Back to Journals » International Journal of General Medicine » Volume 17
Correlation of Peripheral Blood Inflammatory Indicators to Prognosis After Intravenous Thrombolysis in Acute Ischemic Stroke: A Retrospective Study
Authors Zhang T , Fu S, Cao X, Xia Y , Hu M, Feng Q, Cong Y , Zhu Y , Tang X, Wu M
Received 21 December 2023
Accepted for publication 8 March 2024
Published 15 March 2024 Volume 2024:17 Pages 985—996
DOI https://doi.org/10.2147/IJGM.S456144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Redoy Ranjan
Tianrui Zhang,1,* Sha Fu,1,* Xiaofeng Cao,2 Yangjingyi Xia,1 Manyan Hu,1 Qinghua Feng,1 Yujun Cong,1 Yuan Zhu,1 Xiaogang Tang,1 Minghua Wu1
1Department of Neurology, Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Province Hospital of Chinese Medicine, Nanjing, 210029, People’s Republic of China; 2Department of Neurology, Jiangyan Hospital of Chinese Medicine, Taizhou, Jiangsu, 225500, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Minghua Wu; Xiaogang Tang, Department of Neurology, Affiliated Hospital of Nanjing University of Chinese Medicine, 155 Hanzhong Road, Nanjing, 210029, People’s Republic of China, Tel +8613951786719, Email [email protected]; [email protected]
Purpose: According to many previous studies, neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR) and hypersensitive C-reactive protein (CRP) are commonly used as important indicators to assess the prognosis of intravenous thrombolysis in AIS patients. Based on this, we used two novel biomarkers C-NLR (CRP/neutrophil-to-lymphocyte ratio) and C-LMR (CRP×lymphocyte-to-monocyte ratio) to investigate their correlation with 90-day outcomes in AIS patients after intravenous thrombolysis.
Patients and Methods: A total of 204 AIS patients who received intravenous thrombolysis at the Stroke Center of Jiangsu Province Hospital of Chinese Medicine from January 2021 to December 2022 were retrospectively included. All patients were followed up 90 days after thrombolysis to assess their prognosis. Patients with a modified Rankin scale score (mRS) of 3– 6 were included in the unfavorable outcome group, and those with a score of 0– 2 were included in the favorable outcome group. Logistic regression analysis, receiver operating characteristic (ROC) curve, and Kaplan–Meier survival curve were used to investigate the association between C-NLR, C-LMR, and 90-day prognosis in AIS patients treated with early intravenous thrombolysis.
Results: C-NLR (OR=1.586, 95% CI=1.098~2.291, P=0.014) and C-LMR (OR=1.099, 95% CI=1.025~1.179, P=0.008) were independent risk factors for 90-day prognosis of AIS patients treated with early intravenous thrombolysis. The higher C-NLR and C-LMR were associated with unfavorable prognosis.
Conclusion: C-NLR and C-LMR can be used as biomarkers to predict prognosis of AIS patients treated with early intravenous thrombolysis.
Keywords: modified rankin scale, reperfusion therapy, inflammatory reaction, cerebral vascular disease, clinical prognosis
Introduction
Acute ischemic stroke is a cerebrovascular disease that seriously threatens life, health, and safety. It is characterized by high disability rate and high fatality rate, and the incidence population shows an expanding trend.1,2 Clinically, reperfusion therapy is often adopted for AIS patients with a short duration of onset. For example, early rt-PA intravenous thrombolytic therapy for acute ischemic stroke within a time window of 4.5 h has become mainstream practice.3
Although intravenous thrombolysis can be used as a relatively safe treatment in most cases, it also has many adverse reactions such as intracranial hemorrhage and neurological deterioration.4 Many studies have focused on early neurological deterioration after intravenous thrombolysis, while the observation of medium and long-term prognosis of intravenous thrombolysis is relatively lacking.5,6 Considering that rehabilitation of neurological function in the later stage of stroke is also a key link to stroke treatment, our study used the 90-day time point to observe the prognosis of intravenous thrombolysis.
Previous studies with a sample from our stroke center had found that inflammatory mechanisms play an important role in the onset and progression of ischemic stroke.7,8 Other research has confirmed the close correlation between inflammatory factors and neurological deterioration in pathology.9 NLR has been proven to be an important predictor of stroke prognosis,10,11 while studies have also confirmed the predictive function of LMR.12,13 In addition, many studies also used CRP as a joint prediction index.14–16 Based on these convincing studies, this retrospective study attempted to explore the feasibility of C-NLR and C-LMR in predicting the prognosis of AIS after intravenous thrombolysis on the basis of these indicators.
Materials and Methods
Study Design and Patients
This retrospective cohort study was approved by the Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine (2017NL-012-01). Informed consent was waived because of retrospective design. A total of 204 patients with acute ischemic stroke who received intravenous thrombolysis (rt-PA) in the Department of Neurology of Jiangsu Province Hospital of Chinese Medicine from January 2021 to December 2022 were collected. The inclusion criteria were as follows: (1) It met the diagnostic criteria set out in the “Guidelines for the diagnosis and treatment of acute ischemic stroke in China 2018”.17 (2) It was the first onset, and the imaging diagnosis and laboratory diagnosis indicators at admission were consistent with indications of intravenous thrombolysis. (3) The onset time of symptoms was <4.5h. (4) The patients and their families agreed to be included in this study and follow-up. Exclusion criteria: (1) Patients with a history of malignant tumor and antitumor-related therapy; (2) Patients with a history of infection within the past 1 week and treated with anti-infection drugs; (3) Deaths during treatment; (4) The disease has a new cerebral hemorrhage.
Clinical Treatment
According to “Guidelines for the diagnosis and treatment of acute ischemic stroke in China 2018”, the patient received rt-PA intravenous thrombolysis within 4.5 h of onset (The total dose of rt-PA was 0.9 mg/kg, of which 10%rt-PA was injected intravenously within 1 min, and the remaining 90% was pumped intravenously within 1 h).
Data Collection
Clinical data of participants was collected as follows: (1) age, gender. (2) past medical history: cerebral hemorrhage, transient ischemic attack, atrial fibrillation, coronary heart disease, hypertension, diabetes mellitus, hyperlipidemia, myocardial infarction, renal insufficiency. (3) medication use history: anti-hypertension drugs, hypoglycemic drugs, anti-platelet drugs, statins. (4) personal history: smoking history, drinking history. (5) clinical indicators: systolic blood pressure, systolic blood pressure, total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein. According to the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin scale (mRS), the NIHSS score and mRS Score at admission and 24h after intravenous thrombolysis were collected. NLR and LMR were calculated based on the counts of neutrophils, lymphocytes, monocytes, and high-sensitivity C-reactive protein in peripheral venous blood samples of patients when they first received intravenous thrombolysis. Finally, C-NLR (CRP/neutrophil-to-lymphocyte ratio) and C-LMR (CRP×lymphocyte-to-monocyte ratio) were calculated.
Assessment and Definition of Prognosis
All patients were followed up by neurologists 90 days after intravenous thrombolysis, and the prognosis was evaluated using a modified Rankin scale (mRS). Patients with a score of 0–2 were defined as favorable outcome, and patients with a score of 3–6 were defined as unfavorable outcome. The mRS Score of dead patients was also defined as 6 points.
Statistical Analysis
SPSS 27.0 statistical software was used for data statistical analysis. Measurement data conforming to normal distribution were expressed as mean ± standard deviation (x,s), and comparison between groups was analyzed by independent sample t test. The measurement data that did not meet the normal distribution were expressed as median (quartile) [M(Q1, Q3)], and the Mann–Whitney U-test was used for comparison between groups. Count data were expressed as frequency and percentage (n, %), and chi-square test was used for comparison between groups. Binary Logistic regression was used to analyze the influencing factors of poor prognosis. ROC curve was drawn and AUC was calculated to verify the predictive value of C-NLR and C-LMR for the prognosis of patients, P<0.05 was considered statistically significant. Kaplan–Meier survival curve was drawn and log-rank < 0.05 was considered statistically significant between groups.
Results
A total of 234 patients with acute ischemic stroke who received intravenous thrombolysis in the Stroke Center of Jiangsu Province Hospital of Chinese Medicine from January 2021 to December 2022 were retrospectively enrolled in this study. Among them, 6 cases had a history of malignant tumors, 3 cases had a history of infection within 2 weeks, 16 cases did not receive high-sensitivity C-reactive protein test, and 5 cases dropped out during follow-up.
A total of 25 cases were excluded, and the flow chart is shown in (Figure 1). A total of 204 patients met the inclusion criteria. There were 131 males (65.4%) and 73 females (34.5%), with an average age of 69.1±12.3. Overall, 9 (4.4%) patients had cerebral hemorrhage, 56 (27.4%) patients had TIA, 26 (12.7%) patients had atrial fibrillation, 43 (21.0%) patients had coronary heart disease, 147 (72.0%) patients had hypertension, 85 (41.6%) patients had diabetes mellitus, 141 (69.1%) patients had hyperlipidemia, 12 (5.8%) had myocardial infarction, 14 (6.8%) patients had renal insufficiency, 147 (72.0%) were taking anti-hypertension drugs, 80 (39.2%) patients were taking hypoglycemic drugs, 155 (75.9%) patients were taking antiplatelet aggregation drugs, 160 (78.4%) were taking statins, 42 (20.5%) patients had a history of smoking, and 60 (29.4%) patients had a history of drinking. The distribution of basic demographic data, clinical, and laboratory data of the patients are detailed in (Table 1, Figures 2).
 |
Table 1 Baseline Characteristics of AIS Patients Received Early Intravenous Thrombolysis Treatment |
 |
Figure 1 Flow chart. Abbreviations: AIS, acute ischemic stroke; mRS, modified Rankin scale. |
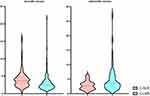 |
Figure 2 Violet plot of C-NLR and C-LMR. Abbreviations: C-NLR, CRP/neutrophil-to-lymphocyte ratio; C-LMR, CRP×lymphocyte-to-monocyte ratio. |
As shown in (Table 1), there were significant differences between groups in age, atrial fibrillation, coronary heart disease, diabetes mellitus, hyperlipidemia, hypoglycemic drugs, statins, systolic blood pressure, and NIHSS score and mRS Score at admission, 24h after thrombolysis (P<0.05). According to univariate logistic regression analysis, C-NLR (OR=1.145, 95% CI=1.034~1.268, P=0.009), C-LMR (OR=1.020, 95% CI=1.006~1.034, P=0.004) were both risk factors for the prognosis of AIS patients after intravenous thrombolysis at 90 days. Then, we included factors such as age, atrial fibrillation, coronary heart disease, diabetes mellitus, hyperlipidemia, hypoglycemic drugs, statins, systolic blood pressure, NIHSS, and mRS at admission and after intravenous thrombolysis in the multivariate logistic regression analysis. C-NLR (OR=1.586, 95% CI=1.098~2.291, P=0.014) and C-LMR (OR=1.099, 95% CI=1.025~1.179, P=0.008) were independent risk factors for 90-day prognosis in AIS patients after intravenous thrombolysis (Table 2, Figure 3).
 |
Table 2 Univariate and Multivariate Logistic Regression of NLR, LMR, CRP, C-NLR, C-LMR |
According to ROC analysis shown in (Figure 4), the results showed that the area under the curve of C-NLR was 0.6, the specificity was 91.4%, the sensitivity was 35.7%, the Youden index was 0.271, and the optimal offcut value was 2.559. The area under the C-LMR curve was 0.608, the specificity was 85.8%, the sensitivity was 40.5%, the Youden index was 0.26, and the optimal cutoff value was 14.917. Therefore, C-NLR and C-LMR have a certain predictive value for the poor prognosis of AIS patients at 90 days.
 |
Figure 4 Receiver operating characteristic(ROC) curve of C-NLR, C-LMR. Abbreviations: C-NLR, CRP/neutrophil-to-lymphocyte ratio; C-LMR, CRP×lymphocyte-to-monocyte ratio. |
According to the optimal cutoff values of C-NLR and C-LMR in ROC analysis, the patients were divided into two groups and survival analysis was performed. The mortality rate was 4.4% (9/204) in C-NLR>2.559 patients, and the mortality rate was 4.4% (9/204) in C-LMR>14.917 patients. Figure 5 shows that patients of C-NLR>2.559 or C-LMR>14.917 have a higher risk of death.
Discussion
For AIS patients with onset within this time window, the current mainstream treatment principle is reperfusion therapy to improve the blood flow of the occluded vessels. Recombinant tissue plasminogen activator (rt-PA), as a recombinant tissue fibrinogen activator, can convert fibrinogen into thrombolytic enzyme to dissolve thrombus, improve vascular recanalization rate, make ischemic brain tissue regain blood perfusion, and thus improve neurological function of patients.18 Intravenous thrombolytic therapy has a strict time window limit in clinical practice, and it cannot effectively improve the vascular recanalization rate for stroke patients with large vessel occlusion. At the same time, about 12%–34% of patients have vascular reocclusion after rt-PA thrombolytic therapy, which seriously affects the prognosis of the disease.19,20 Therefore, we used inflammatory markers to predict the possibility of poor outcome of intravenous thrombolysis.
Previous studies have proposed that inflammatory markers play an important role in the ultra-early onset of ischemic stroke. In the early stage of the disease, the ischemic brain tissue is necrotic, the blood–brain barrier is damaged, and the ischemic penumbra appears, which releases a large amount of oxygen free radicals, thereby mediating the release of inflammatory factors. Leukocytes, neutrophils, monocytes, and other cells aggregate under the action of chemokines, through the destroyed blood–brain barrier, release inflammatory mediators, and aggravate the damage to brain tissue. A retrospective study by Tokgoz found that NLR value can be used as an independent risk factor for neurological deterioration in AIS patients.21 Lattanzi’s study proved the correlation between NLR and early neurological impairment. Compared to uninjured, patients in the early stage, especially those who deteriorated in 1 week, had significantly higher neutrophil counts, and lower lymphocyte counts, resulting in an increase in NLR value.22 The inflammatory response caused by intravenous thrombolytic therapy in the early stage is far more intense than that in the recovery stage of the disease, with strong specificity, which has become the pathological basis for us to observe the prognosis of the disease with inflammatory indicators.
In previous pathological and clinical studies, neutrophils, lymphocytes, and monocytes play an important role in the ultra-early stage of stroke.23 Neutrophils accumulate in the early stage of stroke and play a dominant role in the subsequent inflammatory response. Studies have shown that the extracellular matrix-activating enzyme MMP-9 released by a large number of neutrophils destroy the blood–brain barrier and cause brain tissue necrosis.24 The related inflammatory factors released by neutrophils are also key factors that aggravate brain tissue damage.25–27
Lymphocytes are closely related to the immune inflammatory response in the early stage of stroke, which runs through the processes of brain tissue necrosis, brain edema, neuroinflammation, and nerve repair. In the ultra-early stage of cerebral ischemia, lymphocytes mainly DNT cells infiltrated in the ischemic penumbra. Within 3 days, CD4+T cells began to infiltrate in large numbers, while at about 1 month, CD8+T cells began to infiltrate in large numbers, more numerous than before, and the response was more intense.28–30 After that, NK cells and B cells are also heavily involved in the inflammatory response during the long recovery process of the disease.31 On the other hand, the peripheral inflammatory response caused by AIS focal lesions activates IL-1 and TNF-α, which continuously infiltrate into the center and aggravate the necrosis of brain tissue.32 In addition, studies have shown that the number of T lymphocyte subsets has a significant downward trend within 14 days of ischemic necrosis of brain tissue, but the inflammatory response still maintains a relatively severe degree under the stimulation of various factors.33 Some studies have focused on the neuroprotective effects of lymphocytes. Xiong’s study has shown that the anti-inflammatory cytokine IL-4 secreted by T lymphocytes has nutritional and protective functions in neurons, while another anti-inflammatory cytokine IL-10 significantly reduces the pro-inflammatory function of T lymphocytes.34,35
Monocytes are also an important part of the body’s immune defense mechanism. Inflammatory mediators mediated by monocytes migration to the endothelium are important risk factors for atherosclerosis.36 Another study also confirmed that early neurological deterioration and clinical outcome of ischemic stroke were associated with a surge in the percentage of CD14++/CD16+ monocytes.37
High-sensitivity C-reactive protein (CRP), as a commonly used clinical inflammatory index, is highly sensitive to inflammatory response and also plays an important role in the process of stroke. The inflammatory response caused by ischemic necrosis of brain tissue increases CRP, which leads to the aggravation of arteriosclerosis, activates the complement system, inhibits the fibrinolytic system, and eventually forms thrombosis.38,39 However, CRP is lack of specificity for inflammatory responses after brain ischemia, and stroke patients often have urinary tract infection, pulmonary infection, arteriosclerosis, and other inflammatory reactions. It has great limitations to independently predict the prognosis of stroke by CRP.15,16,40,41
Based on the above discussion, our novel biomarkers, C-NLR and C-LMR, integrate these four inflammatory markers and to some extent avoid the unspecificity of individual indicators. In a retrospective study, it was found that C-NLR could be used as an important indicator to predict the poor prognosis of patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy, which has a certain clinical significance.42 There are also studies that define C-NLR as CRP×neutrophil-to-lymphocyte ratio to predict poor prognosis in patients with heart failure and pancreatic cancer after surgery.43,44 However, studies on the combination of CRP and LMR are relatively lacking, and C-NLR and C-LMR are rarely used in the field of cerebrovascular diseases. Therefore, our study tried to make up for this deficiency.
Regarding the impact of intervention therapy on biomarkers, Gong’s study suggested that intravenous thrombolysis to help revascularization significantly affected the level of inflammatory biomarkers, manifested in early neurological function changes in symptoms.12 However, Ma’s study pointed out that the combination of inflammatory indicators and a long enough observation period can reduce this effect and maximize the prediction of inflammatory indicators for poor prognosis.8 Our retrospective study concluded that higher C-NLR and C-LMR were associated with poor prognosis based on this consideration. Given that recovery from stroke is a long process, it is of great significance for the early treatment and long-term rehabilitation of AIS.
Our research also has unavoidable limitations. First of all, our sample size is insufficient, resulting in a lack of universality, and the influencing factors we collected are insufficient compared with complex clinical situations, and there is a lack of thorough methods to control confounding factors. The observation time is relatively short compared with the recovery period of stroke and cannot fully reflect the prognosis of stroke. DNT time was not included in the study, and individual differences in prognosis of thrombolytic therapy could not be observed. Future studies are expected to make up for these limitations.
Conclusion
Higher C-NLR and C-LMR were associated with unfavorable prognosis. C-NLR and C-LMR can be used as biomarkers to predict prognosis of AIS patients treated with early intravenous thrombolysis.
Ethics Approval and Consent to Participate
Study approval statement: This study protocol was reviewed and approved by Institutional Review Board of Affiliated Hospital of Nanjing University of Chinese Medicine (Jiangsu Province Hospital of Chinese Medicine), approval number [2017NL-012-01].
Informed consent was waived because of retrospective design. During the study, patient data confidentiality and compliance with the Declaration of Helsinki were followed.
Acknowledgments
This publication was made possible by support from the Brain Center at Jiangsu Province Hospital of Chinese Medicine.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82274428, 81973794), National Administration of Traditional Chinese Medicine: Evidence-Based Capacity-Building Project (2019XZZX-NB007), 333 high-level talents training project in Jiangsu (grant no. BRA 2016507), Jiangsu Province Administration of Chinese Medicine (ZT202102).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Montalvo M, Mistry E, Chang AD, et al. Predicting symptomatic intracranial haemorrhage after mechanical thrombectomy: the TAG score. J Neurol Neurosurg Psychiatry. 2019;90(12):1370–1374. doi:10.1136/jnnp-2019-321184
2. Gao Y, Jiang B, Sun H, et al. The burden of stroke in China: results from a nationwide population-based epidemiological survey. PLoS One. 2018;13(12):e0208398. doi:10.1371/journal.pone.0208398
3. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi:10.1161/STR.0000000000000211
4. Simonsen CZ, Schmitz ML, Madsen MH, et al. Early neurological deterioration after thrombolysis: clinical and imaging predictors. Int J Stroke. 2016;11(7):776–782. doi:10.1177/1747493016650454
5. Che F, Wang A, Ju Y, et al. Early neurological deterioration in acute ischemic stroke patients after intravenous thrombolysis with alteplase predicts poor 3-month functional prognosis - data from the Thrombolysis Implementation and Monitor of Acute Ischemic Stroke in China (TIMS-China). BMC Neurol. 2022;22(1):212. doi:10.1186/s12883-022-02737-8
6. Yu WM, Abdul-Rahim AH, Cameron AC, et al. The Incidence and Associated Factors of Early Neurological Deterioration After Thrombolysis: results From SITS Registry. Stroke. 2020;51(9):2705–2714. doi:10.1161/STROKEAHA.119.028287
7. Cao X, Zhu Q, Xia X, et al. The correlation between novel peripheral blood cell ratios and 90-day mortality in patients with acute ischemic stroke. PLoS One. 2020;15(8):e0238312. doi:10.1371/journal.pone.02383123
8. Ma F, Li L, Xu L, et al. The relationship between systemic inflammation index, systemic immune-inflammatory index, and inflammatory prognostic index and 90-day outcomes in acute ischemic stroke patients treated with intravenous thrombolysis. J Neuroinflammation. 2023;20(1):220. doi:10.1186/s12974-023-02890-y
9. Kleinschnitz C, Kraft P, Dreykluft A, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121(4):679–691. doi:10.1182/blood-2012-04-426734
10. Lux D, Alakbarzade V, Bridge L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020;17(1):60. doi:10.1186/s12974-020-01739-y
11. Liu YL, Wu ZQ, Qu JF, et al. High neutrophil-to-lymphocyte ratio is a predictor of poor short-term outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Brain Behav. 2020;10(12):e01857. doi:10.1002/brb3.1857
12. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi:10.1186/s12974-021-02090-6
13. Ren H, Han L, Liu H, et al. Decreased Lymphocyte-to-Monocyte Ratio Predicts Poor Prognosis of Acute Ischemic Stroke Treated with Thrombolysis. Med Sci Monit. 2017;23:5826–5833. doi:10.12659/msm.907919
14. Berkley A, Ferro A. Changes in C-reactive protein in response to anti-inflammatory therapy as a predictor of cardiovascular outcomes: a systematic review and meta-analysis. JRSM Cardiovasc Dis. 2020;9:2048004020929235. doi:10.1177/2048004020929235
15. Karlinski M, Bembenek J, Grabska K, et al. Routine serum C-reactive protein and stroke outcome after intravenous thrombolysis. Acta Neurol Scand. 2014;130(5):305–311. doi:10.1111/ane.12227
16. Topakian R, Strasak AM, Nussbaumer K, et al. Prognostic value of admission C-reactive protein in stroke patients undergoing iv thrombolysis. J Neurol. 2008;255(8):1190–1196. doi:10.1007/s00415-008-0866-y
17. Chinese Society of Neurology, Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chine j Neurol. 2018. doi:10.3760/cma.j.issn.1006-7876.2018.09.004
18. Zhang K, Luan J, Li C, et al. Nomogram to predict hemorrhagic transformation for acute ischemic stroke in Western China: a retrospective analysis. BMC Neurol. 2022;22(1):156. doi:10.1186/s12883-022-02678-2
19. Zhang WB, Zeng YY, Wang F, et al. A high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. 2020;12(3):2428–2439. doi:10.18632/aging.102752
20. Kaesmacher J, Abdullayev N, Maamari B, et al. Safety and Angiographic Efficacy of Intra-Arterial Fibrinolytics as Adjunct to Mechanical Thrombectomy: results from the INFINITY Registry. J Stroke. 2021;23(1):91–102. doi:10.5853/jos.2020.01788
21. Tokgoz S, Keskin S, Kayrak M, et al. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis. 2014;23(8):2163–2168. doi:10.1016/j.jstrokecerebrovasdis.2014.04.007
22. Lattanzi S, Cagnetti C, Provinciali L, et al. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8(34):57489–57494. doi:10.18632/oncotarget.15423
23. Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129(2):259–277. doi:10.1007/s00401-014-1355-2
24. Neumann J, Sauerzweig S, Rönicke R, et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28(23):5965–5975. doi:10.1523/JNEUROSCI.0060-08.2008
25. Rørvig S, Honore C, Larsson LI, et al. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J Leukoc Biol. 2009;86(6):1439–1449. doi:10.1189/jlb.1008606
26. Jickling GC, Liu D, Stamova B, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34(2):185–199. doi:10.1038/jcbfm.2013.203
27. Rosell A, Cuadrado E, Ortega-Aznar A, et al. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39(4):1121–1126. doi:10.1161/STROKEAHA.107.500868
28. Selvaraj UM, Ujas TA, Kong X, et al. Delayed diapedesis of CD8 T cells contributes to long-term pathology after ischemic stroke in male mice. Brain Behav Immun. 2021;95:502–513. doi:10.1016/j.bbi.2021.05.001
29. Ito M, Komai K, Mise-Omata S, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565(7738):246–250. doi:10.1038/s41586-018-0824-5
30. Ahnstedt H, Patrizz A, Chauhan A, et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav Immun. 2020;87:556–567. doi:10.1016/j.bbi.2020.02.001
31. Fury W, Park KW, Wu Z, et al. Sustained Increases in Immune Transcripts and Immune Cell Trafficking During the Recovery of Experimental Brain Ischemia. Stroke. 2020;51(8):2514–2525. doi:10.1161/STROKEAHA.120.029440
32. Kim E, Cho S. CNS and peripheral immunity in cerebral ischemia: partition and interaction. Exp Neurol. 2021;335:113508. doi:10.1016/j.expneurol.2020.113508
33. Li S, Huang Y, Liu Y, et al. Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J Cereb Blood Flow Metab. 2021;41(9):2280–2294. doi:10.1177/0271678X21995694
34. Xiong X, Barreto GE, Xu L, et al. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42(7):2026–2032. doi:10.1161/STROKEAHA.110.593772
35. Singh HV, Pandey A, Shrivastava AK, et al. Prognostic value of neuron specific enolase and IL-10 in ischemic stroke and its correlation with degree of neurological deficit. Clin Chim Acta. 2013;419:136–138. doi:10.1016/j.cca.2013.02.014
36. Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88(6):2039–2046. doi:10.1172/JCI115532
37. Urra X, Villamor N, Amaro S, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29(5):994–1002. doi:10.1038/jcbfm.2009.25
38. Verma S, Yeh ET. C-reactive protein and atherothrombosis--beyond a biomarker: an actual partaker of lesion formation. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1253. doi:10.1152/ajpregu.00170.2003
39. Pedersen ED, Waje-Andreassen U, Vedeler CA, et al. Systemic complement activation following human acute ischaemic stroke. Clin Exp Immunol. 2004;137(1):117–122. doi:10.1111/j.1365-2249.2004.02489.x
40. Matsuo R, Ago T, Hata J, et al. Plasma C-Reactive Protein and Clinical Outcomes after Acute Ischemic Stroke: a Prospective Observational Study. PLoS One. 2016;11(6):e0156790. doi:10.1371/journal.pone.0156790
41. Wang L, Li Y, Wang C, et al. C-reactive Protein, Infection, and Outcome After Acute Ischemic Stroke: a Registry and Systematic Review. Curr Neurovasc Res. 2019;16(5):405–415. doi:10.2174/1567202616666191026122011
42. Okita R, Kawamoto N, Okada M, et al. Preoperative neutrophil-to-lymphocyte ratio correlates with PD-L1 expression in immune cells of patients with malignant pleural mesothelioma and predicts prognosis. Sci Rep. 2023;13(1):5263. doi:10.1038/s41598-023-31448-4
43. Yang F, Zhang L, Huang W, et al. Clinical prognostic impact of C-NLR in heart failure patients with different ejection fractions: a retrospective study. BMC Cardiovasc Disord. 2024;24(1):54. doi:10.1186/s12872-024-03714-4
44. Taniai T, Haruki K, Furukawa K, et al. The novel index using preoperative C-reactive protein and neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with pancreatic cancer. Int J Clin Oncol. 2021;26(10):1922–1928. doi:10.1007/s10147-021-01964-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


