Back to Journals » Journal of Pain Research » Volume 17
Cortical Sulcal Abnormalities Revealed by Sulcal Morphometry in Patients with Chronic and Episodic Migraine
Authors Liu S , Hou X , Shi M, Shen Y, Li Z, Hu Z, Yang D
Received 3 November 2023
Accepted for publication 21 January 2024
Published 1 February 2024 Volume 2024:17 Pages 477—488
DOI https://doi.org/10.2147/JPR.S447148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Alexandre F DaSilva
Shanyu Liu,1,2,* Xiaolin Hou,3,* Min Shi,1 Yuling Shen,1,2 Zhaoying Li,1,2 Zhenzhu Hu,1,2 Dongdong Yang1
1Department of Neurology, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 3Department of Neurosurgery, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongdong Yang, Department of Neurology, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, No. 39, Twelve Bridges Road, Chengdu, Sichuan Province, 610075, People’s Republic of China, Tel/Fax +86 028-87783481, Email [email protected]
Purpose: Previous studies have reported mixed results regarding the importance of cortical abnormalities in patients with migraines. However, cortical sulci, as a component of the cerebral cortex, have not been specifically investigated in migraine patients. Therefore, we aim to evaluate alterations in cortical sulcal morphology among patients with chronic migraine (CM), episodic migraine (EM), and healthy controls (HCs).
Patients and Methods: In this cross-sectional study, structural magnetic resonance images were acquired from 35 patients with CM, 35 with EM, and 35 HCs. Cortical sulci were identified and reconstructed using the BrainVisa 5.0.4 software. We focused on regions involved in pain processing in which abnormal cortical structure were identified in previous neuroimaging studies. Morphometric analysis was performed to calculate sulcal parameters including mean depth, cortical thickness, and opening width. Partial correlation analyses of clinical characteristics and sulcal parameters were performed for CM, EM and the combined migraine (CM + EM) groups.
Results: In comparison with HCs, both CM and EM groups showed increased opening width in bilateral insula. In comparison with HC and EM groups, CM patients showed increased cortical thickness in bilateral superior postcentral sulcus, bilateral median frontal sulcus and left superior parietal sulcus, as well as increased mean depth in the left anterior callosomarginal fissure and decreased mean depth in bilateral superior frontal sulcus and left median frontal sulcus. Migraine frequency and disease duration were both correlated with cortical thickness in bilateral superior postcentral sulcus.
Conclusion: Abnormal sulcal morphometry primarily affected areas associated with pain processing in patients with migraine, with CM exhibiting more extensive abnormalities in areas related to sensory and affective processing. These changes may contribute to understanding the pathology of EM and CM.
Keywords: sulcal morphometry, mean depth, cortical thickness, opening width, chronic migraine, episodic migraine
Introduction
Migraine is a neurological condition characterized by bilateral headaches, nausea, vomiting, photophobia, phonophobia, and sometimes comorbid psychiatric disorders.1 Chronic migraine (CM) and episodic migraine (EM) are migraine subtypes. Notably, CM patients exhibit a twofold higher likelihood of comorbid depression and anxiety compared to EM patients.2 Despite the burden and disability associated with migraine, our understanding of the pathophysiology underlying the condition is limited.
Migraine is commonly associated with the activation of the trigeminovascular system,3 a pathway that originates in trigeminal ganglion neurons, transmitting monosynaptic nociceptive signals to brainstem neulci, hypothalamus, and basal ganglia. These signals are then relayed by trigeminovascular thalamic neurons to various cortical regions such as somatosensory, motor, parietal, temporal and occipital areas. Neuroimaging studies in patients with migraine have identified specific changes in brain regions along this pathway, including the brainstem,4 hypothalamus,5 caudate,6 somatosensory7 and occipital areas.8 Concurrently with functional imaging studies, structural magnetic resonance imaging (sMRI) has revealed altered brain morphology, including gray matter volume and cortical thickness, in migraine patients. Voxel-based morphometry (VBM) has detected gray matter volume changes in various regions including the frontal, temporal, and occipital lobes,9 brainstem, cerebellum,10 and subcortical areas,11–13 while some study report no changes.12 Surface-based morphometry (SBM) has been employed to investigate cortical thickness, cortical folding, and cortical surface, with some studies reporting reduced cortical thickness in the insular cortex, precentral gyrus, and parietal lobe in patients with CM,14 while others revealed increased somatosensory cortex thickness15 or unchanged cortical thickness versus healthy controls (HCs).16,17 Thus, the results regarding cortical changes in migraine are inconsistent. Discrepancies in these results could stem from variations in the heterogeneous study populations, encompassing different migraine subtypes (CM and EM). Additionally, diverse morphometry analysis methods, such as VBM and SBM, along with variations in software, might contribute to inconsistent outcomes. Utilizing a more sensitive morphometry method could yield more stable conclusions.
A previous review that covered clinical, neuroimaging, and neurophysiological evidence suggested pivotal involvement of the cerebral cortex in migraine.18 The cerebral sulci are integral components of the cerebral cortex, undergo morphological changes that can reflect cortical abnormalities. Cerebral sulci serve to expand the surface area of the cerebral cortex, demarcate distinct functional regions, and enhance the efficiency of neuronal information transmission. VBM and SBM are traditionally used to accurately identify gray and white matter boundaries to measure brain volume, have a limited ability to capture concurrent changes in both gray and white matter integrity affecting brain function. In contrast, sulcal morphometry is sensitive and effective for capturing these changes.19 Neuroimaging research on the cortical sulci has gained significance as a potential source of biomarkers for early diagnosis in various conditions including Alzheimer’s disease,20,21 autism spectrum disorder,22 and Tourette’s syndrome.23
Only a few studies have explored changes in cortical sulcal morphology in migraine patients by assessing parameters such as cortical thickness24 and sulcal depth.25–27 However, these studies did not specifically investigate sulcal morphology, resulting in conflicting results and limitations in terms of the number of analyzed sulci and sulcal morphometry parameters. Therefore, it is appropriate to regard this study as a preliminary study. In this study, we used sulcal morphometry to compare various sulcal parameters, including mean depth, cortical thickness, and opening width. We hypothesized that patients would exhibit alterations in sulcal parameters in regions involved in pain processing, and that some of these alterations would correlate with clinical characteristics.
Methods
Participants
In this cross-sectional study, patients with migraines were recruited from a convenience sample of patients seeking treatment at the outpatient clinic of the Department of Neurology, Hospital of Chengdu University of Traditional Chinese Medicine between April 2019 and April 2022. HCs were recruited via advertisements targeting local university and community members. All participants were right-handed and aged 18–65 years. Diagnoses of CM and EM were based on the third edition of the International Classification of Headache Disorders (ICHD-3) criteria.28 Patients who fulfilled ICHD-3 diagnostic criteria for migraine, suffered from migraine for >1 year, and experienced headache attacks in the month preceding the initial visit were included in the study. Exclusion criteria were as follows: comorbidity with another type of headache, as defined by the ICHD-3 criteria (only tension-type-like headache <10 days a month was allowed); comorbidity with medication overuse headache; use of migraine prophylaxis in the past three months; pregnancy or lactation; diabetes mellitus, severe hypertension, major anxiety, or depression preceding the onset of headache (Hamilton anxiety scale [HAMA] ≥24 or Hamilton Depression Scale-17 items [HAMD-17] ≥24), other psychiatric disorders, stroke, or tumors; and having a condition incompatible with MRI, such as metallic or electric implants or claustrophobia. Criteria for inclusion in the HC group were as follows: no history of migraine or other primary headaches (only infrequent episodic tension-type headaches were allowed), no history of headache attacks in the previous month, and no cardiovascular or neurological diseases. This study is part of a larger research project, which was approved by the Medical Ethics Committee of the Chengdu University Hospital of Traditional Chinese Medicine (2019KL-061) and registered at ClinicalTrials.gov (chiCTR1900028542). Subsequently, it has been expanded with an increased number of cases and the addition of an EM cohort. All participants provided written informed consent outlining the purpose, procedures, risks, rights, and data publication of the study. The study was conducted adhering to the ethical principles for medical research involving human subjects in the Declaration of Helsinki. Structural and functional imaging data of a subset of the subjects were previously published.29 This analysis serves as the primary assessment of sulcal morphological alterations in patients with CM and EM.
Clinical Measures and Neuropsychological Tests
Demographic and clinical data including headache characteristics, associated symptoms, and affective and cognitive measurements were collected. The visual analog scale (VAS)30 was used to assess headache intensity. Days individuals experienced headache and migraine were calculated. The HAMD31 was used to assess depression symptoms-based classification into the following categories: no depression (0–7); mild depression (8–16); moderate depression (17–23); and severe depression (≥24). The HAMA32 was used to assess anxiety symptoms based on the following severity ranges: no anxiety (0–7); mild anxiety (8–14); moderate anxiety (15–23) and severe anxiety (≥24). The Montreal cognitive assessment33 was used to measure the global cognitive function.
Magnetic Resonance Imaging Acquisition
Images were acquired using a GE 3.0 T MRI scanner (GE Discovery MR750, General Electric Company, Fairfield, CT, USA) with a 32-channel head coil. All images were axial scans. The field of view extended from the top of the head to the lower edge of the cerebellar tonsils, and the anterior commissure-posterior commissure line served as the reference plane.
Initiate the T2 fluid attenuated inversion recovery (FLAIR) sequence to exclude obvious brain structural abnormalities. Throughout the experiment, no participants with substantial intracranial abnormalities were identified. A high-resolution three-dimensional (3D) T1-weighted scan was used for sulcal morphometry analysis of each participant. Each scan was performed using a 3D fast spoiled gradient recalled sequence with the following parameters: repetition time, 6800 ms; echo time, 84 ms; flip angle, 90°; matrix size, 128 × 128; slice thickness, 0.5 mm; slice gap, 0 mm; number of slices, 312. Voxel size resolution: 0.9 × 0.9 × 0.5 mm3.
Symptoms of different phases of migraine according to ICHD-3 criteria were explained to patients before they underwent MRI on headache-free days. Patients were allowed to take analgesics during attacks; however, none had a history of headache attacks or use of pain medication during the 24 hours prior to the scan. If a headache attack occurred, the scan was stopped immediately and another scan was performed 24 h after the headache had completely resolved. All scans were performed in the afternoon.
Image Processing
Processing of the 3D T1-weighted images was carried out using the Morphologist toolbox of BrainVisa (version 5.0.4) software (https://brainvisa.info). The segmentation pipeline included the following steps: a) image orientation handling; b) T1 bias correction; c) histogram analysis; d) brain mask computation; e) mask splitting; f) Talairach transformation; g) gray/white classification; h) head meshing; i) recognition of sulci; and j) measurement of sulcal morphology. The technical details of these procedures are discussed in prior publications.22,34 For each participant, the steps were manually inspected to ensure quality. The software automatically labelled 61 sulci in the right hemisphere and 62 sulci35 in the left hemisphere. For each sulcus, the parameters were computed using morphometric analysis.
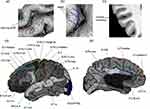
For each sulcus, we measured the following parameters: mean depth, cortical thickness, and opening width (all measurements are in mm) (Figure 1a–c). Sulcal parameters were computed after nine-parameter affine normalization to Talairach space to control for brain volume. Sulcal depth was computed for each point from a convex hull around the brain to the bottom line of the medial sulcal surface. The mean depth was the average value of the depth across all points on the bottom line. Sulcal cortical thickness was defined as the distance between the gray matter/white matter boundary and the gray matter/cerebrospinal fluid boundary averaged across the two walls of the cortical mantle that defined the sulcus. Sulcal opening width was defined as the distance between the two walls of each sulcal fold, namely, between the pial surfaces of the two gyri that formed the sulcus.36,37 Sulcal measurements have high reliability and reproducibility using automated tracing methods in BrainVisa Morphologist,38 and are used in an autism spectrum disorder population study.39
Selection of Cortical Sulci of Interest
Base on a comprehensive review of neuroimaging studies40 and relevant research14,41,42 in migraine, our primary focus was on brain regions involved in pain processing of migraine, specifically those located in the frontal, parietal, temporal, occipital and insula areas. In our analysis, we identified and examined a total of 22 distinct sulci in each hemisphere (shown in Figure 1d and e).
Statistical Analysis
As this was a preliminary study, no statistical power calculation was performed prior to its initiation, and the sample size was based on the available data. Statistical analyses were performed using IBM SPSS 26.0 software (International Business Machines Corporation, Armonk, NY, USA). Before the analysis, all data were tested for normality using the Shapiro–Wilk test. Continuous variables with a normal distribution are expressed as mean ± standard deviation (SD), while variables with a non-normal distribution are expressed as median and interquartile range (IQR). Categorical variables are summarized as frequencies (percentages).
To assess differences between demographic data and clinical characteristics, one-way analysis of variance (ANOVA) was used if continuous variables were normally distributed; otherwise, the Kruskal–Wallis test was employed to assess between-group differences. Chi-square tests were used to compare categorical variables among the three groups.
Prior to the analysis of sulcal parameters (mean depth, cortical thickness, opening width), normality tests were performed on all variables, and, if necessary, a rank-based inverse normal transformation was used to create a normal distribution.43 Multivariate analysis of covariance (MANCOVA) was performed to assess sulcal variables among the three groups, with age and sex as covariates. Each sulcal parameter was analyzed separately. In this preliminary study, both conservative and liberal methods were employed to correct for multiple comparisons. The Bonferroni method was performed to set the P value at <0.001 (0.05/44). We also applied the Benjamini–Hochberg method to control the false discovery rate (FDR) procedure with a Q value <0.05. Subsequently, between-group differences were analyzed via post-hoc pairwise comparisons using the Bonferroni test, and statistical significance was set at a Padj value (adjusted P value) <0.05.
Pearson partial correlation analysis, with age and sex as covariates of no interest, was performed to assess relevant clinical variables (migraine frequency, VAS, disease duration, HAMA, HAMD, and MOCA) and altered sulcal parameters. Due to the high correlation between headache and migraine frequency, headache frequency was temporarily excluded from correlation analyses. Correlation analyses were conducted separately for CM, EM, and combined migraine (CM + EM) groups. Multiple comparisons were corrected using the Bonferroni method. All our analyses were two-tailed tests.
Results
Demographic and Baseline Clinical Characteristics
We initially recruited 37 patients with CM, 35 patients with EM, and 35 HCs who underwent MRI. No patients had a headache attack 24 hours after the MRI scan. Two patients with CM were excluded from the analysis due to blurred images caused by excessive head movement, leaving 35 patients with CM, 35 with EM, and 35 HC included in the final analysis. Demographic and clinical data of all participants are shown in Table 1. There were no significant differences in age or sex among the three groups and all variables were comparable. The CM group had a median number of headache days per month of 19 (IQR, 7), median number of migraine days per month of 14 (IQR, 7), median headache intensity of 7 (IQR, 1), and median disease duration of 18 years (IQR, 8). The EM group had a median number of headache days per month of 3 (IQR, 3), median number of migraine days per month of 3 (IQR, 3), median headache intensity of 7 (IQR, 2), and median disease duration of 10 years (IQR, 10). Compared to the EM group, the headache frequency, migraine frequency, and disease duration (P < 0.001 for all) of the CM group were increased. No significant differences in VAS scores of the two groups (P = 0.077) were observed. In the CM and EM groups, 85.7 and 85.7% of patients experienced nausea, 40.0 and 28.6% reported vomiting, 51.4 and 40.0% reported photophobia, and 45.7 and 44.3% reported phonophobia, respectively. No significant between-group differences in accompanying symptoms were found. The migraine groups had higher HAMA and HAMD scores than the HC group (P < 0.001). In addition, the CM group had a higher HAMD score than the EM group (P = 0.012), and the CM group had lower MoCA scores than EM and HC groups (P = 0.038).
 |
Table 1 Demographic and Clinical Characteristics |
Sulcal Mean Depth
ANCOVA analysis showed significant differences in mean depth in the left anterior callosomarginal fissure (F(2,102) = 8.83, P < 0.001) after applying the Bonferroni correction. Additionally, when using FDR correction, differences in mean depth were also observed in the left superior frontal sulcus (F(2,102) = 7.02, P = 0.001), right superior frontal sulcus (F(2,102) = 6.98,P = 0.001), and left median frontal sulcus (F(2,102) = 6.13, P = 0.003). For pairwise comparisons, in both the left and right superior frontal sulcus, as well as the left median frontal sulcus, CM patients showed decreased mean depth when compared to both HC and EM groups (Padj < 0.05). In the left anterior callosomarginal fissure, CM patients showed increased mean depth when compared to HC groups (Padj < 0.05). No significant results were found between EM and HC patients. The significant results that survived multiple comparisons are shown in Table 2, and additional results and Q values are reported in Supplementary file Table S1.
 |
Table 2 Significant Sulcal Mean Depth Differences Between CM, EM, and HCs Group |
Sulcal Cortical Thickness
ANCOVA analysis showed significant differences in cortical thickness in the left superior postcentral sulcus (F(2,102) = 40.99, P < 0.001), right superior postcentral sulcus (F(2,102) = 39.24, P < 0.001), and left median frontal sulcus (F(2,102) = 10.96, P < 0.001) after applying the Bonferroni correction. Additionally, when using FDR correction, differences in cortical thickness were also observed in the right median frontal sulcus (F(2,102) = 7.15, P = 0.001) and left superior parietal sulcus (F(2,102) = 5.59, P = 0.005). For pairwise comparisons, in both the left and right superior postcentral sulcus, as well as the left median frontal sulcus, CM patients showed increased coritical thicknesswhen compared to both HC and EM groups. In the right median frontal sulcus and left superior parietal sulcus, CM patients showed increased coritical thickness when compared to EM groups. No significant results were found between EM and HC patients. The significant results that survived multiple comparisons are shown in Table 3, and additional results and Q values are reported in Supplementary file TableS 2.
 |
Table 3 Significant Sulcal Cortical Thickness Differences Between CM, EM, and HCs Group |
Sulcal Opening Width
ANCOVA analysis showed significant differences in opening width in the right insula (F(2,102) = 8.71, P < 0.001) after applying the Bonferroni correction, as well as the left insula (F(2,102) = 6.89, P = 0.001) when using FDR correction. For pairwise comparisons, in both the left and right insula, both CM and EM patients showed increased opening width when compared to HC group. No significant results were found between CM and EM patients. The significant results that survived multiple comparisons are shown in Table 4, and additional results and Q values are reported in Supplementary file TableS 3.
 |
Table 4 Significant Sulcal Opening Width Differences Between CM, EM, and HCs Group |
Correlation Analysis
After correction, significant positive correlations between migraine frequency and cortical thickness in the left superior postcentral sulcus (r = 0.56, P < 0.001) (Figure 2a) and in the right superior postcentral sulcus (r = 0.61, P < 0.001) (Figure 2b) were observed within the combined migraine group. There were also significant positive correlations between disease duration and cortical thickness in the left superior postcentral sulcus (r = 0.40, P < 0.001) (Figure 2c) and in the right superior postcentral sulcus (r = 0.42, P < 0.001) (Figure 2d) within the combined migraine group. No correlation results for CM or EM groups survived after applying multiple comparison corrections.
Discussion
This is the first analysis of cortical sulcus morphometry among patients with CM, patients with EM and HCs. In this study, we found abnormal sulci morphometry within areas associated with pain processing in migraine patients. Specifically, when compared to HCs, both CM and EM groups displayed increased opening width in insula. Furthermore, CM, compared to both EM and HC, presented altered mean depth and cortical thickness in various sulci including superior frontal sulcus, median frontal sulcus, superior postcentral sulcus and superior parietal sulcus. Notably, CM revealed more pronounced alterations in cortical sulci within regions implicated in the sensory processing and affective components of pain. Finally, we identified significant correlations between migraine frequency, disease duration and cortical thickness in the bilateral superior postcentral sulcus.
The insula, which plays a crucial role in pain processing, has been previously identified in various studies. It serves as a central hub in the cortex, responsible for processing multisensory and affective components associated with migraine.44 Additionally, the insula is an integral part of the salience network and exhibits robust connections with other networks, like central executive network.45 Previous studies using VBM and SBM have consistently reported a reduction in gray matter volume46 and cortical thickness14 within the insula cortex among migraine patients. Increased opening width reflects cortical atrophy47 and has been identified in Alzheimer’s disease.48 Our study reveals that increased opening width in the insular sulci may reflect atrophy in insula and is in accordance with the reductions in gray matter volume and cortical thickness reflecting atrophy in the insula in VBM and SBM studies. However, we did not found concomitant changes in cortical thickness and previous study sometimes also found an absence of structural changes in the insula.16 Cortical thickness and opening width both reflect cortical atrophy, and they may did not changed together. Opening width may serve as complementary biomarkers in migraine.
We found increased cortical thickness in the bilateral superior postcentral sulcus in CM patients when compared to both EM and HC. The superior postcentral sulcus is a part of somatosensory cortex known to receive noxious afferents from the trigeminovascular system, which plays a crucial role in migraine pathophysiology. These findings are consistent with previous study that has reported increased cortical thickness in the somatosensory cortex.15,41 Our study is also in alignment with previous research that identified changes between low-frequency and high-frequency migraines.49 Moreover, our correlation analysis revealed a positive association between migraine frequency and cortical thickness in the bilateral superior postcentral sulcus, suggesting that these changes may be attributed to repetitive and prolonged stimulation from migraines. Notably, alterations in the superior part of the postcentral sulcus were specifically observed in CM. Traditionally, neuroimaging studies have implicated the inferior part of the postcentral cortex in representing the head and face.49 However, some studies have reported alterations in the superior part of the postcentral sulcus in the context of migraine.41 In addition to these findings, functional magnetic resonance imaging (fMRI) indicated that CM patients exhibit a broader tactile sensitivity beyond the head.50 This broader tactile sensitivity may help explain the observed changes in the superior part of the postcentral sulcus. Furthermore, we also observed increased cortical thickness in the parietal lobe of CM patients compared to EM patients. Given that the superior parietal sulcus shares approximately half of its cortical thickness with the superior postcentral sulcus, this may contribute to the observed changes in cortical thickness in the superior parietal sulcus.
The prefrontal cortex plays a multifaceted role, encompassing executive functions and the processing of pain.51 Previous studies have reported inconsistent findings regarding the morphology of the prefrontal cortex.9,11,14,27 While most studies have reported decreased gray matter volume or thickness in the prefrontal cortex in migraine patients,14,42,46 one have suggested an positive association between migraine frequency and gray matter volume in frontal gyrus.11 We found increased cortical thickness in the bilateral median frontal sulcus in CM. The discrepancy between our findings and previous study may be attributed to the possibility that sulci and gyri do not exhibit coordinated changes. Additionally, we identified decreased sulcal mean depth in the median frontal sulcus and superior frontal sulcus. Interestingly, sulcal depth was previously reported to be increased in the temporal pole in migraine patients with aura when compared to those without aura.25 Conversely, one study found no differences in sulcal depth in migraine patients without aura compared to healthy controls.26 These discrepancies may be linked to variations in study cohorts. Our findings primarily focus on changes in CM. In line with similar studies on pain,52 trigeminal neuralgia research indicated decreased sulcal depth in the bilateral superior frontal cortex, an area within the same region as our evaluated superior frontal sulcus. Trigeminal neuralgia and migraine may share common pain processing pathways, leading to consistent morphological changes in the sulci.
Additionally, our study revealed that patients with CM showed increased sulcal depth in the left anterior callosomarginal fissure compared to the HC group, a region associated with the anterior cingulate cortex. The anterior cingulate cortex, which is situated in the trigeminovascular pain pathway, plays a role in affective processing and receives input from the thalamus and various regions of the frontal, parietal, and temporal cortex.53 Reduced grey matter volume in the anterior cingulate cortex has been linked to increased headache frequency.49 Our findings, in conjunction with previous research, suggest that regions involved in affective processing may exhibit adaptive responses to recurrent migraine attacks. Furthermore, CM patients demonstrated more pronounced abnormal changes in affective regions compared to EM patients, aligning with our clinical characteristics results.
Our study had several limitations. First, due to the preliminary nature of this study, a larger sample size is needed to enhance the robustness and reproducibility of the results.54 Second, migraine patients only underwent MRI testing during migraine-free days. This was a cross-sectional study in which no MRI images of the patients at multiple time points were acquired. We could not confirm whether migraine caused changes in sulcal parameters or whether changes in sulcal morphology caused migraine. Future research comparing different migraine phases and longitudinal follow-up is required.
Conclusion
Our study revealed abnormal sulcal morphology primarily within regions associated with pain processing in migraine patients, and CM exhibiting more extensive abnormal sulcal morphology in areas related to sensory and affective processing. These cortical sulcal abnormalities may contribute to understanding the pathology of EM and CM. Sulcal morphometry may emerge as an MRI biomarker for diagnosing migraine and their subtypes.
Abbreviations
CM, chronic migraine; EM, episodic migraine; HC, healthy controls; ANCOVA: analysis of covariance; VBM, voxel-based morphometry; SBM, surface-based morphometry; VAS, visual analogue scale; HAMA, Hamilton Anxiety Scale; HAMD-17, Hamilton Depression Scale-17; MoCA, Montreal Cognitive Assessment; F.C.M.ant., anterior callosomarginal fissure; INSULA, insula sulci; S.F.inter., intermediate frontal sulcus; S.F.median., median frontal sulcus; S.F.sup., superior frontal sulcus; S.Pa.sup., superior parietal sulcus S.Pe.C.median., median precentral sulcus; S.Po.C.sup., superior postcentral sulcus.
Consent for Publication
All the details can be published.
Data Sharing Statement
The raw data of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Medical Ethics Committee of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (2019KL-061). Written informed consent was obtained from participants.
Acknowledgment
The authors would like to thank all study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the 2019 “Xinglin Scholars” Subject Talent Research Promotion Plan (Project No: ZYTS2019028), Chengdu university of traditional Chinese medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peres MFP, Mercante JPP, Tobo PR, Kamei H, Bigal ME. Anxiety and depression symptoms and migraine: a symptom-based approach research. J Headache Pain. 2017;18(1). doi:10.1186/s10194-017-0742-1
2. Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clinics. 2019;37(4):631–649. doi:10.1016/j.ncl.2019.06.001
3. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619–6629. doi:10.1523/JNEUROSCI.0373-15.2015
4. Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3(11):e3799. doi:10.1371/journal.pone.0003799
5. Messina R, Rocca MA, Valsasina P, Misci P, Filippi M. Clinical correlates of hypothalamic functional changes in migraine patients. Cephalalgia. 2022;42(4–5):279–290. doi:10.1177/03331024211046618
6. Yuan Z, Wang W, Zhang X, et al. Altered functional connectivity of the right caudate nucleus in chronic migraine: a resting-state fMRI study. J Headache Pain. 2022;23(1):154. doi:10.1186/s10194-022-01506-9
7. Hougaard A, Amin FM, Hoffmann MB, et al. Structural gray matter abnormalities in migraine relate to headache lateralization, but not aura. Cephalalgia. 2015;35(1):3–9. doi:10.1177/0333102414532378
8. Palm-Meinders IH, Arkink EB, Koppen H, et al. Volumetric brain changes in migraineurs from the general population. Neurology. 2017;89(20):2066–2074. doi:10.1212/WNL.0000000000004640
9. Chen XY, Chen ZY, Dong Z, Liu MQ, Yu SY. Regional volume changes of the brain in migraine chronification. Neural Regen Res. 2020;15(9):1701–1708. doi:10.4103/1673-5374.276360
10. Bilgic B, Kocaman G, Arslan AB, et al. Volumetric differences suggest involvement of cerebellum and brainstem in chronic migraine. Cephalalgia. 2016;36(4):301–308. doi:10.1177/0333102415588328
11. Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB. Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache. 2017;57(3):400–416. doi:10.1111/head.13012
12. Coppola G, Petolicchio B, Di Renzo A, et al. Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain. 2017;18(1):115. doi:10.1186/s10194-017-0825-z
13. Chen Z, Chen X, Liu M, Ma L, Yu S. Volume of hypothalamus as a diagnostic biomarker of chronic migraine. Front Neurol. 2019;10:606. doi:10.3389/fneur.2019.00606
14. Lai KL, Niddam DM, Fuh JL, Chen WT, Wu JC, Wang SJ. Cortical morphological changes in chronic migraine in a Taiwanese cohort: surface- and voxel-based analyses. Cephalalgia. 2020;40(6):575–585. doi:10.1177/0333102420920005
15. Kim JH, Kim JB, Suh SI, Seo WK, Oh K, Koh SB. Thickening of the somatosensory cortex in migraine without aura. Cephalalgia. 2014;34(14):1125–1133. doi:10.1177/0333102414531155
16. Woldeamanuel YW, DeSouza DD, Sanjanwala BM, Cowan RP. Clinical features contributing to cortical thickness changes in chronic migraine - A pilot study. Headache. 2019;59(2):180–191. doi:10.1111/head.13452
17. Datta R, Detre JA, Aguirre GK, Cucchiara B. Absence of changes in cortical thickness in patients with migraine. Cephalalgia. 2011;31(14):1452–1458. doi:10.1177/0333102411421025
18. Barbanti P, Brighina F, Egeo G, Di Stefano V, Silvestro M, Russo A. Migraine as a cortical brain disorder. Headache. 2020;60(9):2103–2114. doi:10.1111/head.13935
19. Lamont AJ, Mortby ME, Anstey KJ, Sachdev PS, Cherbuin N. Using sulcal and gyral measures of brain structure to investigate benefits of an active lifestyle. NeuroImage. 2014;91:353–359. doi:10.1016/j.neuroimage.2014.01.008
20. Reiner P, Jouvent E, Duchesnay E, Cuingnet R, Mangin JF, Chabriat H. Sulcal span in Azheimer’s disease, amnestic mild cognitive impairment, and healthy controls. J Alzheimer’s Dis. 2012;29(3):605–613. doi:10.3233/jad-2012-111622
21. Hamelin L, Bertoux M, Bottlaender M, et al. Sulcal morphology as a new imaging marker for the diagnosis of early onset Alzheimer’s disease. Neurobiol Aging. 2015;36(11):2932–2939. doi:10.1016/j.neurobiolaging.2015.04.019
22. Shokouhi M, Williams JH, Waiter GD, Condon B. Changes in the sulcal size associated with autism spectrum disorder revealed by sulcal morphometry. Autism Res. 2012;5(4):245–252. doi:10.1002/aur.1232
23. Muellner J, Delmaire C, Valabrégue R, et al. Altered structure of cortical sulci in Gilles de la Tourette syndrome: further support for abnormal brain development. Mov Disord. 2015;30(5):655–661. doi:10.1002/mds.26207
24. Magon S, May A, Stankewitz A, et al. Cortical abnormalities in episodic migraine: a multi-center 3T MRI study. Cephalalgia. 2019;39(5):665–673. doi:10.1177/0333102418795163
25. Petrusic I, Dakovic M, Kacar K, Zidverc-Trajkovic J. Migraine with Aura: surface-Based Analysis of the Cerebral Cortex with Magnetic Resonance Imaging. Korean J Radiol. 2018;19(4):767–776. doi:10.3348/kjr.2018.19.4.767
26. Masson R, Demarquay G, Meunier D, et al. Is Migraine Associated to Brain Anatomical Alterations? New Data and Coordinate-Based Meta-analysis. Brain Topogr. 2021;34(3):384–401. doi:10.1007/s10548-021-00824-6
27. Messina R, Rocca MA, Colombo B, et al. Cortical abnormalities in patients with migraine: a surface-based analysis. Radiology. 2013;268(1):170–180. doi:10.1148/radiol.13122004
28. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia. 2018; 38(1):1–211. doi:10.1177/0333102417738202
29. Shi M, Yang J, Yang D, Yang X, Zhao H. Microstructural white matter changes in chronic migraine patients with liver-yang hyperactivity and qi-blood deficiency syndrome: a diffusion tensor imaging study. Neuroreport. 2022;33(10):422–428. doi:10.1097/WNR.0000000000001800
30. Aicher B, Peil H, Peil B, Diener HC. Pain measurement: visual Analogue Scale (VAS) and Verbal Rating Scale (VRS) in clinical trials with OTC analgesics in headache. Cephalalgia. 2012;32(3):185–197. doi:10.1177/03331024111430856
31. Zimmerman M, Martinez J H, Young D, Chelminski I and Dalrymple K. (2013). Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders, 150(2), 384–388. 10.1016/j.jad.2013.04.028
32. Matza L S, Morlock R, Sexton C, Malley K and Feltner D. (2010). Identifying HAM‐A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psych Res, 19(4), 223–232. 10.1002/mpr.323
33. Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011;24(4):184–190. doi:10.1177/0891988711422528
34. Fischer C, Operto G, Laguitton S, Perrot M, Rivière D. Morphologist 2012: the new morphological pipeline of BrainVISA. 2012.
35. Perrot M, Rivière D, Mangin J-F. Cortical sulci recognition and spatial normalization. Med Image Anal. 2011;15(4):529–550. doi:10.1016/j.media.2011.02.008
36. Mangin JF, Jouvent E, Cachia A. In-vivo measurement of cortical morphology: means and meanings. Curr Opin Neurol. 2010;23(4):359–367. doi:10.1097/WCO.0b013e32833a0afc
37. Auzias G, Viellard M, Takerkart S, et al. Atypical sulcal anatomy in young children with autism spectrum disorder. NeuroImage Clin. 2014;4:593–603. doi:10.1016/j.nicl.2014.03.008
38. Snyder W, Patti M, Troiani V. An evaluation of automated tracing for orbitofrontal cortex sulcogyral pattern typing. J Neurosci Methods. 2019;326:108386. doi:10.1016/j.jneumeth.2019.108386
39. Nordahl CW, Dierker D, Mostafavi I, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 2007;27(43):11725–11735. doi:10.1523/JNEUROSCI.0777-07.2007
40. Chong CD, Schwedt TJ, Dodick DW. Migraine: what Imaging Reveals. Curr Neurol Neurosci Rep. 2016;16(7):64. doi:10.1007/s11910-016-0662-5
41. DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69(21):1990–1995. doi:10.1212/01.wnl.0000291618.32247.2d
42. Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–117. doi:10.1111/j.1526-4610.2007.00723.x
43. Bishara AJ, Hittner JB. Testing the significance of a correlation with nonnormal data: comparison of Pearson, spearman, transformation, and resampling approaches. Psychol Methods. 2012;17(3):399–417. doi:10.1037/a0028087
44. Borsook D, Veggeberg R, Erpelding N, et al. The insula: a”hub of activity” in migraine. Neuroscientist. 2016;22(6):632–652. doi:10.1177/1073858415601369
45. Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One. 2012;7(12):e52927. doi:10.1371/journal.pone.0052927
46. Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi:10.1111/j.1468-2982.2008.01550.x
47. Kochunov P, Rogers W, Mangin JF, Lancaster J. A library of cortical morphology analysis tools to study development, aging and genetics of cerebral cortex. Neuroinformatics. 2012;10(1):81–96. doi:10.1007/s12021-011-9127-9
48. Fumagalli GG, Basilico P, Arighi A, et al. Parieto-occipital sulcus widening differentiates posterior cortical atrophy from typical Alzheimer disease. NeuroImage Clin. 2020;28:102453. doi:10.1016/j.nicl.2020.102453
49. Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012;32(8):607–620. doi:10.1177/0333102412445622
50. Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68(1):81–91. doi:10.1002/ana.21994
51. Ong WY, Stohler CS, Herr DR. Role of the Prefrontal Cortex in Pain Processing. Mol Neurobiol. 2019;56(2):1137–1166. doi:10.1007/s12035-018-1130-9
52. Li M, Yan J, Wen H, et al. Cortical thickness, gyrification and sulcal depth in trigeminal neuralgia. Sci Rep. 2021;11(1):16322. doi:10.1038/s41598-021-95811-z
53. Tolner EA, Chen S-P, Eikermann-Haerter K. Current understanding of cortical structure and function in migraine. Cephalalgia. 2019;39(13):1683–1699. doi:10.1177/0333102419840643
54. Marek S, Tervo-Clemmens B, Calabro FJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):7902):654–660. doi:10.1038/s41586-022-04492-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.