Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
Cost-Effectiveness Of The SQ® Grass SLIT-Tablet In Children With Allergic Rhinitis: A German Payer Perspective
Authors Vogelberg C, Hamelmann E, Wahn U, Domdey A , Pollock RF , Grand TS
Received 16 July 2019
Accepted for publication 11 October 2019
Published 6 November 2019 Volume 2019:11 Pages 637—649
DOI https://doi.org/10.2147/CEOR.S223383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Christian Vogelberg,1 Eckard Hamelmann,2 Ulrich Wahn,3 Anne Domdey,4 Richard F Pollock,5 Tobias S Grand4
1Technische Universität Dresden, Dresden, Germany; 2Klinik Für Kinder- und Jugendmedizin, Evangelisches Klinikum Bethel GmbH, Akademisches Lehrkrankenhaus der Universität Münster, Bielefeld, Germany; 3Klinik Für Pädiatrie m.S. Pneumologie, Immunologie und Intensivmedizin, Charité, Berlin, Germany; 4ALK-Abelló A/S, Hørsholm, Denmark; 5Covalence Research Ltd, London, UK
Correspondence: Tobias S Grand
ALK-Abelló A/S, Hørsholm, Denmark
Tel +45 45 74 75 76
Email [email protected]
Background: The Grazax Asthma Prevention (GAP) trial has recently demonstrated significant reductions in the odds of asthma symptoms or medication use in patients treated with SQ® grass SLIT-tablet relative to placebo, both in combination with allergy and asthma pharmacotherapy. The objective of the present analysis was to evaluate the cost-effectiveness of SQ grass SLIT-tablet relative to placebo in children with AR from the perspective of a German healthcare payer.
Methods: A cost-utility model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to evaluate the cost-utility of SQ grass SLIT-tablet in combination with pharmacotherapy versus pharmacotherapy alone in patients with AR. Transition probabilities were derived from the GAP trial, and costs were taken from a real-world insurance database analysis. Future costs and effects were discounted at 3% per annum, and extensive deterministic and probabilistic sensitivity analyses were performed.
Results: Over a 10-year time horizon, the base case analysis showed an increase in overall treatment costs of €897 per child being treated with SQ grass SLIT-tablet relative to pharmacotherapy alone. The increased treatment costs were accompanied by an improvement in patient quality of life of 0.10 quality-adjusted life years (QALYs) yielding an ICER of €8978 per QALY gained, falling well below a willingness-to-pay threshold of €17,800 per QALY gained. The base case results were insensitive to changes in all individual model parameters.
Discussion: Improvements in quality of life with the SQ grass SLIT-tablet would be accompanied by only a modest increase in costs over a 10-year time horizon, with the SQ grass SLIT-tablet therefore representing excellent value for money from the German healthcare payer perspective.
Keywords: rhinitis, allergic, asthma, costs and cost analysis, Germany
Introduction
Respiratory allergic diseases such as allergic rhinitis (AR) and allergic asthma (AA) are increasingly prevalent. While exact estimates of the prevalence of AR vary, a 2016 study by Bergmann and colleagues reported the lifetime prevalence of AR in Germany to be 14.8% in adults and 10.7% in children based on the Study on Adult Health in Germany, and the Study on the Health of Children and Adolescents in Germany, respectively.1 Previous studies have reported lifetime AR prevalence rates of up to 23%, with grass pollen being the most commonly diagnosed allergen.2 AR is regarded as one of the major risk factors for the development of AA and more than 80% of people with asthma have rhinitis, while 10–40% of people with rhinitis suffer from asthma.3,4 In a 2007 longitudinal study, childhood AR was shown to increase the risk of developing new-onset asthma by two- to seven-fold, and childhood AR in combination with AA increased the likelihood of asthma persisting into adulthood.5
Respiratory allergic disease can be a significant burden on patients due to the deterioration in overall quality of life, and with an estimated 500 million people worldwide suffering from AR, the overall societal costs are considerable, including substantial direct and indirect costs.3,6–9 A survey conducted in Sweden found that the mean annual direct and indirect costs of AR per person were €210 and €751 resulting in a total cost of €961 per year. With a population of 9.8 million, the total cost of AR in Sweden was estimated to be €1.3 billion annually.7
Allergy pharmacotherapy, used to treat symptoms of both AR and AA, represents a substantial direct cost associated with respiratory allergic diseases. When pharmacotherapy is not sufficient to control symptoms in patients with moderate-severe AR, allergy immunotherapy (AIT) may be prescribed.3 AIT can be administered either subcutaneously (SCIT) or sublingually (SLIT) as tablets or drops. The SQ® grass SLIT-tablet (GRAZAX®, ALK-Abelló, Denmark) is a standardised sublingual allergy immunotherapy for the treatment of grass pollen-induced allergic rhinitis/conjunctivitis (AR/C) in adults and children (5 years or older). The Grazax Asthma Prevention (GAP) trial, conducted over 5 years in children with AR, compared the risk of developing asthma in patients treated with SQ grass SLIT-tablet relative to those taking placebo.10 In line with European Medicines Agency (EMA) guidelines on the clinical development of AIT, all patients were offered allergy and asthma pharmacotherapy during the trial.11 Asthma medications included β2-agonists, systemic corticosteroid, inhaled corticosteroids, leukotriene receptor antagonists, long-acting β2-agonist, sustained-release theophylline, and cromolyn sodium, while allergy pharmacotherapy included antihistamines, eye drops and nasal spray. The full trial protocol has been published previously, but in brief, the trial was a double-blind placebo-controlled trial with 812 children randomly assigned to receive 3 years of treatment with the SQ grass SLIT-tablet or placebo, and 2 years of follow-up after the end of treatment. At randomisation, patients were required to be aged 5 to 12 years, and have a diagnosis of grass pollen AR and clinically relevant symptoms. Patients were not eligible for the trial if they had experienced asthma symptoms or had a medical history of asthma during the past 2 years.10
The primary endpoint of the GAP trial was time to asthma onset, with asthma defined as the fulfilment of one or more respiratory criteria (Table 1). The trial showed that a numerically lower proportion of patients in the SQ grass SLIT-tablet arm were diagnosed with asthma (34/398 or 8.54%) than the placebo arm (39/414 or 9.42%), but the finding did not reach statistical significance (hazard ratio 0.9; p=0.667).10 While the primary endpoint was not statistically significant, treatment with the SQ grass SLIT-tablet did significantly reduce the odds of experiencing asthma symptoms or using asthma medication at the end of trial (i.e. after 3 years of treatment and 2 years of follow-up) relative to placebo with an odds ratio of 0.66 (p=0.036).10 Furthermore, relative to the placebo arm, AR symptoms were reduced by between 22% and 30% in each year of the trial (p≤0.002 for all 5 years). The observed effects in the SQ grass SLIT-tablet arm were added benefits on top of pharmacotherapy. These positive results from the secondary endpoints of the GAP trial complement previous findings on the efficacy of AIT in reducing the incidence of asthma. For example, in 2015, a large retrospective cohort study based on data from the German population found that the relative risk of incident asthma was 0.60 (95% CI: 0.42–0.84, p=0.003) times lower in patients that had received AIT compared to the control group.12 Similarly, a retrospective database analysis published in 2018 reported that asthma onset was less frequent in patients treated with grass pollen SLIT tablets than in a control group of non-AIT (odds ratio 0.696, p=0.002).13
 |
Table 1 Asthma Diagnosis Criteria In The Grazax Asthma Prevention (GAP) Trial |
Despite the substantial burden of AR and the proven benefits of using AIT to reduce the incidence of asthma symptoms and asthma medication use, the health economic aspects of AIT use in the context of seasonal pollen-induced AR and onset of AA are yet to be quantified using data directly from a SLIT tablet trial including asthma endpoints. The objective of the present study was therefore to use the findings of the GAP trial and a bespoke cost-utility model to evaluate the relative costs and quality-adjusted life expectancy associated with using the SQ grass SLIT-tablet relative to with placebo in children with grass pollen-induced AR.
Methods
Model Structure
A cost-utility model was developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to evaluate the cost-utility of SQ grass SLIT-tablet in combination with pharmacotherapy versus pharmacotherapy alone in patients with AR. A Markov model was designed to capture the co-morbid nature of AR and AA, as well as differing levels of AR severity across four health states (Figure 1). Health states were based on the Allergic Rhinitis and its Impact on Asthma (ARIA) classification, which separates AR into mild and moderate-severe AR.3 Moderate-severe AR is defined by the presence of one or more of the ARIA quality of life items: sleep disturbance, impairment of daily activities, impact of school/work, and troublesome symptoms, while mild AR is defined by the absence of all four items. Within the mild and moderate-severe AR health states, patients were subdivided into categories based on the presence or absence of AA in addition to AR, resulting in the four health states depicted in Figure 1. Treatment of AA follows a stepwise approach with the objective of keeping AA in a controlled state, but AA may become uncontrolled over time.14 As it was not possible to subdivide asthma into controlled or uncontrolled asthma based on data from the GAP trial, it was conservatively assumed that all modeled patients with asthma had controlled asthma. The cycle length for the model was 1 year based on the annual occurrence of the grass pollen season.
Each of the four health states were adjusted for intermittent or persistent AR. The division of patients into intermittent and persistent groups was implemented to capture the number of days per year on which symptoms of seasonal AR were experienced, which has been shown to have a marked effect on both cost and health-related quality of life.7,15–18 AR was classified as persistent when symptoms were present every day during the allergy season, and intermittent when symptoms were present for less than 4 days a week or for less than 4 consecutive weeks.4,16 Based on data from www.pollenstiftung.de, the length of the grass pollen season in Germany was taken to be 10 weeks. Based on this season duration, patients with persistent AR would experience symptoms for 10 weeks (i.e. 70 days) a year, while patients with intermittent AR would experience symptoms for a minimum of 1 day, and a maximum of 56 days (symptoms every day for a total of 8 weeks of the grass pollen season and 2 weeks without symptoms to account for non-consecutive weeks). The mean duration of symptoms for patients with intermittent AR was taken to be 28 days based on the midpoint between the minimum and maximum duration of intermittent AR symptoms and assuming that symptom duration is normally distributed. In the base case analysis, 70 days of symptoms were therefore modeled for patients with persistent AR and 28 days for patients with intermittent AR. The effect of these different durations of symptoms was examined in sensitivity analyses.
Clinical Data
Clinical data to support the first 5 years of analysis in the SQ grass SLIT-tablet and placebo arms were taken from the respective arms of the GAP trial. Data from children who discontinued treatment in the GAP trial were not included in the analysis, and 608 children (300 from active group, 308 from placebo group) were therefore included in the clinical dataset.10 The number of transitions between the four health states was recorded at each annual visit in the GAP trial; these trial transitions were used to derive annual transition probabilities for years 1–5. Children were assigned to the different health states based on AR severity and duration of symptoms (i.e. intermittent or persistent) as determined annually at trial visits, and based on asthma symptoms and medication use reported annually. Children were assigned to an asthma health state if they had experienced asthma symptoms and used asthma medication during the year leading up to the visit.
Data on the severity of AR up to 10 years after treatment initiation were not available and costs and treatment effects beyond the GAP trial period were therefore extrapolated based on the mean transition probabilities derived from years 4 and 5 of the GAP trial data. The key assumption underpinning this extrapolation was the sustained efficacy of the SQ grass SLIT-tablet in the treatment of AR beyond the trial period, as demonstrated previously.19
For clinical data relating to asthma, one long-term prospective study of AIT for asthma prevention was identified by the literature review: the PAT trial.20 The PAT trial included data on asthma prevention up to 10 years after treatment initiation, and has previously been used to model long-term outcomes for SQ grass SLIT-tablet in similar economic evaluations.20–22 The study reported data from an open randomised controlled trial investigating the efficacy of grass or birch SCIT in relation to prevention of asthma in children with allergic rhinitis. A total of 205 children aged 6 to 14 with grass and/or birch pollen allergy were randomly assigned to either birch SCIT, grass SCIT or placebo, with all groups having access to allergy and asthma pharmacotherapy. The children were treated with extract of grass pollen and/or birch pollen administered subcutaneously every 6 weeks during the 3-year treatment period and were followed for a period of 10 years.23 Clinical data from all three treatment groups were included in the base case analysis. Asthma symptoms and medication use were not available from the PAT trial, and children were assigned to having asthma based on the definition of asthma used in the PAT trial (see online repository for exact definition), and thus, the definition of asthma in the model is different in years 1–5 and years 6–10.
The PAT trial was used to extrapolate the treatment effect up to 10 years based on the established disease modifying effect of the SQ grass SLIT-tablet, and it contains the same pollen extract as the grass pollen SCIT used in the PAT trial.19 Thus, extrapolation of data on asthma prevention was based on the assumption that the immunotherapy products used for treatment were similar between GAP and PAT, and that both treatments exhibit a disease-modifying effect. The base case analysis used linear regression to extrapolate AA to years 6–10 using the PAT trial data (Figure 2).
Cost And Resource Use Data
Cost and resource use for the moderate-severe AR health states were taken from a 2014 German analysis of data from a Saxonian insurance database (n=1,739,440).24 In the analysis, ICD-10 codes J30 (“vasomotor and allergic rhinitis”) and J45 (“asthma”) were used to define whether people had AR or AA, respectively. In addition to the ICD-10 code, at least two filled prescriptions for inhaled corticosteroids (ICS) within four consecutive quarters were required to confirm the diagnosis of asthma. The study did not report differences in asthma severity, and the reported costs and resource use estimates therefore represent asthma of any severity. The study included a comparison of costs in patients using AIT and pharmacotherapy versus patients using pharmacotherapy only. In Europe, AIT is indicated for patients with moderate-severe rhinitis, and the cost reported in the AIT and pharmacotherapy group was therefore assigned to the moderate/severe rhinitis health states in the SQ grass SLIT-tablet arm of the analysis. Costs reported in the pharmacotherapy only group were assigned to the moderate/severe rhinitis health states in the placebo arm of the analysis, i.e. patients with access to pharmacotherapy only. The cost of pharmacotherapy did not include over-the-counter (OTC) drugs as these are not typically reimbursed by insurers.
Patients with mild AR typically use OTC products such as anti-histamines. Thus, the costs associated with mild AR were based on the assumption that there would be no difference in cost between the SQ grass SLIT-tablet group and placebo, except the cost of the SQ grass SLIT-tablet itself. For the mild AR health states, it was assumed that patients with co-reported asthma experienced mild controlled asthma. Based on the Global Initiative for Asthma (GINA) guidelines, patients with mild asthma can be controlled with either symptom-driven or daily low-dose ICS.14 Low-dose ICS-formoterol or short-acting beta-agonists (SABA) may also be taken to relieve symptoms as required.14 For mild AR and co-morbid AA, it was therefore assumed that patients received ICS at an average daily dose of 100 mcg (i.e. 1 inhaled dose per day). The guidelines also recommend that SABA inhalers should be used to relieve daytime symptoms when less frequent than twice a month so it was assumed that mild patients require 4 doses of a SABA inhaler once a month. These asthma medications were assumed to be administered throughout the year for the treatment of AA. The cost of the SQ grass SLIT-tablet itself was included as a once a day treatment for 31 months. The total time on treatment was assumed to be 31 months based on the mean values reported from the GAP trial. The assignment of cost and resource use for all four health states is shown in Table 2.
 |
Table 2 Resource Use For Health States |
Utility Values
Utility values associated with each of the health states were taken from a belief elicitation study by Retzler et al, which was based on 1454 adults and 1082 children from four European countries (UK, France, Germany and Slovakia; Table 3).25 Adults and children were asked to complete an online survey on AR and AA, and the utility values estimated for children were applied to each of the model health states. Children with AR and no comorbid AA were assumed to have the same utility as the general population outside the grass pollen season.26,27 Annual utility values for mild and moderate/severe AR with no co-morbid asthma were therefore adjusted for the proportion of days with no allergic symptoms. For children with co-morbid asthma, asthma symptoms were assumed to be persistent regardless of pollen season, and utility values for well/partly controlled asthma were therefore applied to asthma health states all year around. Annual quality of life (in quality-adjusted life years) was then calculated using the pollen season utility and the utility assigned to the remainder of the year.
 |
Table 3 Utility Values |
Perspective, Discounting And Time Horizon
The analysis was conducted in a simulated cohort of 1000 pediatric patients over a 10-year time horizon. All future health outcomes (i.e. quality-adjusted life expectancy) and costs were discounted at 3% per annum based on guidelines from the German Institute for Quality and Efficiency in Health Care (IQWiG).28 As there is no formal cost-effectiveness threshold in Germany, a threshold of €17,800 per QALY was adopted in the current study based on recommendations from the National Institute for Health and Care Excellence (NICE) and an exchange rate of £0.89 to €1.00.29,30
Sensitivity And Scenario Analyses
A series of one-way sensitivity analyses were conducted to investigate the impact of individual model parameters on the results relative to the base case analysis. The discount rate was varied from 3% (as recommended by IQWiG guidelines) to values within a range that covers 127 international Health Technology Assessment (HTA) agencies as described in Mathes et al.28,31 Duration of treatment was varied from 31 months (the mean treatment duration in the GAP trial) to 36 months which is the recommended treatment duration for AIT.32 The price of the SQ grass SLIT-tablet was varied from the price used in the base-case analysis for Germany (€3.23) to prices within a range of ±10%. Costs were varied within the ranges of the standard deviations provided in Domdey et al, and utility values were varied according to the interquartile ranges reported in Retzler et al.24–25
The base case analysis for asthma was based on extrapolation of the treatment effect from the PAT trial. To establish the extent to which the extrapolation had an impact on the results of the analysis, a conservative scenario analysis was performed to consider another scenario where data on the development of asthma were extrapolated using the average proportion of patients with AA from years 4–5 of the GAP trial (Figure 3).
A series of two-way sensitivity analyses were conducted to explore the potential effects of persistence on the results. In these persistence analyses, two variables were analyzed: the rate of persistence with SQ grass SLIT-tablet treatment, and the percentage reduction in quality of life associated with non-persistence. Reported levels of persistence and adherence to SLIT and SCIT vary widely in the literature and a range of assumed degrees of persistence were therefore analyzed from 100% down to 60% in 2% intervals. The effect of non-persistence on quality of life was also analyzed over a range of 0% to 10% reductions in quality of life in each of the four health states in 0.5% intervals. Since the costs of pharmacotherapy in the base case analysis were based on published data from an insurance database in Germany, the existing costs of pharmacotherapy were assumed to capture a realistic level of persistence with treatment; in the two-way sensitivity analysis, only the costs of SQ grass SLIT-tablet were therefore affected by the level of persistence, assuming that non-persistent patients would incur no further costs of treatment. ICERs from the 441 analyses (21 persistence levels × 21 quality of life decrements) were then plotted on a contour plot showing areas of dominance, domination, and cost-effectiveness for SQ grass SLIT-tablet relative to placebo.
A probabilistic sensitivity analysis was conducted in which uncertainty around multiple parameters was captured. In the analysis, 1000 Monte Carlo simulations were conducted, simultaneously drawing from distributions around key model parameters. The key model parameters were: cost of pharmacotherapy and inpatient visits for the M/S AR, M/S AR + AA health states, utility values for all health states, length of grass pollen season, and number of days with symptoms for intermittent patients and long-term effectiveness for SQ grass SLIT-tablet and placebo. The modelled estimates of cost and quality of life were recorded for each model iteration and used to generate a cost-effectiveness plane and cost-effectiveness acceptability curves.
Results
Base Case Analysis
Over a 10-year time horizon, the base case analysis showed an increase in overall treatment costs of €897 per child being treated with SQ grass SLIT-tablet. The increased treatment costs were accompanied by an improvement in patient quality of life of 0.10 QALYs (Table 4), yielding an ICER of €8978 per QALY gained. As the ICER falls below the cost-effectiveness threshold of €17,800 per QALY gained, SQ grass SLIT-tablet would be considered a cost-effective intervention for children with AR at risk of developing AA, relative to pharmacotherapy alone.
 |
Table 4 Base Case Analysis Results |
Sensitivity And Scenario Analyses
One-way sensitivity analysis results showed that for all sensitivity analyses, the ICER remained below the cost-effectiveness threshold (Table 5). The parameters that had the highest impact on the ICER were hospital costs associated with the health state M/S AR + AA and utility values for the health state M/S AR + AA.
 |
Table 5 Sensitivity Analyses |
The result from the scenario analysis shows that when data on the development of asthma were extrapolated for years 6–10 using the average from years 4–5 of the GAP trial, the value for the ICER increased from €8,978 to €16,367 but remained well below the €17,800 per QALY willingness-to-pay (WTP) threshold adopted in the present study.
The two-way sensitivity analysis of persistence and quality of life (Figure 4) showed that SQ grass SLIT-tablet would remain either cost-effective or dominant with persistence rates of between 60% and 100%, assuming that non-persistence was associated with up to a 3% reduction in patient quality of life. Assumptions of larger reductions in quality of life with persistence rates of 90% and below were associated with less favourable outcomes for SQ grass SLIT-tablet relative to placebo, including domination and less effective but less costly outcomes (Figure 6).
Plotting the results of 1000 probabilistic sensitivity analysis iterations on a cost-effectiveness plane showed the majority (94.3%) of results to be in the northeast quadrant, corresponding to increased costs and improved quality of life (Figure 5). A cost-effectiveness acceptability curve was generated by plotting the proportion of PSA iterations that would be cost-effective at a range of WTP thresholds from €0 to €100,000 per QALY (Figure 6). At a WTP threshold of €17,800 per QALY gained, there was an 82.3% probability of the SQ grass SLIT-tablet being cost-effective compared to pharmacotherapy. Above the pre-specified WTP threshold, the likelihood of cost-effectiveness with the SQ grass SLIT-tablet continued to increase, reaching 84.9% at €20,000 per QALY gained, and 90.4% €30,000 per QALY gained.
Discussion
The present study showed that the SQ grass SLIT-tablet is cost-effective relative to placebo in children with grass pollen-induced AR in Germany. Over a 10-year time horizon, the SQ grass SLIT-tablet was projected to increase quality of life by 0.10 QALYs relative to placebo, and incur an additional €897 in costs, translating to an ICER of €8978 per QALY gained, falling below the €17,800 per QALY threshold converted from the £20,000 per QALY threshold recommended by NICE in the absence of a specific recommendation from IQWiG.29
SQ grass SLIT-tablet has already been shown to be an efficacious and well-tolerated treatment for grass pollen-induced AR.33–35 The data from the GAP trial confirmed the safety profile of the SQ grass SLIT-tablet (with similar proportions of patients experiencing adverse events in the active and placebo arms), while also demonstrating additional benefits in terms of reducing asthma symptoms and/or asthma medication use.10 When considering sublingual tablets relative to other AIT administration routes, SLIT-tablets confer many benefits over SCIT products, the most significant economic benefit being safe for at-home oral administration; whereas SCIT products require frequent interactions with healthcare professionals (HCP) for the first 3–12 months of treatment, SLIT-tablets offer simple, once-daily, at-home administration with no up-dosing schedule.36,37 Even when compared with SLIT-drops, SLIT-tablets are easier to store and transport, require a shorter sublingual holding time, and offer more flexible dosing.38–40
This is the first analysis to show cost-effectiveness for the SQ grass SLIT-tablet in children with AR who develop concomitant asthma, made possible due to novel data on asthma medication use and symptoms obtained from the GAP trial. The cost-effectiveness of the SQ grass SLIT-tablet has been investigated previously, and the results from this analysis broadly corroborate previous results and the conclusion that the SQ grass SLIT-tablet is a cost-effective treatment for AR.21,22,41,42 More clinical studies on the effect of AIT on asthma could further strengthen the analysis, especially because more severe and uncontrolled forms of asthma represent large costs to healthcare budgets. These costs were not included in the current analysis as a conservative approach to asthma was adopted such that all asthma was assumed to be mild.
The results shown in this analysis are similar to other economic evaluations including SQ grass SLIT-tablet despite differences in model structure and different input parameters, which serves as a form of external or “third-order” validation of the model.21,22,41–43 Notably, in all sensitivity analyses, the SQ grass SLIT-tablet remained cost-effective when compared to placebo, demonstrating that the base case analysis was robust to changes in individual model parameters. The key drivers of cost-effectiveness identified through sensitivity analyses were hospital costs associated with the moderate-severe AR + AA health state, utility values for the moderate-severe AR + AA health state, and the proportion of children with asthma in years 6–10.
The results obtained in the current analysis are backed by high-quality and clinically relevant data used to inform transition probabilities between health states. These included results from a large clinical trial in children (the GAP trial) that provided data on asthma outcomes during a 5-year period,10 and cost data from a real-world insurance database.24 Furthermore, the clinical assumptions used to inform the model structure and parameters were carefully aligned with expert clinical opinion, which was solicited to validate key model parameters, including the time horizon, length of pollen season, duration of symptoms for intermittent and persistent AR, and the model structure. Other important strengths of the model include its novel use of health states informed by the ARIA classifications,3 the use of cost data specific to the German setting, and the use of a true Markov structure with time-dependent transition matrices to model transitions between AR and AA states, which allows for more sophisticated analyses to be conducted relative to a decision tree, for example.
The comprehensive analysis of persistence and a range of assumed effects on patient quality of life was also a key strength of the present analysis, illustrating a range of health economic outcomes for SQ grass SLIT-tablet relative to placebo depending on the assumed rates of persistence and the effect of non-persistence on patient quality-of-life. With persistence of 60% or above, SQ grass SLIT-tablet was found to be either highly cost-effective or dominant when treatment discontinuation was associated with quality of life reductions of up to 3%. Persistence is a well-established driver of health economic analyses of AIT products, with a recent German cost-effectiveness analysis of increased SCIT uptake moving from cost-effective to dominant when reducing the discontinuation rates to a minimal value identified in the literature.44 The contour plot used to present the results of the treatment persistence analysis reflects the current uncertainty around SLIT persistence rates and the effect of non-persistence on quality of life and represents a potential approach for comprehensively exploring these factors in future health economic analyses.
The base case analysis was conservative in a number of ways, perhaps most notably with regard to the severity of disease in the patients included in the GAP trial. Recruiting a sufficient number of patients with moderate-severe AR without asthma symptoms was challenging, and the GAP trial therefore also included pediatric patients with mild AR, who would be less likely to benefit from AIT treatment or likely to see a reduced effect relative to those patients with more severe AR. In the present study, we relied on the secondary endpoints of risk of experiencing asthma symptoms, using asthma medication, and asthma symptom severity, all of which are highly relevant in a health economic analysis where cost and quality of life are the exclusive determinants of the incremental cost–utility ratio.
As with all health economic modeling analyses, there are a number of limitations that should be acknowledged when interpreting the findings of the analysis. One of the inherent weaknesses of the current study included a scarcity of data beyond the 10-year time horizon, meaning that the analysis could not predict the development of asthma, asthma exacerbations or worsening of existing asthma beyond 10 years after treatment initiation. Lack of data on SLIT-tablet treatment beyond 5 years after treatment initiation meant that AR severity was extrapolated from years 4 and 5 of the GAP trial, while the development of asthma in years 6–10 was based on data from the PAT trial, which represents a possible weakness in the analysis. Notably, the PAT trial and the GAP trial also utilised different definitions of asthma, which represents a potential disjunction in the modeling analysis. Crucially, however, the assumptions concerning this extrapolation were tested in a scenario analysis that extrapolated based exclusively on years 4 and 5 of the GAP trial, which supported the outcome of the base case analysis based on PAT trial data. Furthermore, the findings of the PAT trial (i.e. a significant reduction in the proportion of patients with incident asthma in the 7 years after cessation of allergy immunotherapy) have been corroborated by large-scale retrospective studies.12,13
In conclusion, the SQ grass SLIT-tablet has already been demonstrated to be an efficacious and safe AIT product, with a straightforward administration route, and resulting in marked improvements in quality of life in pediatric patients with AR. Based on the present health economic analysis, the improvements in quality of life would be accompanied by only a modest increase in costs over a 10-year time horizon, with the SQ grass SLIT-tablet therefore representing excellent value for money from the German healthcare payer perspective.
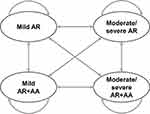 |
Figure 1 Overview of Markov model structure. Abbreviations: AA, allergic asthma; AR, allergic rhinitis. |
 |
Figure 2 Base case extrapolation with linear regression using PAT trial data. |
 |
Figure 3 Scenario analysis of extrapolation. |
 |
Figure 5 Cost-effectiveness plane presenting results of the probabilistic sensitivity analysis, dotted line indicates the cost-effectiveness threshold.Note: Solid line indicates cost-effectiveness threshold of €17,800 per QALY (converted from £20,000 per QALY using an exchange rate of €1 to £0.89).30 |
 |
Figure 6 Cost-effectiveness acceptability curve. |
Disclosure
Prof. Dr Christian Vogelberg reports personal fees from Allergopharma, personal fees from AstraZeneca, grants, personal fees from Boehringer Ingelheim, personal fees from Bencard Allergy, personal fees from DBV Technologies, grants, personal fees from Novartis Pharma, and personal fees from Sanofi Avensis, outside the submitted work. Mrs Anne Domdey was employed by ALK-Abelló A/S while conducting the work. This study was funded by an unrestricted research grant from ALK-Abelló A/S. Medical writing assistance was provided by Covalence Research Ltd, which received consultancy fees from ALK-Abelló A/S. The authors report no other conflicts of interest in this work.
References
1. Bergmann KC, Heinrich J, Niemann H. Current status of allergy prevalence in Germany: position paper of the Environmental Medicine Commission of the Robert Koch Institute. Allergo J Int. 2016;25:6–10. doi:10.1007/s40629-016-0092-6
2. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764. doi:10.1183/09031936.04.00013904
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63 Suppl 86:8–160. doi:10.1111/j.1398-9995.2007.01620.x
4. Bousquet J, Van Cauwenberge P, Khaltaev N; Aria Workshop Group, World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. doi:10.1067/mai.2001.118891
5. Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007;120:863–869. doi:10.1016/j.jaci.2007.07.020
6. Canonica GW. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62 supp:17–25. doi:10.1111/j.1398-9995.2007.01549.x
7. Cardell LO, Olsson P, Andersson M, et al. TOTALL: high cost of allergic rhinitis-a national Swedish population-based questionnaire study. NPJ Prim Care Respir Med. 2016;26:15082. doi:10.1038/npjpcrm.2015.82
8. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7:12. doi:10.1186/1939-4551-7-12
9. Price D, Scadding G, Ryan D, et al. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. doi:10.1186/s13601-015-0083-6
10. Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141:529–538. doi:10.1016/j.jaci.2017.06.014
11. European Medicines Agency. Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. Rep. CHMP/EWP/18504/2006. London, UK: European Medicines Agency; 2008.
12. Schmitt J, Schwarz K, Stadler E, Wustenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136:1511–1516. doi:10.1016/j.jaci.2015.07.038
13. Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. 2018;73(1):165–177. doi:10.1111/all.13213
14. GINA Board of Directors and Committees. Global Strategy for Asthma Management and Prevention. Asthma management and prevention for adults and children over 5 years: a pocket guide for healthcare professionals. 2019. Available from: https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf.
15. Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy. 2015;60:350–353. doi:10.1111/j.1398-9995.2005.00751.x
16. Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117:158–162. doi:10.1016/j.jaci.2005.09.047
17. Colas C, Brosa M, Anton E, et al. Estimate of the total costs of allergic rhinitis in specialized care based on real-world data: the FERIN Study. Allergy. 2017;72:959–966. doi:10.1111/all.13099
18. Petersen KD, Kronborg C, Gyrd-Hansen D, Dahl R, Larsen JN, Lowenstein H. Quality of life in rhinoconjunctivitis assessed with generic and disease-specific questionnaires. Allergy. 2008;3:284–291. doi:10.1111/j.1398-9995.2007.01583.x
19. Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial. J Allergy Clin Immunol. 2012;2012(129):717–725. doi:10.1016/j.jaci.2011.12.973
20. Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–948. doi:10.1111/j.1398-9995.2007.01451.x
21. Bachert C, Vestenbaek U, Christensen J, Griffiths UK, Poulsen PB. Cost-effectiveness of grass allergen tablet (GRAZAX) for the prevention of seasonal grass pollen induced rhinoconjunctivitis - a Northern European perspective. Clin Exp Allergy. 2007;37:772–779. doi:10.1111/j.1365-2222.2007.02706.x
22. Ronaldson S, Taylor M, Bech PG, Shenton R, Bufe A. Economic evaluation of SQ-standardized grass allergy immunotherapy tablet (Grazax®) in children. Clinicoecon Outcomes Res. 2014;6:187–196.
23. Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J Allergy Clin Immunol. 2002;109:251–256. doi:10.1067/mai.2002.121317
24. Domdey A, Grand TS, Elliot L, Tesch F, Schmitt J, Küster D. Costs and resource use in allergic rhinitis. Allergy. 2018;73(S105):471. doi:10.1111/all.13244
25. Retzler J, Grand TS, Domdey A, Smith A, Romano RM. Utility elicitation in adults and children for allergic rhinoconjunctivitis and associated health states. Qual Life Res. 2018;27:2383–2391. doi:10.1007/s11136-018-1910-8
26. Janssen MF, Szende A, Cabases J, Ramos-Goni JM, Vilagut G, Konig HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2018.
27. Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York, UK: Centre for Health Economics; 1999.
28. Institute for Quality and Efficiency in Health Care. General Methods for the Assessment of the Relation of Benefits to Costs. Cologne, Germany; 2009. Available from: https://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf.
29. National Institute for Health and Care Excellence. Guide to the Methods of Technology Appraisal 2013. London, UK; 2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781.
30. OANDA Corporation. OANDA FX Rate® GBP: EUR exchange rate. Available from: https://www1.oanda.com/fx-for-business/exchange-rates-api.
31. Mathes T, Jacobs E, Morfeld JC, Pieper D. Methods of international health technology assessment agencies for economic evaluations–a comparative analysis. BMC Health Serv Res. 2013;13:371. doi:10.1186/1472-6963-13-438
32. Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73(4):765–798. doi:10.1111/all.13317
33. Kleine-Tebbe J, Ribel M, Herold DA. Safety of a SQ-standardised grass allergen tablet for sublingual immunotherapy: a randomized, placebo-controlled trial. Allergy. 2006;61(2):181–184. doi:10.1111/j.1398-9995.2006.00959.x
34. Dahl R, Stender A, Rak S. Specific immunotherapy with SQ standardized grass allergen tablets in asthmatics with rhinoconjunctivitis. Allergy. 2006;61(2):185–190. doi:10.1111/j.1398-9995.2005.00949.x
35. Dahl R, Kapp A, Colombo G, et al. Sublingual grass allergen tablet immunotherapy provides sustained clinical benefit with progressive immunologic changes over 2 years. J Allergy Clin Immunol. 2008;121(2):512–518.e2. doi:10.1016/j.jaci.2007.10.039
36. Moingeon P. Update on immune mechanisms associated with sublingual immunotherapy: practical implications for the clinician. J Allergy Clin Immunol Pract. 2013;1(3):228–241. doi:10.1016/j.jaip.2013.03.013
37. Nolte H, Maloney J. The global development and clinical efficacy of sublingual tablet immunotherapy for allergic diseases. Allergol Int. 2018;67(3):301–308. doi:10.1016/j.alit.2018.03.008
38. Staloral® Monograph. 7268-3. June 2011.
39. GRAZAX® Summary of Product Characteristics. June 2018.
40. Demoly P, Passalacqua G, Calderon MA, Yalaoui T. Choosing the optimal dose in sublingual immunotherapy: rationale for the 300 index of reactivity dose. Clin Transl Allergy. 2015;5:44. doi:10.1186/s13601-015-0088-1
41. Nasser S, Vestenbaek U, Beriot-Mathiot A, Poulsen PB. Cost-effectiveness of specific immunotherapy with Grazax in allergic rhinitis co-existing with asthma. Allergy. 2008;63(12):1624–1629. doi:10.1111/j.1398-9995.2008.01743.x
42. Canonica GW, Poulsen PB, Vestenbaek U. Cost-effectiveness of GRAZAX for prevention of grass pollen induced rhinoconjunctivitis in Southern Europe. Respir Med. 2007;101(9):1885–1894. doi:10.1016/j.rmed.2007.05.003
43. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–743. doi:10.1177/0272989X12454579
44. Richter AK, Klimek L, Merk HF, et al. Impact of increasing treatment rates on cost-effectiveness of subcutaneous immunotherapy (SCIT) in respiratory allergy: a decision analytic modelling approach. Eur J Health Econ. 2018;19(9):1229–1242. doi:10.1007/s10198-018-0970-6
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

