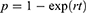Back to Journals » ClinicoEconomics and Outcomes Research » Volume 13
Cost-Effectiveness Analysis of Sacubitril/Valsartan Compared to Enalapril for Heart Failure Patients in Indonesia
Authors Zakiyah N , Sinuraya RK , Kusuma ASW, Suwantika AA , Lestari K
Received 3 June 2021
Accepted for publication 15 September 2021
Published 5 October 2021 Volume 2021:13 Pages 863—872
DOI https://doi.org/10.2147/CEOR.S322740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Xing Lin Feng
Neily Zakiyah,1,2 Rano K Sinuraya,1– 3 Arif SW Kusuma,2,4 Auliya A Suwantika,1,2,5 Keri Lestari1,2
1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 2Center of Excellence in Higher Education for Pharmaceutical Care Innovation, Universitas Padjadjaran, Bandung, Indonesia; 3Unit of Global Health, Department of Health Sciences, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; 4Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Bandung, Indonesia; 5Center for Health Technology Assessment, Universitas Padjadjaran, Bandung, 40132, Indonesia
Correspondence: Neily Zakiyah Tel/Fax +62 22-7796200
Email [email protected]
Objective: Sacubitril/valsartan is a relatively new medication that is more effective than the usual enalapril for heart failure patients with reduced ejection fraction. Therefore, this study aims to determine the cost-effectiveness of sacubitril/valsartan compared to enalapril in Indonesia’s healthcare system.
Methods: In this study, a Markov decision-analytic model was developed to estimate the total cost, health outcomes, and cost-effectiveness of sacubitril/valsartan compared to enalapril from Indonesia’s healthcare perspective. The input parameters for the cost-effectiveness were predominantly from the PARADIGM-HF trial. Subsequently, the country-specific data were synthesized for medication and hospitalization costs, cardiovascular and non-cardiovascular death, as well as re-hospitalization rate. The incremental cost-effectiveness ratio (ICER) per quality-adjusted life years (QALYs) gained was estimated to determine the cost-effectiveness. Deterministic and probabilistic sensitivity analyses were conducted to assess the impact of parameter uncertainty.
Results and Discussion: In the base case, sacubitril/valsartan was more costly and effective than enalapril with a total cost of IDR 91,783,325,865 (USD 6,487,522) vs IDR 68,101,971,241 (USD 4,813,653) and a total QALYs of 19,680 vs 18,795, resulting in an ICER of IDR 26,742,098 (USD 1890). Based on the willingness to pay threshold GDP per capita in Indonesia, it can be considered cost-effective. The most influential drivers of cost-effectiveness in deterministic sensitivity analysis were risk of mortality outside hospitalization, hospital admission rate, and cost of sacubitril/valsartan. The vast majority of simulation results from probabilistic analysis suggested that sacubitril/valsartan was likely resulted in higher cost and improved QALYs compared with enalapril, indicating the robustness of the model.
Conclusion: Based on the current price in Indonesia, sacubitril/valsartan can be considered a cost-effective option, although this depends heavily on the willingness to pay threshold. Further studies that incorporate real-world evidence with sacubitril/valsartan are needed to inform the decision-making process.
Keywords: cost-effectiveness, heart failure, Markov model, sacubitril/valsartan
Introduction
Heart failure (HF) is a global public health and economic problems associated with significant morbidity and mortality.1,2 In Indonesia, cardiovascular disease such as HF, is a leading cause of hospitalization and mortality in adults, which is responsible for the third major cause of deaths in Indonesia.3,4 Previous studies have shown that there is an increase in the prevalence of HF in younger patients, especially with a poor ejection fraction (HFrEF) and a high rate of comorbidities.3,5,6
Previous international guidelines recommended angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), beta-blockers, and aldosterone receptor antagonists for patients with HF.7,8 The use of these treatments for decades appeared to decrease hospitalization, death risk, and improve the quality of life.9 Recently, a novel oral pharmacotherapy, namely dual-acting angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan has been introduced to treat HF patients with reduced left ventricular ejection fraction (LVEF), based on the PARADIGM-HF trial results (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure).10 The trial compared morbidity and mortality in terms of hospitalization and a composite of death due to cardiovascular causes between sacubitril/valsartan and one of ACEIs, enalapril, in HFrEF patients.10 The result showed that sacubitril/valsartan was associated with a reduced risk of composite cardiovascular death as a primary outcome and HF hospitalization by approximately 20% compared to enalapril.10 Therefore, this treatment is recommended by several guidelines as an alternative for HFrEF patients that tolerate enalapril,11,12 including in Indonesia.13 In 2020, the Indonesian Cardiac Association started including sacubitril/valsartan for the treatment of heart failure in their recommendation. However, due to constraints in budget resources, the use of this drug is still limited. Currently, the universal healthcare system in Indonesian managed by Indonesia’s National Healthcare Security Agency, namely Badan Penyelenggara Jaminan Sosial Kesehatan (BPJS Kesehatan), is being implemented gradually.14 Given the limited healthcare budgets, health economic evaluation of relatively new drugs or medical interventions is needed to allow informed decisions by stakeholders and policymakers.
The uptake and cost-effectiveness analysis result of this medication varied among countries due to variations in drug cost, hospitalization rate, healthcare system, and the willingness to pay thresholds. Several cost-effectiveness analyses conducted in high-income countries showed that sacubitril/valsartan seems to be a cost-effective option compared to the current standard of care.15–18 However, conflicting results that were also observed in several countries showed that sacubitril/valsartan is likely not to be cost-effective.19–21 Therefore, this study aims to assess the cost-effectiveness of sacubitril/valsartan compared to enalapril from the perspective of Indonesia’s healthcare system.
Methods
Model Overview
The reporting standard for the evaluations was based on the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.22 As depicted in Figure 1, a Markov model was developed to simulate a hypothetical cohort of 10,000 patients suffering from HF, using Markov cycles of 3 months. The cohort characteristics were based on the PARADIGM-HF trial, which includes patients categorized as having New York Heart Association (NYHA) functional class II–IV, with left ventricular ejection fraction (LVEF) of 40% or less.10
 |
Figure 1 A schematic representation of the Markov model. The model consists of three health states, and the arrows indicate transition probabilities between the health states per three-month cycle. |
The starting age of the cohort was 60 years old, which corresponds to the mean age of patients suffering from HF in several Indonesian hospitals.3 The cohort was followed over the time horizon of ten years, which is equivalent to recent data on life expectancy in Indonesia.23 This model analyzed the total cost, health outcomes in terms of quality-adjusted life-years (QALYs), and cost-effectiveness of sacubitril/valsartan compared to one of the usual ACEIs drug, namely enalapril.13,24 The primary outcome for the cost-effectiveness analysis was expressed as the incremental cost-effectiveness ratios (ICERs) per QALYs gained. The cost-effectiveness model was built in Microsoft Excel. Validation of the model was conducted using extreme value testing on transition probabilities. The detail of CHEERS checklist is provided in Supplementary Material.
Input Parameters
In the schematic representation of the model (Figure 1), oval shapes represented the health states, and the arrows indicated the transition probabilities between the health states during the 3-month cycle. There were three health states in the model, ie:
- HF state represented outpatients/ambulatory care
- Hospitalization state comprised patients receiving treatment in the hospital, and
- Death due to natural cause or due to HF complications.
All patients in the cohort started in the HF state with a probability of being hospitalized or remained in the HF state. Moreover, patients can be re-hospitalized, returned to HF state (outpatients), or transit to the death state. The following formula (1) was used to transform the incidence rate of input parameters to 3-month transition probability:25,26
And rate to probability, formula (2):
where r denoted the rate, p denoted the probability, and t was the time period of interest.25,26
The mortality risks were differentiated into death due to HF complications and death due to other causes.3,27 The age-specific mortality rates from the World Health Organization (WHO) life tables were used to estimate the mean mortality rate due to other causes apart from HF complications.27 Data on both hospitalization and non-hospitalization mortality rates of HF were derived from a prospective cohort study assessing predictors of mortality and HF-related re-hospitalization in several hospitals in Indonesia.3 The 3-month HF hospitalization and non-hospitalization mortality risks were 2.3% and 5.1%, respectively, while the mortality risk due to natural cause based on cohort age in the model was 0.76%.3,27 To derive HF mortality rates for both enalapril and sacubitril/valsartan, the country-specific mortality rates were adjusted for both hospitalization and non-hospitalization with cardiovascular mortality risk described in PARADIGM-HF. The result showed a 16.45% cardiovascular death in patients in the enalapril group, and 13.33% in the sacubitril/valsartan group (hazard ratio 0.80; 95% CI 0.71–0.89).10 The adjusted values are presented in Table 1.
 |
Table 1 Input Parameters |
The mean incidence rate ratio of HF hospitalization in patients aged 50–69 years old, in correspondence to the age cohort, was used to estimate the probabilities of hospitalization in the model.28 In the absence of country-specific data on the incidence rate of hospitalization in these patients, estimation from the best available data that represented the probabilities was used. Furthermore, the estimation from previous cost-effectiveness study was used,15 which obtained the risk of HF hospitalization from the total number of hospitalizations for HF reported in PARADIGM-HF29 assessing sacubitril/valsartan group compared with enalapril (rate ratio 0.77; 95% CI 0.67–0.89) to adjust the risk of HF hospitalization. The reported 3-month probabilities were 2.62% and 3.44% for sacubitril-valsartan and enalapril, respectively. The estimations on the risk of HF hospitalization for both interventions are shown in Table 1. The 3-month re-hospitalization rate was obtained from country-specific data and estimated to be 17.12%.3 This rate was adjusted with the converted relative risk of hospital re-admission for sacubitril/valsartan compared to enalapril, as reported in the PARADIGM-HF trial,30 which was previously used in previous cost-effectiveness analysis21 (Table 1).
The healthcare payer perspective which considered only direct medical costs was used for analysis. Subsequently, the estimated average cost of HF hospitalization covered by the Indonesian National Healthcare Insurance (BPJS Kesehatan) was from the official tariffs from the public access government database,31 considering the cost incurred by private and government hospitals in Indonesia. Since BPJS Kesehatan did not cover both sacubitril/valsartan and enalapril, the cost for both medications using the official highest retail price was considered.32 The cost per cycle was estimated by calculating the average cost of medications in each group and its usage percentage in 3 months. Meanwhile, other direct costs such as outpatient visits and laboratory assessments were assumed to be similar in both groups and were not included in the analysis. In addition, all cost estimations in the input parameters were in Indonesian rupiah (IDR) 2020, which is shown in Table 1. The results were also presented in US dollars (USD) 2019 and were converted using official exchange rates from The World Bank’s annual consumer index.
Due to incomplete data on preference-based health outcomes in Indonesian HF patients, the average value of utility parameters was from published data that measured the impact of chronic HF on health-related quality of life (HRQoL).33 Similarly, utility values from a cost-effectiveness study that assessed the economic evaluation of different treatments for HF patients were also used.34 These data were differentiated for both hospitalized and non-hospitalized patients and the estimated utility value for both patients were 0.70 and 0.65, respectively (Table 1). Attributable to a long-time horizon in the analysis, all costs and health outcomes were discounted at 3%, a rate commonly used in many countries,35 in the absence of the specific guideline regarding discounting in Indonesia.
Analyses
Base-Case
The base-case analysis was performed using the deterministic value to estimate the ICER in IDR per QALYs gained, which was calculated as the difference in total costs divided by the difference in health outcomes measure of sacubitril/valsartan and enalapril. Due to paucity in the cost-effectiveness thresholds in Indonesia, a treatment choice was considered to be cost-effective when the estimated ICER was not greater than willingness-to-pay (WTP) thresholds of one to three times gross domestic product (GDP) per capita (GDP per capita Indonesia) (USD 4135 or IDR 58,000,000).36
Sensitivity Analysis
Deterministic and probabilistic sensitivity analyses were carried out to determine the effects of uncertainty in the model. Subsequently, a one-way sensitivity analysis was conducted in the deterministic analysis by varying all transition probabilities, costs, and utilities in ranges of plausible values. A 95% confidence interval (CI) was used to vary the parameters whenever possible. When the data on 95% CI were unavailable, the values were ranged from 75% to 125% interval of their primary values and the individual effect on the ICER was displayed as a Tornado diagram.
In addition to the deterministic analysis, a probabilistic sensitivity analysis was performed. All parameters were varied simultaneously using their respective distributions to determine the uncertainty in the model. Furthermore, the expected outcomes in terms of costs and QALYs were estimated using Monte Carlo simulations with 10,000 iterations. Dirichlet distributions were used for transition probabilities since the data were multinomial, while beta distributions were used for utility variables, and gamma distributions for costs, using predefined standard errors when the data on 95% CI were not available.
Results
Base-Case Analysis
The base-case analysis results showed that the total costs of sacubitril/valsartan were higher than enalapril. The total cost of sacubitril/valsartan was IDR 91,783,325,865 (USD 6,487,522) compared to a total cost of enalapril, which was IDR 68,101,971,241 (USD 4,813,653). In terms of effectiveness, total QALYs of sacubitril/valsartan were also higher than total QALYs of enalapril (19,680 vs 18,795), which showed that sacubitril/valsartan was more effective than enalapril. Compared to enalapril, sacubitril/valsartan resulted in 11% and 2% lower number of hospitalization and cardiovascular death, respectively. Considering both total costs and QALYs, the ICER was estimated to be IDR 26,742,098 or USD 1890. Considering the threshold of GDP per capita of Indonesia, sacubitril/valsartan can be considered cost-effective. The summary of total cost and effectiveness results is provided in Table 2.
 |
Table 2 Base-Case Results for Cohort of 10,000 HF Patients |
Sensitivity Analysis
The tornado diagram in Figure 2 shows the deterministic sensitivity analysis results. Input parameters considered in the model were ordered from top to bottom, from the most influential driver for the ICERs to the least. Risk of mortality outside hospitalization for sacubitril/valsartan and enalapril were the most influential driver on the cost-effectiveness, followed by hospital admissions and cost of sacubitril/valsartan. Other input parameters seemed to result in fewer changes on ICER, including the cost of hospitalizations, cost of enalapril, and utility value of hospitalizations.
The probabilistic sensitivity analysis result was presented in a form of a cost-effectiveness plane in Figure 3. The results of 10,000 iterations from the simulation showed that all ICERs were scattered in all quadrants, dominantly in the northeast, which indicated that sacubitril/valsartan was likely associated with higher cost and improved QALYs compared to enalapril as shown in 77% of the simulations. Moreover, approximately 19% of the simulations showed that sacubitril/valsartan can be a dominant option compared to enalapril, with a lower total cost and more QALYs. The result from Monte Carlo simulations also showed that there were chances for sacubitril/valsartan to be more costly but less effective (1% of the simulations) and less cost and less effective (3% of simulations) compared to enalapril. Figure 4 shows the cost-effectiveness acceptability curve of sacubitril/valsartan in the range of willingness to pay (WTP) of GDP per capita. The result showed that at the current price, the probability of sacubitril/valsartan to be cost-effective was approximately more than 80% at a WTP threshold of IDR 58,000,000 per QALYs gained (equal to Indonesia’s GDP per capita), and more than 98% at a WTP threshold of IDR 100,000,000.
 |
Figure 3 Scatter plots of 10,000 iterations of incremental costs and effects for sacubitril/valsartan compared with enalapril in a hypothetical cohort of 10,000 patient. |
 |
Figure 4 Cost-effectiveness acceptability curve. |
Discussion
The base-case result showed that HF therapy with sacubitril/valsartan had an ICER of IDR 26,742,098 or USD 1890 per QALY gained compared to enalapril, based on Indonesia’s health care system perspective. When willingness to pay thresholds of GDP per capita was used, this result was considered cost-effective. The model results were robust to variations in most assumptions and the uncertainty parameters when assessed in the sensitivity analysis. Moreover, reductions in mortality, hospitalization, and the cost of sacubitril/valsartan also appeared to have the most influential impact on the ICER. When several assumptions on input parameters were varied in sequence, the results showed that the changes had a relatively moderate impact on the ICER. In the probabilistic sensitivity analysis, when all model input parameters were varied, the results were scattered between four quadrants of the cost-effectiveness plane. However, the majority of simulations showed that the ICER was still below Indonesia’s GDP per capita per QALYs gained.
It should be highlighted that the WTP threshold that was used to determine that the therapy was cost-effective was solely based on the recommendation from WHO,37 in the absence of a formal cost-effectiveness threshold in Indonesia. Given the considerable variation among countries in terms of the healthcare system, available resources, affordability, and cultural value perceived on health, country-specific cost-effectiveness thresholds were prominent to reflect each countries’ needs.38 However, there are very few countries that explicitly disclosed a WTP threshold, including Indonesia. The results also showed that using the choice of GDP per capita as a WTP threshold was favorable for sacubitril/valsartan, as shown in the base case. Subsequently, probabilistic sensitivity analysis results showed that sacubitril/valsartan was likely to be cost-effective below the threshold in 80% of the simulations. Although general WTP was used for analysis, the results provided a valuable quantitative assessment of this relatively new therapy for HF to assist the decision-making process.
The results of previous cost-effectiveness study of sacubitril/valsartan were varied among different countries. While most analyses conducted in high-income countries suggested that sacubitril/valsartan was considered cost-effective as seen from countries’ implemented cost-effectiveness threshold,15–17,39 some results indicated otherwise.20,21 Furthermore, two previous studies in Singapore and Thailand indicated that sacubitril/valsartan was not considered cost-effective at ICER per QALY gained of USD 55,490 in Singapore and USD 4857 in Thailand since the ICERs were above the country’s thresholds. Based on country classification by income, Indonesia is currently categorized as an upper-middle-income country,40 and Thailand was the only upper-middle-income country that assessed the cost-effectiveness of sacubitril/valsartan compared to enalapril. The difference in Thailand’s results compared to this study was mainly due to the decision to describe intervention as cost-effective. Thailand has set their country-specific cost-effectiveness thresholds, which is 160,000 THB/QALY gained or approximately USD 4789/QALY gained,21 that was lower than Thailand’s GDP per capita (USD 7806). Therefore, the result of USD 4857/QALY gained was slightly above the threshold, thus was considered as not cost-effective. Meanwhile, discrepancies were also observed in the model structure, and several input parameters, especially cost, that inevitably affected total costs and effects. For instance in Thailand, the cost for sacubitril/valsartan was approximately 225 times more expensive than enalapril (USD 452 vs USD 2), while in Indonesia, the price difference was not significantly high (USD 331 vs USD 47). This might be because generic enalapril was unavailable in Indonesia;41 therefore, the price was higher. In Indonesia, the first line of ACEIs covered by BPJS Kesehatan was captopril at approximately USD 9 for 3 months of usage, which was still more expensive than generic enalapril in Thailand. We tried to incorporate this in the deterministic sensitivity analysis, where we assumed the same effectiveness for enalapril and captopril and used the price of generic captopril. The result showed that the ICER was higher, with a value of 2345 per QALY gained but was still below the country’s GDP per capita, which showed that the price of enalapril had a relatively modest impact on the final results. According to the result from deterministic sensitivity analysis, the uncertainty in the risk of mortality in the sacubitril/valsartan group was accountable and had the highest impact on the ICER. When the upper risk of mortality was used, the ICER resulted in IDR 8432 per QALY gained, above the threshold of one GDP per capita. This estimation was because avoiding death due to HF was a significant parameter of medication effectiveness, which directly resulted in more QALYs than enalapril.
A previous study suggested that the immediate initiation of sacubitril/valsartan during HF hospitalization can be considered cost-effective and even cost-saving compared to the initial use of enalapril or switching to sacubitril/valsartan after hospitalization with enalapril, with reduced hospitalizations and increased QALYs. This result indicates that if the present estimation also considers the immediate initiation of sacubitril/valsartan during HF hospitalization, the results might be even more in favour of sacubitril/valsartan. However, this estimation needs to be confirmed in further research.42
To the best of our knowledge, this study was the first cost-effectiveness study of sacubitril/valsartan compared to enalapril based on input parameters and costs that were specific to Indonesia. The concept of universal national health insurance system is relatively new in Indonesia, as it was started only recently, in 2014.43 Several policies related to medicines were introduced by the Ministry of Health to support the new system, one of which was the compilation of a national formulary consisting of a list of medicines covered by BPJS Kesehatan.14 Due to the limited healthcare budgets, health economic evaluation of drugs or medical interventions can be as critical as effectiveness and safety. As for Indonesia, cardiovascular disease, including heart failure, posed a tremendous economic burden, with the rough estimation showed that management of cardiovascular disease accounted for almost half of total healthcare expenses.41 Therefore, comprehensive consideration of the long-term cost and effectiveness of the treatment is fundamental in heart failure therapy.
Inevitably, this study has some limitations. Although we used country-specific data for death rate (cardiovascular and non-cardiovascular), re-hospitalization rate, and costs, other input parameters, including treatment effect, especially on hospitalization and mortality rate, were derived from the PARADIGM-HF trial. Because the baseline and clinical characteristics of the patients may be different between countries, this could lead to different estimation on the actual effect of sacubitril/valsartan and also enalapril in Indonesia, even though subgroup analyses in PARADIGM-HF that was geographically diverse study, indicated that the treatment effect was considered similar.10 Therefore, further research needs to incorporate real-world data to better inform the decision-making process. Another potential limitation was that we did not consider adverse events (AEs) due to the use of both sacubitril/valsartan and enalapril in the model. Based on the trial, there are several AEs that were associated with both drugs. Elevated serum creatinine and potassium level, hypotension, and cough were the few AEs associated with sacubitril/valsartan. However, the reported AEs in the trial were considered mild, did not require special treatment, and did not lead to medication discontinuation. Therefore, the main analysis might not differ significantly from the present results if we also consider the AEs.10,44 Furthermore, due to limited data on HRQoL, the utility values were also from previous studies, while in reality, these estimations may differ between countries. These uncertainties were addressed by conducting both deterministic and probabilistic sensitivity analyses, where the results showed the impact of the variations of these parameters on the ICER.
Conclusion
In conclusion, based on the perspective of Indonesia’s healthcare system, the results from this analysis showed that sacubitril/valsartan was likely to be considered cost-effective compared to enalapril when a willingness to pay threshold of Indonesia GDP per capita was used. Furthermore, the ICER for sacubitril/valsartan was estimated to be IDR 26,742,098 (USD 1890) per QALY gained.
Abbreviations
HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ARNI, angiotensin receptor neprilysin inhibitor; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; LVEF, left ventricular ejection fraction; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years; IDR, Indonesian rupiah; USD, United States dollar; GDP, gross domestic product; CHEERS, Consolidated Health Economic Evaluation Reporting Standards; NYHA, New York Heart Association; HRQoL, health-related quality of life; BPJS Kesehatan, Indonesian National Healthcare Insurance; WTP, willingness-to-pay.
Data Sharing Statement
Data will be available upon reasonable request.
Funding
This study is supported by a grant from Universitas Padjadjaran (grant number: 1427/UN6.3.1/LT/2020).
Disclosure
The authors declared that they have no conflicts of interest that are relevant to this work or the content of this study.
References
1. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. J Am Med Assoc. 2003;289(2):194–202. doi:10.1001/jama.289.2.194
2. Ponikowski P, Voors AA, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18(8):891–975. doi:10.1002/ejhf.592
3. Siswanto BB, Radi B, Kalim H, et al. Heart failure in NCVC Jakarta and 5 hospitals in Indonesia. CVD Prev Control. 2010;5(1):35–38. doi:10.1016/j.cvdpc.2010.03.005
4. World Health Organization. WHO | noncommunicable diseases country profiles Indonesia. World Health Organization; 2018. Available from: http://www.who.int/nmh/publications/ncd-profiles-2018/en/.
5. MacDonald MR, Tay WT, Teng TK, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN‐HF registry. J Am Heart Assoc. 2020;9(1). doi:10.1161/JAHA.119.012199
6. Maharani A, Sujarwoto S, Praveen D, Oceandy D, Tampubolon G. Cardiovascular disease risk factor prevalence and estimated 10-year cardiovascular risk scores in Indonesia: the smarthealth extend study. PLoS One. 2019;14(4):e0215219. doi:10.1371/journal.pone.0215219.
7. Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi:10.1016/j.jacc.2013.05.019
8. McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi:10.1093/eurheartj/ehs104
9. Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10(1):325. doi:10.1161/CIRCHEARTFAILURE.116.003529
10. McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New Engl J Med. 2014;371(11):993–1004. doi:10.1056/NEJMoa1409077
11. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–e161. doi:10.1161/CIR.0000000000000509
12. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37(27):2129–2200m. doi:10.1093/eurheartj/ehw128
13. Perhimpunan Dokter Spesialis Kardiovaskular Indonesia (PERKI). Pedoman Tatalaksana Gagal Jantung Edisi Kedua [Guidelines for the Management of Heart Failure; Second Edition]; 2020. Indonesian.
14. Menteri Kesehatan Republik Indonesia. Peraturan Menteri Kesehatan Republik Indonesia Nomor 28 Tahun 2014 Tentang Pedoman Pelaksanaan Program Jaminan Kesehatan Nasional [Minister of Health of the Republic of Indonesia Regulation Number 28 of 2014 concerning Guidelines for the Implementation of the National Health Insurance Program]; 2014. Indonesian.
15. King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost-effectiveness of sacubitril-valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2016;4(5):392–402. doi:10.1016/j.jchf.2016.02.007
16. Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD, Heidenreich PA. Cost-effectiveness of sacubitril-valsartan in patients with heart failure with reduced ejection fraction. Ann Intern Med. 2016;165(10):681–689. doi:10.7326/M16-0057
17. McMurray JJV, Trueman D, Hancock E, et al. Cost-effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart. 2018;104(12):1006–1013. doi:10.1136/HEARTJNL-2016-310661
18. A Z, Am P, E H, et al. Cost-effectiveness of sacubitril/valsartan in chronic heart-failure patients with reduced ejection fraction. Swiss Med Wkly. 2017:147. doi:10.4414/SMW.2017.14533.
19. Perera K, Ademi Z, Liew D, Zomer E. Sacubitril-valsartan versus enalapril for acute decompensated heart failure: a cost-effectiveness analysis. Eur J Prev Cardiol. 2019. doi:10.1177/2047487319878953
20. Liang L, Bin-Chia Wu D, Aziz MIA, et al. Cost-effectiveness of sacubitril/valsartan versus enalapril in patients with heart failure and reduced ejection fraction. J Med Econ. 2018;21(2):174–181. doi:10.1080/13696998.2017.1387119
21. Krittayaphong R, Permsuwan U. Cost-effectiveness analysis of sacubitril-valsartan compared with enalapril in patients with heart failure with reduced ejection fraction in Thailand. Am J Cardiovasc Drugs. 2018;18(5):405–413. doi:10.1007/s40256-018-0288-x
22. Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–e5. doi:10.1016/j.jval.2013.02.010
23. The World Bank. Life expectancy at birth, total (years) - Indonesia | data; 2021. Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=ID.
24. Perhimpunan Dokter Spesialis Kardiovaskular Indonesia (PERKI). Panduan Praktik Klinis (PPK) dan Clinical Pathway (CP) Penyakit Jantung dan Pembuluh Darah [Clinical Practice Guide and Clinical Pathway for Cardiovascular Disease]; 2016. Available from: http://www.inaheart.org/upload/image/Buku_PPK_CP_05Apr16.pdf.
25. Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. OUP Oxford; 2006.
26. Gidwani R, Russell LB. Estimating transition probabilities from published evidence: a tutorial for decision modelers. PharmacoEconomics. 2020;38(11):1153–1164. doi:10.1007/s40273-020-00937-z
27. World Health Organization. GHO | by category | life tables by country - Indonesia. World Health Organization; 2020. Available from: https://apps.who.int/gho/data/view.main.60750?lang=en.
28. McAllister DA, Read SH, Kerssens J, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138(24):2774–2786. doi:10.1161/CIRCULATIONAHA.118.034986
29. Packer M, McMurray JJV, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61. doi:10.1161/CIRCULATIONAHA.114.013748
30. Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68(3):241–248. doi:10.1016/j.jacc.2016.04.047
31. Kementrian Kesehatan Republik Indonesia. Standar tarif pelayanan kesehatan dalam penyelenggaraan program jaminan kesehatan. Jakarta, Indonesia: Kementrian Kesehatan Republik Indonesia [Standard tariffs for health services in the implementation of health insurance programs]; 2014. Available from: https://www.google.com/search?q=Standar+Tarif+Pelayanan+Kesehatan+dalam+Penyelenggaraan+Program+Jaminan+Kesehatan&rlz=1C5CHFA_enID915ID915&oq=Standar+Tarif+Pelayanan+Kesehatan+dalam+Penyelenggaraan+Program+Jaminan+Kesehatan&aqs=chrome.69i57j0l2.313j0j9&s.
32. MIMS Indonesia. The monthly index of medical specialities Indonesia; 2021. Available from: https://www.mims.com/indonesia/.
33. Calvert MJ, Freemantle N, Cleland JGF. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7(2):243–251. doi:10.1016/j.ejheart.2005.01.012
34. van der Pol S, Degener F, Postma MJ, Vemer P. An economic evaluation of sacubitril/valsartan for heart failure patients in the Netherlands. Value Health. 2017;20(3):388–396. doi:10.1016/j.jval.2016.10.015
35. World Health Organization. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization; 2001.
36. The World Bank. GDP per capita (current US$) - Indonesia | data. The World Bank; 2021. Available from: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=ID.
37. Tan-Torres Edejer T, Baltussen TA R, Hutubessy R, Acharya A, Evans CJLM DB. Making choices in health: WHO guide to cost-effectiveness analysis. World Health Organization; 2003.
38. Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–935. doi:10.1016/J.JVAL.2016.02.017
39. Qi LX, Shan HL, Qing HJ, Juan XL, Xia C, Yan LH. Cost-effectiveness analyses of sacubitril-valsartan for heart failure. Heart Fail Rev. 2020. doi:10.1007/s10741-020-09956-6
40. The World Bank. World bank country and lending groups; 2021. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
41. Kesehatan BPJS. Badan Penyelenggara Jaminan Sosial Kesehatan [Health Social Security Agency]; 2020. Available from: https://www.bpjs-kesehatan.go.id/bpjs/.
42. Gaziano TA, Fonarow GC, Velazquez EJ, Morrow DA, Braunwald E, Solomon SD. Cost-effectiveness of sacubitril-valsartan in hospitalized patients who have heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(11):1236–1244. doi:10.1001/JAMACARDIO.2020.2822
43. Republik Indonesia. Undang-Undang Republik Indonesia Nomor 24 Tahun 2011 Tentang Badan Penyelenggara Jaminan Sosial [Law of the Republic of Indonesia Number 24 of 2011 concerning Health Social Security Agency]; 2011. Available from: https://djsn.go.id/storage/app/uploads/public/58c/24d/0e4/58c24d0e4ed39439756046.pdf.
44. Park S-K, Hong H, K H, Kim S, Lee E-K. Cost-utility analysis of sacubitril/valsartan use compared with standard care in chronic heart failure patients with reduced ejection fraction in South Korea. Clin Ther. 2019;41(6):1066–1079. doi:10.1016/J.CLINTHERA.2019.04.031
45. Yap J, Tay WT, Teng THK, et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J Am Heart Assoc. 2019;8(17):e013114. doi:10.1161/JAHA.119.013114
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.