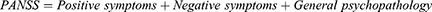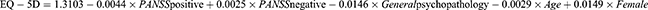Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Cost-Effectiveness of Aripiprazole Tablets with Sensor versus Oral Atypical Antipsychotics for the Treatment of Schizophrenia Using a Patient-Level Microsimulation Modeling Approach
Authors Chopra AS, Hadzi Boskovic D, Kulkarni A, Cochran JM
Received 17 November 2022
Accepted for publication 2 May 2023
Published 22 May 2023 Volume 2023:15 Pages 375—386
DOI https://doi.org/10.2147/CEOR.S396806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Avijeet S Chopra,1 Dusica Hadzi Boskovic,2 Amit Kulkarni,2 Jeffrey M Cochran2
1Manticore Consultancy, North Potomac, MD, USA; 2Otsuka Pharmaceutical Development and Commercialization, Princeton, NJ, USA
Correspondence: Dusica Hadzi Boskovic, Otsuka Pharmaceutical Development and Commercialization, Inc., 508 Carnegie Center, Princeton, NJ, 08540, USA, Email [email protected]
Objective: Strategies designed to track drug ingestion may improve medication adherence and clinical outcomes in adults with schizophrenia. This study aimed to estimate the cost-effectiveness of aripiprazole tablets with sensor (AS; Abilify MyCite®) versus generic oral atypical antipsychotics (AAPs) in schizophrenia from the United States payer and societal perspectives over 12 months.
Methods: An individual-level microsimulation was developed to generate individual trajectories using data from a phase 3b multicenter, open-label, mirror-image trial in adults with schizophrenia treated prospectively for 6 months with AS. The patient’s clinical characteristics and outcomes were computed as a function of the Positive and Negative Syndrome Scale (PANSS) scores. Direct and indirect medical cost estimates were sourced from the literature; EuroQol 5-Dimensions (EQ-5D) utilities were derived using risk equations based on patient and clinical characteristics. Scenario analyses were also conducted to assess outcomes under the assumption of treatment durability over 12 months.
Results: Over 12 months, AS showed a 12.2% improvement in PANSS score. AS had an incremental cost of $2168 and incremental cost savings of $22,343 from the payer and societal perspectives, respectively, with an incremental quality-adjusted life-year (QALY) gain of 0.0298 versus oral AAPs. Further, AS resulted in a 28.2% reduction in hospitalizations over 12 months. At a willingness-to-pay of $100,000 per QALY, the net monetary benefit over 12 months was $25,323 from the payer perspective. Under the assumption of the durability of the treatment effect of AS, the findings were similar to those of the base case analyses, though with greater cost savings and QALYs gained with AS. The results from the sensitivity analyses were consistent with those of the base case analysis.
Conclusion: AS may be a cost-effective strategy, with lower costs and improved quality of life among patients with schizophrenia over 12 months, from the payer and societal perspectives.
Keywords: aripiprazole tablets with sensor, Abilify MyCite, digital medicine system, cost-effectiveness analysis, schizophrenia
Key Points for Decision Makers
- The use of aripiprazole tablets with sensor (AS) reduces disease severity and subsequently psychiatric hospitalizations compared to oral atypical antipsychotics (AAPs).
- The economic analysis showed that AS results in cost savings and improvement in patient quality of life compared to oral AAPs, making it a potentially cost-effective strategy.
Introduction
Schizophrenia, a chronic psychiatric disorder is characterized by a combination of psychotic symptoms, such as delusions, disorganized speech, and hallucinations.1 Schizophrenia is one of the leading causes of disability with a prevalence ranging between 0.25% and 0.64% in the United States (US).2–4 Schizophrenia has a considerable clinical, economic, and social burden albeit low prevalence. About 50% of individuals with schizophrenia have co-morbid mental or behavioral health disorders.5 Combined with an increased risk of premature mortality and a suicidal rate of 4.9%, the average potential life lost for individuals with schizophrenia in the US is 28.5 years.6–9 The financial burden of schizophrenia is disproportionately high relative to other chronic mental and physical health conditions with an estimated annual economic burden of $155.7 billion in the US. Indirect costs account for approximately 76% of the total financial burden attributed to high unemployment rates and caregiver burden. Psychiatric hospitalization and medications are the key drivers of direct costs.10
The goal of treatment is to improve the quality of care and treatment outcomes for patients with schizophrenia as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition.11 The American Psychiatric Association recommends the use of antipsychotic medications for the treatment of patients with schizophrenia. Atypical antipsychotics (AAPs) have largely replaced traditional agents as first-line therapy in the treatment of schizophrenia. Despite the availability of effective treatments for schizophrenia,12 many patients with mental health issues continue to have poor treatment outcomes. Suboptimal response to treatment in clinical practice may be due to several factors, alone or in combination, including poor adherence, under-dosing, and limited medication effectiveness despite adherence.11,13 Medication nonadherence is one of the most important underlying causes of suboptimal clinical outcomes.14 Medication nonadherence is associated with an increased risk of relapse, rehospitalization, self-harm, and increased inpatient costs.15 A large US administrative claims database study of patients who initiated treatment for schizophrenia between January 1, 2006 to September 30, 2009 showed that early nonadherence results in more all-cause and schizophrenia-related hospitalizations with a greater length of stay and cost of care.16 Therefore, there is a need to improve evaluation and medication adherence for patients with serious mental health illnesses.
Strategies designed to monitor drug ingestion may improve patient adherence and disease-related outcomes.11,13 A digital medicine system (DMS) is an approach to monitoring and enhancing medication adherence. A DMS comprises evidence-based therapeutic interventions that simplify clinical care and patient self-management, resulting in improved clinical and economic outcomes.17 Aripiprazole tablets with sensor (AS; Abilify MyCite®, Otsuka America Pharmaceutical, Inc., Princeton, New Jersey, USA), a DMS, is a prescription antipsychotic medication tablet embedded with an ingestible event-marker sensor, a wearable sensor patch, and a smartphone app to monitor tablet ingestion.18 A phase 3b multicenter, open-label, mirror-image, trial in adults with schizophrenia treated prospectively for 6 months with AS (MONARCH trial; ClinicalTrials.gov identifier: NCT03892889).19 Participants were recruited via site databases of patients with schizophrenia. Eligible patients had ≥1 inpatient psychiatric hospitalization in the preceding 48 months and had been prescribed AAPs, including aripiprazole, for ≥6 months. Eligible participants were taking aripiprazole, had a history of tolerating oral aripiprazole, or, for those with unknown aripiprazole tolerance, were progressively cross-titrated to aripiprazole over ≤45 days. The study evaluated the impact of AS on inpatient psychiatric hospitalizations over 3 and 6 months compared to retrospective study of oral AAPs.19 AS significantly improved patients’ medication adherence, reduced symptom severity, and reduced the rate of inpatient psychiatric hospitalizations from baseline compared to oral AAPs.19
Although there is evidence of a favorable clinical profile of AS, data on the economic benefit of using AS for the treatment of patients with schizophrenia is limited. The objective of this study was to estimate the cost-effectiveness of AS versus oral AAPs in schizophrenia from the US payer and societal perspectives over 12 months. It is anticipated that the higher cost of AS may be offset by the cost-savings from improved outcomes and quality of life for the patients compared to those on oral AAPs.
Methods
Model Population
The MONARCH trial was used as the primary source to simulate the population in the model.19 The trial comprised 277 participants (intent-to-treat [ITT] population) with a mean age of 44.2 years, 34.3% females, and a Positive and Negative Syndrome Scale (PANSS) score between 60 to 90. Patients included in the trial had ≥1 inpatient psychiatric hospitalization in the preceding 48 months and had been prescribed oral AAPs.19 This analysis used the modified intent-to-treat (mITT) population (n = 113), defined as participants who completed 3 months of AS use or took ≥80% of their study medication during prospective months 1 to 3.19 The mITT population assesses the clinical efficacy of aripiprazole in patients with the integration of the AS system into the routine care plan and, therefore, provides a better estimation of the efficacy of AS on the patient population. Further, the reduction in psychiatric hospitalizations at 3 and 6 months relative to the retrospective period of AS in the ITT population was similar to that in the mITT population, albeit with a slightly lower reduction.19
Model Design
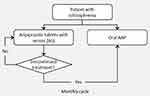
The model structure for one cycle is presented in Figure 1. An individual-level state transition was used to generate individual patient trajectories. The patient trajectories describe a patient’s clinical outcomes considering disease severity and intervention. Each patient’s history starts when they enter the model at cycle 1 with a defined PANSS score. Treatment decisions are made in 1-month cycles, for 1000 patients initiating either AS or oral AAPs. For each patient, the starting scores on the three PANSS subscales are derived by Monte-Carlo simulation using a multivariate gamma distribution, which is estimated using the shape and scale of the parameters from the MONARCH trial.19 The time series correlation was adjusted using the double exponential smoothing function (Holt linear method).20 The baseline adherence to AS was assumed to be equivalent to the historical adherence for patients on oral AAPs in the MONARCH mirror-image study.19 During each cycle, patients who discontinue AS switch to oral AAPs. In the base case analysis, patients who switch from AS to oral AAPs experienced the relevant clinical outcomes of the oral AAP group for that monthly cycle, based on the results from the MONARCH trial.19 The model estimates the costs and quality of life based on the patient profile at the end of each monthly cycle. The incremental cost-effectiveness ratio (ICER) of AS versus oral AAPs was evaluated using direct medical costs, indirect costs, and quality-adjusted life-years (QALYs) over 12 months from the US payer and societal perspectives. The model was developed in Microsoft® Excel 2019 (Redmond, Washington, US).
The effect of AS was modeled by shifts in the distribution means, reflecting improvements in the PANSS subscales and Clinical Global Impression – Severity Scale (CGI-S) scores.19 Each patient is given a score on the PANSS subscales—positive symptoms (7 to 49), negative symptoms (7 to 49) and general psychopathology (7 to 112), and CGI-S (1 to 7). The overall PANSS score was calculated as the sum of all three subscale scores:
These shifts were again generated using a multivariate gamma distribution as estimated by the sample means and covariance as observed in the MONARCH trial (using 3- and 6-month data). While the shifts in the means capture the improvements in the scores, the covariance matrix captures the positive correlation between the three subscales. Since this correlation is not perfect, patients may improve in one subscale but not necessarily on another subscale of PANSS.
Demographic and Clinical Inputs
Clinical data included baseline PANSS subscale scores, CGI-S scores, and adherence to AS or oral AAPs (Table 1). Baseline data for patients on AS was assumed to be identical to those on oral AAPs from the retrospective arm of the study. The medication ingestion with AS was based on the overall proportion of days covered (PDC) as a proxy for medication adherence21 in the ITT population (those who received ≥1 dose of study medication). For the retrospective phase, PDC was calculated from pharmacy records as the proportion of days a participant had medication available versus the number of days in a defined period.21 For participants who completed prospective months 1–6 using AS. PDC was determined from study drug dispensation when participants collected their medication from the study site. For participants who discontinued before 6 months, PDC during AS use was determined from study drug dispensation (ie, when participants collected their medication), whereas PDC after AS cessation was based on pharmacy records.19 In the subsequent monthly cycles, the PANSS scores were selected from the treatment distributions, considering the PANSS score of the prior cycle and the patient’s medication status.
 |
Table 1 PANSS Score at Baseline, 3 Months, and 6 Months of Treatment with AS |
Healthcare Resource Utilization and Costs
Healthcare resource utilization, direct costs, and indirect costs are presented in Table 2. Psychiatric hospitalization rate and length of stay were obtained from the MONARCH trial.19 Wholesale acquisition costs for AS and generic oral AAPs were obtained from IBM® Micromedex RED BOOK.22 Outpatient visits, laboratory testing, and emergency or crisis care visits were obtained from the published literature. The resource utilization was based on disease severity—mild-to-moderate (PANSS <58) and moderate-to-severe disease (PANSS ≥58).23 Costs associated with these utilizations were sourced from the literature.
 |
Table 2 Health Care Resource Utilization and Costs |
Indirect costs were included in the model for estimating the cost-effectiveness of AS from the societal perspective. Indirect resource utilization included absence from work, unemployment, informal care, and premature death. Indirect resource utilization was imputed by anchoring the costs to the PANSS score reported by Ignatova et al24 and generating utilization for the trial population. The reduction in indirect resource utilization after treatment was estimated using the average reduction of the indirect resource utilization before and after psychiatric hospitalization reported by Ignatova et al.24 Indirect cost estimates were calculated using $51,480 per capita income in the US.25
Unless otherwise specified, all costs are reported in 2021 United States Dollars (USD). Costs were inflated to 2021 USD using Consumer Price Index for medical care (June 2021).26
Health Utilities
EuroQol 5-Dimensions (EQ-5D) utilities were computed using risk equations based on patient and clinical characteristics, consistent with the published methodology.27
Base Case Analysis
In this analysis, the total costs and QALYs were calculated for AS and oral AAPs over 6 and 12 months based on the assumption that adherence to AS declines over 12 months. Adherence to AS was calculated using logarithmic distributions based on the 3- and 6-month data from the MONARCH trial.19 Patients who discontinued AS treatment were assumed to have switched to an oral AAP, and experienced the corresponding hospitalization rates and PANSS changes of the oral AAP group for that monthly cycle. From a payer perspective, total costs included direct medical costs (costs paid by third-party payers), and from a societal perspective, total costs comprised indirect costs (productivity loss, work-related training, and caregiving) in addition to direct medical costs. The primary outcome was ICER defined as the difference in costs divided by the difference in QALYs of AS and oral AAPs. Secondary outcomes included change in PANSS score, hospitalization, and CGI-S.
Sensitivity Analysis
Sensitivity analyses around the estimates in the base case analysis were conducted to account for uncertainty in the parameters sourced from literature and the MONARCH trial.19 Deterministic sensitivity analysis was conducted using percent variation in parameter estimates, comprising the length of psychiatric hospital stay, cost of emergency or crisis center, and emergency or crisis center use (Supplementary Table 1). Different estimates for variation were used to reflect the estimated variation in the inputs observed in the literature. In the case of comparative parameters (eg, psychiatric hospitalization with AS and oral AAPs), variation in only estimates related to AS was considered. For distributions, mean ± 0.7 times the standard deviation was considered for baseline, 3-month, and 6-month PANSS subscale scores, accounting for approximately 10% variation in the scores. Probabilistic sensitivity analysis was conducted using random draws from uncertainty distributions to determine the robustness of the results to variations in several parameters at once. The number of iterations was set to 1000. By bootstrapping the data from the prospective study using appropriate distributions, uncertainty margins surrounding the parameters were obtained.
Scenario Analysis
Adherence to medication affects a patient’s health outcomes. Given the unknown durability of the effect of AS after discontinuation, a scenario analysis was conducted in which the clinical benefit of treatment was independent of medication adherence. Total costs and utilities were computed at the end of each cycle as in the base case analysis.
Results
Base Case Analysis
AS resulted in an improvement in PANSS scores for patients on AS. With AS, the reduction in PANSS from baseline to 12 months was 8.8 points (baseline: 71.9, at 12 months: 63.1), translating to a 12.2% improvement in schizophrenia symptoms. In the same period, patients on AS also showed an improvement in CGI-S score showing a reduction of 0.57 points from baseline, translating to a 15.1% improvement. Further, AS resulted in a 28.2% reduction in psychiatric hospitalizations over 12 months.
Over 12 months, AS had an incremental cost of $2168 and incremental cost savings of $22,343 from the payer and societal perspectives, respectively, with an incremental QALY gain of 0.0298 versus oral AAPs (Table 3). This resulted in an ICER of $72,752 per QALY compared to oral AAPs from the payer perspective. Over 6 months, the results were similar to those over 12 months; the use of AS incurred costs from the payer perspective but showed cost savings from the societal perspective compared to oral AAPs. A breakdown of costs is provided in Supplementary Table 2. At a willingness-to-pay (WTP) of $100,000 per QALY, the net monetary benefit (NMB) over 12 months was $25,323 from the payer perspective.
 |
Table 3 Cost-Effectiveness of AS versus Generic Oral AAPs Estimated Using PANSS Scores and Treatment Status (Base Case) |
Sensitivity Analyses
The findings from the deterministic and probabilistic sensitivity analysis results were consistent with those of the base case analysis. Length of psychiatric hospital stay, cost of emergency/crisis care, and baseline medicate adherence rate were the factors most influenced by variation in the parameter estimates (Figure 2). The PSA randomly sampled parameters from within chosen distributions over 1000 iterations. The findings from the PSA were consistent with the base case analysis; AS is a cost-effective treatment strategy compared to the generic oral AAPs over 12 months (incremental costs: $1797; incremental QALYs: 0.0438) (Figure 3). The cost-effectiveness acceptability curve showed that AS is cost-effective in 92.5% of the iterations at a WTP threshold of $100,000/QALY from the payer perspective.
Scenario Analysis
In the scenario analysis, the treatment effect of AS at 6 months in the MONARCH trial was assumed to be durable through 12 months irrespective of discontinuation. In this analysis, AS resulted in a 37.7% reduction in hospitalization over 12 months (Table 4). From the payer perspective, the use of AS was a dominant strategy that resulted in an incremental cost savings of $2092 and an incremental QALY gain of 0.0606. From the societal perspective, AS resulted in incremental cost savings of $23,264 and an incremental QALY gain of 0.0606, resulting in an ICER of $74,072/QALY over 12 months.
 |
Table 4 Cost-Effectiveness of AS versus Generic Oral AAPs Using Durability of Treatment Effect Assumption (Scenario Analysis) |
Discussion
Schizophrenia is a chronic psychiatric disorder and presents a substantial clinical, financial, and humanistic burden in the US. Control of symptoms and prevention of relapses is crucial to reducing health care resource utilization, medical costs, and indirect costs. Despite treatments with antipsychotics, suboptimal response to treatment in clinical practice may be due to several factors, including poor medication adherence. Strategies designed to track drug ingestion may improve patient adherence and disease-related outcomes. Decision-making for serious mental illnesses should consider both clinical, economic, and patient-related implications of treatment. The current analysis aimed to evaluate the potential health economic implications of AS for the treatment of schizophrenia. To the best of our knowledge, this is the first economic analysis to assess the cost-effectiveness of AS versus oral AAPs for the treatment of schizophrenia.
Schizophrenia is a highly heterogeneous disorder with various underlying causes that are not well understood.28 A microsimulation model was developed to accurately reflect individual clinical pathways, incorporate the impact of history on future events, and more easily capture the variation in patients’ characteristics at baseline.29,30 Microsimulation models are different from traditional cohort models because they simulate the impact of an intervention or policy on individual patient trajectories instead of the average trajectory of a population.31–33 In a microsimulation model, outcomes are generated for each individual and are used to estimate the distribution of an outcome for a sample of potentially heterogeneous individuals. These models allow the inclusion of stochastic variation in disease progression as well as variation due to individual characteristics. Further, microsimulation models retain information about the transition states and account for disease severity, costs, and health outcomes unlike cohort models.34 Studies assessing the economic impact of therapies for patients with schizophrenia generally have used the microsimulation approach.35–37
Cost-effectiveness analyses offer an objective assessment of the value of treatments using the incremental clinical benefit approach. Cost-effectiveness models enable payers, physicians, and patients to make informed healthcare decisions. Evidence suggests that improvement in medication adherence to antipsychotics may reduce hospitalization rates and healthcare resource utilization, resulting in a reduction in direct healthcare costs.38 Patients who are adherent to antipsychotics have fewer relapses and require less psychiatric inpatient care compared to those who are non-adherent to medication. Further, longer psychiatric inpatient admissions have been observed in patients who discontinue treatment compared to those who continue treatment.38 A recent study evaluating the cost-effectiveness of improving patient adherence using a discrete event simulation approach noted that adherence to guideline recommendations reduces healthcare costs and improves quality-of-life for patients with schizophrenia.39
The MONARCH trial showed that AS improves medication adherence, which results in improved clinical outcomes and reduced psychiatric hospitalization.19 The improved outcomes with AS may potentially alleviate the economic burden associated with schizophrenia. However, due to the high acquisition cost of AS, the use of AS may be limited. Our analyses suggest that AS may be a cost-effective strategy compared to oral AAPs over 12 months from both payer and societal perspectives using the mITT population from the MONARCH trial. Our analysis showed a gain in QALYs with AS compared to oral AAPs over 6 and 12 months. The initial cost of AS is offset by the reduced cost of psychiatric hospitalizations. Reduction in psychiatric hospitalization costs is a key driver of overall cost savings among patients with schizophrenia.10 Our analysis showed a considerable reduction in psychiatric hospitalization, consistent with the findings of the clinical trial. Improvement in schizophrenia symptom severity and reduction in costs may be attributable to increased medication adherence with AS and improved quality of life of patients with schizophrenia. Analysis conducted in the ITT population showed similar results (data not shown); patients on AS had lower outcomes and lower costs due to limited use of AS. Findings from the sensitivity analysis corroborated the results of the base case analysis, suggesting the robustness of the analysis.
The prognostic balance in a randomized study is preserved in the analysis of the ITT population and mitigates the risk of potential biases during statistical comparisons.40 In the MONARCH trial, the ITT population included patients who were engaged with the system for <3 months. In a scenario analysis using the ITT population, the analysis predicted results similar to those in the mITT population; AS is a cost-effective treatment strategy compared to oral AAPs from the payer and societal perspectives at 12 months. These results are anticipated since the increase in total health care utilization and costs were offset by the reduction in hospitalization and improvement in the quality-of-life.
A scenario analysis was conducted to evaluate the economic and quality of life impact under the assumption of more robust durability of the treatment effect of AS. This increased durability resulted in a greater reduction in hospitalization rate compared to the base case analysis (37.7% versus 28.2%). In this scenario analysis, AS is anticipated to be a dominant strategy with 12-month cost savings of $2092 from a payer perspective, compared to the base case, which was cost-effective at a WTP threshold of $100,000/QALY (ICER: $72,752/QALY) from the payer perspective. This finding suggests that adherence is crucial for improving clinical outcomes and reducing the economic burden of schizophrenia on patients.
To the best of our knowledge, this is the first economic analysis to assess the cost-effectiveness of AS compared with oral AAPs at a WTP of $100,000 per QALY. Our study adds to the nascent literature on the value of AS in improving patient outcomes. Further, this analysis considered the societal perspective including costs accrued due to increased disability, caregiver costs, and costs due to lost productivity of patients in addition to direct medical costs. Patients with schizophrenia experience functional disability and require ongoing care and support that adds to the overall economic and patient burden. In a comprehensive cost-effectiveness analysis, costs should include healthcare costs downstream of the intervention and indirect costs due to productivity loss and caregiver burden, in addition to the direct costs of the intervention.
AS is an effective treatment strategy that may improve medication adherence in patients with schizophrenia. Clinical and economic evidence demonstrates the health economic value of AS over oral AAPs. Treatment costs are central to concerns of access and affordability; however, it the important to consider the clinical, economic, and humanistic value of treatments.
Limitations
PANSS score, CGI-S, and rate of psychiatric hospitalizations are derived from the MONARCH trial, which may not reflect real-world outcomes. Further, the real-world durability of the effect of AS is uncertain and may influence the results. The base case and scenario analyses were designed to explore the consequence of variability in this durability of effect. The results were derived from a population with a mean baseline PANSS score between 60 and 90, and thus, patients with more severe schizophrenia are not included. The model assumes some heterogeneity in the schizophrenia population; however, the presence of other comorbid mental health conditions may further exacerbate schizophrenia symptoms, with an uncertain effect on the value of AS. A simplified care paradigm was implemented for the model, ie the patients who discontinue AS switch to an oral AAP. Conversely, patients discontinuing oral AAPs switch to another oral AAP. The base model assumes that patients experience the benefits of AS for the duration of the model cycle even after discontinuation. Finally, the data on healthcare utilization and costs were obtained from the published literature and may result in under or over-estimation. A probabilistic sensitivity analysis was conducted to account for uncertainty in the parameters. The findings were consistent with base case analyses.
Conclusions
AS may be a cost-effective strategy with lower costs and improved quality of life among adult patients with schizophrenia over 12 months from the payer and societal perspectives. Reduction in psychiatric hospitalization is the key driver of overall cost savings with AS. This study suggests that the use of AS for patients with schizophrenia and low medication adherence to antipsychotics may considerably improve a patient’s clinical and health outcomes and provide economic benefits.
Data Sharing Statement
To submit inquiries related to Otsuka clinical research, or to request access to individual participant data (IPD) associated with any Otsuka clinical trial, please visit https://clinical-trials.otsuka.com/. For all approved IPD access requests, Otsuka will share anonymized IPD on a remotely accessible data-sharing platform.
Compliance with Ethics Guidelines
This study does not involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors. No ethics committee or institutional review board approval was required for this study.
Acknowledgments
Ishveen Chopra of Manticore Consultancy provided medical writing support in the development of this manuscript and review of the materials and inputs from the authors.
Disclosure
AC is an employee of Manticore Consultancy which provided paid consulting services to Otsuka America Pharmaceutical, Inc. DHB, AK, and JC are employees at Otsuka Pharmaceuticals Development and Commercialization. This study was sponsored by Otsuka America Pharmaceutical, Inc. The authors report no other conflicts of interest in this work.
References
1. Marder SR, Cannon TD, Ropper AH. Schizophrenia. N Engl J Med. 2019;381(18):1753–1761. doi:10.1056/NEJMra1808803
2. Kessler RC, Birnbaum H, Demler O, et al. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R). Biol Psychiatry. 2005;58(8):668–676. doi:10.1016/j.biopsych.2005.04.034
3. Wu EQ, Shi L, Birnbaum H, et al. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med. 2006;36(11):1535–1540. doi:10.1017/S0033291706008191
4. National Institute of Mental Health. Schizophrenia; 2020. Available from: https://www.nimh.nih.gov/health/statistics/schizophrenia.
5. Tsai J, Rosenheck RA. Psychiatric comorbidity among adults with schizophrenia: a latent class analysis. Psychiatry Res. 2013;210(1):16–20. doi:10.1016/j.psychres.2013.05.013
6. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. doi:10.1001/jamapsychiatry.2015.1737
7. Palmer BA, Pankratz VS, Bostwick JM. The lifetime risk of suicide in schizophrenia: a reexamination. Arch Gen Psychiatry. 2005;62(3):247–253. doi:10.1001/archpsyc.62.3.247
8. Schoenbaum M, Sutherland JM, Chappel A, et al. Twelve-month health care use and mortality in commercially insured young people with incident psychosis in the United States. Schizophr Bull. 2017;43(6):1262–1272. doi:10.1093/schbul/sbx009
9. Simon GE, Stewart C, Yarborough BJ, et al. Mortality rates after the first diagnosis of psychotic disorder in adolescents and young adults. JAMA Psychiatry. 2018;75(3):254–260. doi:10.1001/jamapsychiatry.2017.4437
10. Cloutier M, Sanon Aigbogun M, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. doi:10.4088/JCP.15m10278
11. Keepers GA, Fochtmann LJ, Anzia JM, et al. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 2020;177(9):868–872. doi:10.1176/appi.ajp.2020.177901
12. Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia-a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1–3):83–92. doi:10.1016/j.schres.2010.11.020
13. Au-Yeung KY, Moon GD, Robertson TL, et al. Early clinical experience with networked system for promoting patient self-management. Am J Manag Care. 2011;17(7):e277–e287.
14. Hatch A, Docherty JP, Carpenter D, et al. Expert consensus survey on medication adherence in psychiatric patients and use of a digital medicine system. J Clin Psychiatry. 2017;78(7):e803–e812. doi:10.4088/JCP.16m11252
15. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. doi:10.2147/PROM.S42735
16. Offord S, Lin J, Mirski D, et al. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30(3):286–297. doi:10.1007/s12325-013-0016-5
17. Kaufman N. Digital therapeutics: leading the way to improved outcomes for people with diabetes. Diabetes Spectr. 2019;32(4):301–303. doi:10.2337/ds19-0012
18. Knights J, Heidary Z, Peters-Strickland T, et al. Evaluating digital medicine ingestion data from seriously mentally ill patients with a bayesian hybrid model. NPJ Digit Med. 2019;2(1):20. doi:10.1038/s41746-019-0095-z
19. Cohen EA, Skubiak T, Boskovic DH, et al. Phase 3b multicenter, prospective, open-label trial to evaluate the effects of a digital medicine system on inpatient psychiatric hospitalization rates for adults with schizophrenia. J Clin Psychiatry. 2022;83(3). doi:10.4088/JCP.21m14132
20. LaViola JJ. Double exponential smoothing: an alternative to Kalman filter-based predictive tracking.2003:199–206. doi:10.1145/769953.769976
21. Canfield SL, Zuckerman A, Anguiano RH, et al. Navigating the wild west of medication adherence reporting in specialty pharmacy. J Manag Care Spec Pharm. 2019;25(10):1073–1077. doi:10.18553/jmcp.2019.25.10.1073
22. International Business Machines. Micromedex® RED BOOK; 2020. Available from: https://www.micromedexsolutions.com/home/dispatch/ssl/true.
23. Kane JM, Sanchez R, Zhao J, et al. Hospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophrenia. J Med Econ. 2013;16(7):917–925. doi:10.3111/13696998.2013.804411
24. Ignatova D, Kamusheva M, Petrova G, et al. Costs and outcomes for individuals with psychosis prior to hospital admission and following discharge in Bulgaria. Soc Psychiatry Psychiatr Epidemiol. 2019;54(11):1353–1362. doi:10.1007/s00127-019-01700-2
25. United States Department of Labor. Usual weekly earnings of wage and salary workers second quarter 2021; 2021. Available from: https://www.bls.gov/news.release/pdf/wkyeng.pdf.
26. Federal Reserve Economic Data. Consumer price index for all urban consumers: medical care in U.S. city average; 2021. Available from: https://fred.stlouisfed.org/series/CPIMEDSL.
27. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192–213. doi:10.1093/schbul/sbs150
28. Liang SG, Greenwood TA. The impact of clinical heterogeneity in schizophrenia on genomic analyses. Schizophr Res. 2015;161(2–3):490–495. doi:10.1016/j.schres.2014.11.019
29. Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices—overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803. doi:10.1016/j.jval.2012.06.012
30. Statistics Canada. Microsimulation approaches; 2016. Available from: https://www.statcan.gc.ca/en/microsimulation/modgen/new/chap2/chap2.
31. Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–1310. doi:10.1002/hec.1148
32. Kreke JE, Schaefer AJ, Roberts MS. Simulation and critical care modeling. Curr Opin Crit Care. 2004;10(5):395–398. doi:10.1097/01.ccx.0000139361.30327.20
33. Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Med Decis Making. 2012;32(5):690–700. doi:10.1177/0272989X12455463
34. Davis S, et al. In NICE DSU Technical Support Document 15: Cost-Effectiveness Modelling Using Patient-Level Simulation. London: National Institute for Health and Care Excellence (NICE); 2014.
35. Ascher-Svanum H, Furiak NM, Lawson AH, et al. Cost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the United States. J Med Econ. 2012;15(3):531–547. doi:10.3111/13696998.2012.662923
36. Lin Z, Xuan J. Cost-effectiveness of aripiprazole orally disintegrating tablets in the treatment of schizophrenia in China. Expert Rev Pharmacoecon Outcomes Res. 2020;20(5):549–557. doi:10.1080/14737167.2020.1807331
37. Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness model comparing olanzapine and other oral atypical antipsychotics in the treatment of schizophrenia in the United States. Cost Eff Resour Alloc. 2009;7(1):4. doi:10.1186/1478-7547-7-4
38. Dilla T, Ciudad A, Alvarez Á. Systematic review of the economic aspects of nonadherence to antipsychotic medication in patients with schizophrenia. Patient Prefer Adherence. 2013;78:e803–e812. doi:10.2147/PPA.S41609
39. Jin H, Tappenden P, MacCabe JH, et al. Cost and health impacts of adherence to the National Institute for Health and Care Excellence schizophrenia guideline recommendations. Br J Psychiatry. 2020;1–6 . doi:10.1192/bjp.2020.241
40. McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med. 2017;18(6):1075–1078. doi:10.5811/westjem.2017.8.35985
41. Citrome L, Kamat SA, Sapin C, et al. Cost-effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the United States. J Med Econ. 2014;17(8):567–576. doi:10.3111/13696998.2014.917089
42. Sicras-Mainar A, Maurino J, Ruiz-Beato E, et al. Impact of negative symptoms on healthcare resource utilization and associated costs in adult outpatients with schizophrenia: a population-based study. BMC Psychiatry. 2014;14(1):225. doi:10.1186/s12888-014-0225-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.