Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Correlation of Body Mass Index and Oxygen Saturation in Chronic Obstructive Pulmonary Disease Patients at a Tertiary Care Center in Nepal: A Cross-Sectional Study
Authors Sangroula P, Ghimire S , Srivastava B , Adhikari D , Dhonju K , Shrestha A , Ghimire S
Received 20 March 2023
Accepted for publication 3 July 2023
Published 11 July 2023 Volume 2023:18 Pages 1413—1418
DOI https://doi.org/10.2147/COPD.S412118
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Prativa Sangroula,1,* Sandip Ghimire,1,* Brajendra Srivastava,2 Divas Adhikari,3 Kiran Dhonju,4 Amit Shrestha,5 Sapana Ghimire6
1Department of Internal Medicine, Lumbini Medical College and Teaching Hospital, Palpa, Lumbini, Nepal; 2Department of Internal Medicine, Nepalese Army Institute of Health Sciences, Kathmandu, Bagmati, Nepal; 3Department of Emergency Medicine, Bharatpur Hospital, Chitwan, Bagmati, Nepal; 4Department of Internal Medicine, Sukraraj Tropical and Infectious Disease Hospital, Kathmandu, Bagmati, Nepal; 5Department of Psychiatry, Patan Academy of Health Sciences, Lalitpur, Bagmati, Nepal; 6Department of Pathology, Shahid Dharma Bhakta National Transplant Centre, Bhaktapur, Bagmati, Nepal
*These authors contributed equally to this work
Correspondence: Sandip Ghimire, Department of Internal Medicine, Lumbini Medical College and Teaching Hospital, Palpa, Lumbini, Nepal, Tel +9609369402 ; +9779851119799, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) is a major cause of mortality and morbidity worldwide. COPD is associated with clinically relevant pulmonary and extrapulmonary manifestations, including hypoxemia and weight loss. The correlation of body mass index (BMI) and oxygen saturation (SpO2) with COPD grades may provide a useful additional marker for understanding and managing the disease. The aim of the study was to study the correlation of BMI and oxygen saturation with COPD in patients presenting to a tertiary care center in Nepal.
Patients and Methods: A descriptive cross-sectional study was conducted among 145 COPD patients visiting the Department of Medicine in Shree Birendra Hospital between 1 March 2019 and 28 February 2020. A non-probability purposive sampling method was used and data were analyzed using SPSS version 21. A p-value of < 0.05 was considered significant.
Results: Out of 145 COPD patients, 58 (40%) were underweight, 53 (36.55%) were of normal weight, 20 (13.79%) were overweight, and 14 (9.6%) were obese. The number of underweight patients was highest in COPD grade 4 and lowest in COPD grade 1. The proportion of subjects with underweight BMI and hypoxia increased with COPD severity, and both were statistically significant (p-values < 0.01).
Conclusion: Our study shows that BMI and oxygen saturation have an inverse association with COPD severity. The correlation of BMI and oxygen saturation with COPD grade could provide a supplementary marker of disease severity, which could be useful in the understanding of the disease process and subsequent management of COPD.
Keywords: body mass index, chronic obstructive pulmonary disease, oxygen saturation, chronic obstructive airway disease
Introduction
In the twenty-first century, chronic obstructive pulmonary disease (COPD) is recognized as a disease of global public health concern. From 1990 to 2015, mortality due to COPD increased by almost 11% and the prevalence of the disease increased by 44%.1 Airflow limitation due to loss of lung elastic recoil and/or airway narrowing is characteristic of COPD.2 COPD affects the lungs and also has significant systemic consequences.3 COPD patients with low oxygen saturation (SpO2) have a greater chance of having the worst degree of breathlessness, with poor quality of life, reduced exercise tolerance, increased risk of cardiovascular morbidity, and greater risk of death.4,5 Low body weight in COPD patients is associated with increased mortality, independently of lung function.6
This study was carried out to identify the association of the severity of COPD with SpO2 and body mass index (BMI) in the Nepalese population. The correlation of BMI and oxygen saturation with COPD grades could provide a supplementary marker of disease severity which could be useful in the understanding and management of COPD.
Materials and Methods
A descriptive cross-sectional study was conducted among COPD patients visiting the Department of Medicine in Shree Birendra Hospital between 1 March 2019 and 28 February 2020. Ethical approval for the study was granted by the Institutional Review Committee, Nepalese Army Institute of Health Sciences (NAIHS-IRC). This study was conducted in compliance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all the participants and the confidentiality of the information was ensured. All adult patients of age 18 years and above with COPD, using inhaled bronchodilators or steroids, and giving written consent, were enrolled in the study. Patients with acute exacerbation of COPD within the last 2 weeks before enrollment, taking oral steroid treatment, or receiving domiciliary oxygen therapy, and patients with active pulmonary tuberculosis, heart failure, or malignancies, were excluded from the study. A non-probability purposive method of sampling was used for the collection of data. The sample size was calculated on the basis of the prevalence of COPD globally, 8.4–15%, as reported by Adeloye et al.7 Taking a prevalence of 10%, with a confidence interval of 95% and allowable error of 5%, the minimum sample size was calculated as 144.
Data were collected on a self-designed semi-structured pro forma, which included questions related to demographic variables and relevant clinical history. Following an interview by the researcher, participants underwent anthropometric measurements and spirometry. Each patient’s weight was taken using a platform weighing scale. Standing height was measured with the participants barefoot, eyes looking ahead, and standing straight with the heels, buttocks, and occiput touching the wall. The BMI was computed by dividing body weight (kilograms) by the square of the height in meters (m2). The BMI was categorized according to the new classification for Asians (Table 1).8
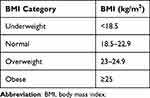 |
Table 1 BMI Classification for Asians |
Spirometry was performed using the Medical International Research Spirolab 4 Portable, Desktop, and PC-Based Spirometer. Prior to performing spirometry, patients were instructed not to take any inhalational bronchodilators on the day of spirometry. Post-bronchodilator spirometry was used to diagnose and grade COPD patients according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (Table 2).9 Oxygen saturation was measured using a pulse oximeter.
 |
Table 2 GOLD Criteria for Severity of Airflow Obstruction in COPD |
Data were entered into SPSS for Windows version 21 for statistical analysis. Descriptive statistics were calculated using the frequency and percentage for qualitative variables, and the mean and standard deviation for quantitative variables. The chi-squared (χ2) test was used to compare categorical variables. A 95% confidence interval was used in this study and a p-value of <0.05 was considered to be statistically significant.
Results
A total of 145 patients with COPD were enrolled in the study, of whom 81 (55.9%) were male and 64 (44.1%) were female. The mean age of the participants was 62.74±8.04 years. The mean BMI was 20.42±3.50 kg/m2. There were 20 (13.79%), 44 (30.34%), 47 (32.41%), and 34 (23.45%) participants with COPD grade 1, grade 2, grade 3, and grade 4, respectively.
Among the 145 patients, the mean SpO2 was found to be 95.85±1.18%, 92.32±1.84%, 88.17±1.48%, and 84.50±1.66% in those with COPD grade 1, grade 2, grade 3, and grade 4, respectively. There was a statistically significant difference in the mean SpO2 among the different COPD grades (p<0.01). Similarly, there was a significant decrease in SpO2 with the increase in COPD grade (Table 3).
 |
Table 3 Correlation of COPD Grades and Mean Oxygen Saturation |
Out of 145 COPD patients, 58 (40%) were underweight, 53 (36.55%) were of normal weight, 20 (13.79%) were overweight, and 14 (9.6%) were obese. The mean BMI of participants with grade 1 COPD was 24.50±3.82 kg/m2, grade 2 COPD was 21.69±2.84 kg/m2, grade 3 COPD was 19.33±2.76 kg/m2, and grade 4 COPD was 17.90±1.86 kg/m2. On comparing the mean BMI of the participants with the grade of COPD, a statistically significant difference in the mean BMI was found among the various grades of COPD (p<0.01). The mean BMI decreased as the severity of the COPD increased (Table 4).
 |
Table 4 Comparison Between COPD Grades and Mean BMI (kg/m2) |
Discussion
In our study, the increase in COPD severity was found to be associated with the statistically significant decrease in the mean oxygen saturation (p<0.01). This finding was consistent with the results of studies by Gupta et al and Kumar et al, in which the mean SpO2 progressively decreased with the increase in the severity of COPD.8,10 A possible explanation for reduced SpO2 with increased severity of COPD is the progressive increase in ventilation/perfusion (V/Q) mismatch as the disease progresses.5,11 Altered ventilatory control is another important factor responsible for the occurrence of hypoxemia in COPD patients.12,13 Similarly, with disease progression there is a deterioration of pulmonary function and this increases the risk of alveolar hypoxia and hypoxemia, leading to a decrease in SpO2.14 Hypoxemia and the decrease in SpO2 with progression of COPD severity are important findings because they lead to poor quality of life, reduced exercise tolerance, poor neurocognitive function, and increased risk of exacerbation and death.15,16 Hence, as there is an inverse relationship between COPD stage and oxygen saturation, SpO2 could be used as a marker of COPD severity, especially in resource-limited settings where pulmonary function tests are not readily available.8
In our study, 40% of the total patients were underweight according to the BMI criteria, and the number of underweight patients was highest in COPD grade 4 and lowest in COPD grade 1. A similar result was found in the study conducted by Schols et al,17 in which approximately 50% of patients with severe COPD suffered from weight loss. Similarly, a study performed in India by Gupta et al8 showed that patients with grade 1 COPD were less undernourished (25%) than those with grade 4 COPD (80%), which is consistent with the results of our study. However, in a study by Cochrane and Afolabi18 on 103 COPD patients in the UK, only 23% of COPD patients were malnourished, unlike in our study where the prevalence of underweight patients was 40%. In our study, the percentage of underweight subjects was high in comparison to the study by Cochrane and Afolabi,18 probably because of differences in geographical distribution and dietary intake patterns between the two countries, and also because of low socioeconomic status, and late disease identification and treatment in our setting.8,10 Hence, there is a need for nutritional counseling in patients with severe COPD, as low BMI is possibly an important and independent risk factor for mortality and morbidity in subjects with severe COPD.19
Our study also revealed that, with increasing COPD grade, the proportion of subjects with underweight BMI status increased significantly (p<0.01). This finding was similar to that of a study conducted by Steuten et al20 in the Netherlands, in which the prevalence of low body weight was strongly increased in patients with COPD GOLD grade 4. Similarly, Montes de Oca et al21 and Yang et al22 found that patients with higher BMI had COPD of lower grade, and predicted that low BMI could be an indicator of mortality among COPD patients.
Malnutrition, reflected as underweight BMI, in COPD patients is related to the imbalance between energy intake and energy expenditure.8,10,23,24 COPD patients have symptoms such as postprandial dyspnea, early satiety, fatigue, and loss of appetite, leading to reduced intake of food.25–27 Meanwhile, there is increased energy expenditure because of the increased work of breathing, thermogenic effects of bronchodilators, and systemic inflammation.24,25 In addition, loss of body muscle mass in COPD can also be due to the adverse effects of corticosteroids on skeletal muscle function.28 At the microscopic level, the increase in severity of COPD is associated with muscle fiber atrophy, muscle fiber alterations, and loss of mitochondrial function.28,29 These findings suggest that COPD impairs metabolic function at the cellular level, resulting in weight loss.29 This weight loss, leading to low BMI, correlates with mortality in COPD patients.22,30,31
Our study has some limitations. The study was conducted on a sample of modest size, so the effects of modest sample size should be considered while interpreting the results. In addition, as BMI may not be an ideal tool to measure the lean body mass of COPD patients who have edema due to cor-pulmonale and pulmonary hypertension, the results should be interpreted cautiously in this group of patients. Also, the presence of pulmonary hypertension can hinder the use of SpO2 as the sole indicator for assessing COPD severity. Moreover, the possibility of selection bias needs to be considered when interpreting the results, as a non-probability purposive sampling method was used in our study.
Conclusion
Our study suggests that BMI and SpO2 have an inverse association with COPD severity. Hence, BMI and oxygen saturation, in addition to COPD grades, could be used as markers of disease severity, and could add a new dimension in our understanding of the disease process and subsequent management of COPD.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sutradhar I, Das Gupta R, Hasan M, Wazib A, Sarker M. Prevalence and risk factors of chronic obstructive pulmonary disease in Bangladesh: a systematic review. Cureus. 2019;11(1):e3970–e3970.
2. Ogawa E, Nakano Y, Ohara T, et al. Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64(1):20–25.
3. Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932.
4. Enocson A, Jordan R, Adab P, Dickens A, Fitzmaurice D. Prevalence and characteristics of low oxygen saturation (SpO2) in a primary care COPD cohort. Eur Respir J. 2016;48:PA3937.
5. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208.
6. Mador MJ. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):787–789.
7. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415.
8. Gupta SS, Gothi D, Narula G, Sircar J. Correlation of BMI and oxygen saturation in stable COPD in Northern India. Lung India. 2014;31(1):29–34.
9. Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2023;207(7):819–837.
10. Kumar DTP, Ramulu G, KrishnaMurthy M, Vikas DM.Correlation of BMI, Oxygen Saturation and CRP Levels in COPD Patients. Sch J App Med Sci. 2017;5(10F):4257–4266.
11. Sandek K, Bratel T, Hellström G, Lagerstrand L. Ventilation-perfusion inequality and carbon dioxide sensitivity in hypoxaemic chronic obstructive pulmonary disease (COPD) and effects of 6 months of long-term oxygen treatment (LTOT). Clinical Physiology. 2001;21(5):584–593.
12. Flenley DC, Franklin DH, Millar JS. The hypoxic drive to breathing in chronic bronchitis and emphysema. Clin Sci. 1970;38(4):503–518.
13. Bradley CA, Fleetham JA, Anthonisen NR. Ventilatory control in patients with hypoxemia due to obstructive lung disease. Am Rev Respir Dis. 1979;120(1):21–30.
14. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555.
15. Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):513–518.
16. Faganello MM, Tanni SE, Sanchez FF, Pelegrino NR, Lucheta PA, Godoy I. BODE index and GOLD staging as predictors of 1-year exacerbation risk in chronic obstructive pulmonary disease. Am J Med Sci. 2010;339(1):10–14.
17. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–1156.
18. Cochrane WJ, Afolabi OA. Investigation into the nutritional status, dietary intake and smoking habits of patients with chronic obstructive pulmonary disease. J Hum Nutr Diet. 2004;17(1):3–11; quiz 13–15.
19. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861.
20. Steuten LM, Creutzberg EC, Vrijhoef HJ, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91.
21. Montes de Oca M, Tálamo C, Perez-Padilla R, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–650.
22. Yang L, Zhou M, Smith M, et al. Body mass index and chronic obstructive pulmonary disease-related mortality: a nationally representative prospective study of 220,000 men in China. Int J Epidemiol. 2010;39(4):1027–1036.
23. Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501.
24. Agustí AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360.
25. Sergi G, Coin A, Marin S, et al. Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(11):1918–1924.
26. Rawal G, Yadav S. Nutrition in chronic obstructive pulmonary disease: a review. J Transl Int Med. 2015;3(4):151–154.
27. Grönberg AM, Slinde F, Engström CP, Hulthén L, Larsson S. Dietary problems in patients with severe chronic obstructive pulmonary disease. J Hum Nutr Diet. 2005;18(6):445–452.
28. Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(4):637–658.
29. Rabinovich RA, Bastos R, Ardite E, et al. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J. 2007;29(4):643–650.
30. Guo Y, Zhang T, Wang Z, et al. Body mass index and mortality in chronic obstructive pulmonary disease: a dose-response meta-analysis. Medicine. 2016;95(28):e4225.
31. Ringbaekl TJ, Viskuml K, Lange P. BMI and oral glucocorticoids as predictors of prognosis in COPD patients on long-term oxygen therapy. Chron Respir Dis. 2004;1(2):71–78.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
