preferences for 1-year health profiles describing fluctuations in health: the case of chronic obstructive pulmonary disease. Pharmacoeconomics. 2009;27(6):465–477. doi:10.2165/00019053-200927060-00003
22. Fang CS. The cost effectiveness analysis of indacaterol versus tiotropium in Chinese medical cost setting. Drug Eval. 2016;13(1):34–39. doi:10.3969/j.issn.1672-2809.2016.01.008
23. Chen X, Wang N, Chen Y, et al. Costs of chronic obstructive pulmonary disease in urban areas of China: a cross-sectional study in four cities. Int J Chron Obstruct Pulmon Dis. 2016;11:2625–2632. doi:10.2147/COPD.S118523
24. Rodrigo GJ, Neffen H. A systematic review on the efficacy and safety of a fixed-dose combination of umeclidinium and vilanterol for the treatment of COPD. Chest. 2015;148(2):397–407. doi:10.1378/chest.15-0084
25. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158–1165. doi:10.1016/j.chest.2019.03.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
