Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
Correlation of acidosis-adjusted potassium level and cardiovascular outcomes in diabetic ketoacidosis: a systematic review
Authors Usman A , Makmor Bakry M , Mustafa N , Rehman IU, Bukhsh A , Lee SWH , Khan TM
Received 12 March 2019
Accepted for publication 5 June 2019
Published 6 August 2019 Volume 2019:12 Pages 1323—1338
DOI https://doi.org/10.2147/DMSO.S208492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Atif Usman,1 Mohd Makmor Bakry,2 Norlaila Mustafa,3 Inayat Ur Rehman,1,4 Allah Bukhsh,1,5 Shaun Wen Huey Lee,1 Tahir Mehmood Khan1,5,6
1School of Pharmacy, Monash University, Bandar Sunway, Selangor, Malaysia; 2Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia; 3Department of Endocrinology, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia; 4Department of Pharmacy, Abdul Wali Khan University, Mardan, Pakistan; 5Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan; 6Asian Centre for Evidence Synthesis in Population, Implementation and Clinical Outcomes, Health and Well-being Cluster, Global Asia in the 21st Century Platform, Monash University Malaysia, Selangor, Malaysia
Background: During the progress and resolution of a diabetic ketoacidosis (DKA) episode, potassium levels are significantly affected by the extent of acidosis. However, none of the current guidelines take into account acidosis during resuscitation of potassium level in DKA management, which may increase the risk of cardiovascular adverse events.
Objective: To assess literature regarding the adjustment of potassium level using pH to calculate pH-adjusted corrected potassium level, and to observe the relationship of cardiovascular outcomes with reported potassium level and pH-adjusted corrected potassium in DKA.
Methodology: Seven databases were searched from inception to January 2018 for studies which had reported people with diabetes developing diabetic ketoacidosis, in relation to prevalence or incidence, fluid resuscitation or potassium supplementation treatment, treatment or cardiovascular outcomes, and experimentation with DKA management or insulin. Quality of studies was evaluated using Cochrane Risk of Bias and Newcastle Ottawa Scale.
Results: Forty-seven studies were included in qualitative synthesis out of a total of 10,292 retrieved studies. Forty-one studies discussed the potassium level and blood pH at the time of admission, ten studies discussed cardiovascular outcomes, and only four studies concurrently discussed potassium level, pH, and cardiovascular outcomes. Only two studies were graded as good on the Newcastle Ottawa Scale. The reported potassium level was well within normal range (5.8 mmol/L), whereas pH rendered patients to be moderately acidotic (7.13). Surprisingly, none of the included studies mentioned pH-adjusted corrected potassium level and, hence, this was calculated later. Although mean corrected potassium was within the normal range (3.56 mmol/L), 13 studies had corrected potassium below 3.5 mmol/L and five had it below 3.0 mmol/L. Nevertheless, with the exception of one study, none discussed cardiovascular outcomes in the context of potassium or pH-adjusted potassium level.
Conclusion: The evidence surrounding cardiovascular outcomes during DKA episodes in light of a pH-adjusted corrected potassium level is scarce. A prospective observational, or preferably, an experimental study in this regard will ensure we can modify and enhance safety of existing DKA treatment protocols.
Keywords: diabetic ketoacidosis, potassium, hypokalemia, blood gasses, acidosis, pH, treatment outcomes, cardiovascular, insulin
Background
Diabetic ketoacidosis
Diabetic ketoacidosis is a prevalent acute hyperglycemic complication of diabetes mellitus.1,2 It is a severe and life threatening complication, and requires immediate therapeutic interventions which may otherwise lead to a fatal outcome.3 The most common causes of any DKA episode are poor compliance, infection, and physical stress, ie, cardiovascular (CV) disorder.2,3 Initial insulin deficiency causes hyperglycemia, ketonemia, and acidosis, all of which promote diuresis. This causes notable hypovolemia and loss of electrolytes. In response to the metabolic and hypovolemic stress, the concentration of counter regulatory hormones (CRH), ie, catecholamines, increases significantly. Increase in CRH further exacerbates circulatory distress.4 Metabolic, hormonal, and circulatory impairment in DKA requires swift action. In this regard, recommended treatment of DKA encompass of correction of blood volume, achieving euglycemia, and restoration of normal pH utilizing intravenous fluid resuscitation, insulin, and bicarbonate buffer, respectively.4–7
Potassium in diabetic ketoacidosis
Hypokalemia is a frequently observed complication in DKA. The role of potassium during an episode of DKA is very crucial where the abovementioned factors have an arguable influence on its regulation.8–10 Initially, DKA patients experience hyperkalemia; insulin deficiency renders cellular inability to allow potassium re-entry into the cell, and catecholamines induced cellular insensitivity hinders cellular uptake of potassium.11 Upon further progress, acidosis causes desensitization of cognate receptors of insulin and catecholamines, resulting in exacerbating hyperkalemia.12 Nevertheless, hyperkalemia swiftly changes to hypokalemia. Primary renal clearance of excessive glucose, and secondary renal clearance of ketone bodies cause polyurea and hypovolemia. In order to maintain homeostasis, the kidneys retain sodium and bicarbonate at the expense of potassium excretion.10,13 Diuresis induced by hyperglycemia and ketosis alone depletes as much as 3–15 mmol/kg of potassium.14,15 Additionally, hyperkalemia-induced vomiting in DKA causes a loss of potassium too.16
Potassium in cardiovascular outcomes
Cardiovascular disorders are also among precipitating factors of DKA whereby they cause physical stress in DM patients.17 Among CV disorders, myocardial infarction (MI) is the most observed cardiac incident which triggers an episode of DKA.1–3,18 Moreover, the concentration of CRH increases significantly during acidosis, which further increases the risk of adverse CV outcome in DKA patients. Although an episode of MI may not be directly associated with potassium level, potassium remains an important repolarization electrolyte in the cardiac cycle. At any point of an episode of acute MI, the level of potassium is often considered crucial as the patient may also experience life threatening arrhythmia secondary to catecholamine driven influx of potassium ion.19,20 Physiologic effects of hypokalemia involving pulse induction are altered primarily due to changes in refractory periods of myocardia, and result in the form of premature ventricular contractions, fibrillation, and tachycardia. These effects are also visible when considering an electrocardiograph (ECG) in DKA patients; a relative flat ST with a prominent U wave fusing with a T wave is observed among patients with DKA experiencing hypokalemia.21,22 This leads to the notion that ECG and CVS stability shall also be considered as prime objectives when treating a DKA patient.
Rationale and objectives
The consequences of hypokalemia accompanied by acidosis are relatively deleterious when physiologic cardiac conduction is hindered.19,23 Moreover, deterioration of a patient’s condition may be observed when pH impairs the regular function of insulin and catecholamines. On the other hand, besides that acidosis is a major factor for hypokalemia in DKA, it also has CVS suppressive effects.11 Hydrogen ions reduce cardiac output and peripheral vasodilation, hinder inotropic effect, and cause bradycardia. Additionally, the association of outcomes of DKA patients with elevated cardiac troponin I, although not extensive, somehow depicts that CVS does undergo enormous stress during a DKA episode.24 All these effects of DKA on CVS encourage us to signify the effect of potassium on CV outcomes in light of acidosis in DKA patients. Hence, this systematic review aims to explore the difference between reported potassium level and pH-adjusted corrected potassium level among the DKA patients. Second, it also focuses on reports of CV complications among DKA patients and its correlation with admission and pH-adjusted corrected potassium level.
Methodology
A systematic review of the published literature was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.25 Seven databases – EBSCOhost, PubMed, Scopus, Ovid databases (Cochrane, Embase, and MEDLINE), and clinical trial registries – were searched from the date of inception until January 30, 2018. Main MeSH terms for DKA, adapted form Andrade-Castellanos CA et al,26 were as follows: “Diabetic ketoacidosis”, “Diabetic Coma”, and (Hyperglycaemic OR Hyperglycemic OR Diabet* AND (Keto* OR acidos* OR coma) OR Emergen*) OR DKA. Similarly, MeSH terms related to treatment, treatment modalities, the outcome of treatment, and potassium were adapted from Tran et al.27 Finally, terms for insulin and cardiovascular were added to conclude the search. All the terms were connected with relevant key words of varying management aspects of DKA (Supplement). Boolean operators were used to combine text terms, keywords, and MeSH terms. This was further supplemented by a backward and forward manual search of relevant references. Once finalized, search terms were evaluated by TMK, NM, IR, and AB.
Registration of study protocol
The protocol was registered with PROSPERO (Registration Number: CRD42018098772).
Study selection
An article was included if it met the following criteria: 1) the study reported the prevalence of DKA in adult diabetic patients and assessed admission potassium level and pH; 2) the study qualitatively observed cardiovascular outcomes in DKA patients; 3) the design of the study was a cross-sectional or cohort, or randomized controlled trial. Studies were excluded if 1) it was a case-control, case report, conference proceeding, systematic review, letter to editor, an opinion, or research brief; 2) published in languages other than English; 3) reported DKA secondary to pregnancy or among pediatrics, or 4) studies evaluating therapeutic intervention which includes DKA secondary to sodium-glucose co-transporter 2 inhibitor.
Outcomes of interest
Primary outcome
Level of serum potassium, and pH-adjusted corrected level of potassium at the time of admission.
Secondary outcome
Specifically, the CV outcomes were noted in relation to the level of potassium at the time of occurrence of CV event and included ECG, report of fibrillation, tachycardia or bradycardia, central venous pressure, cardiac arrest, myocardial infarction, and troponin. Moreover, reports of CV outcomes or signs and symptoms were recorded if the CV outcome was reported as the reason for fatality. It was further noted if any relationship was given by the authors between hypokalemia and the reason for such CV outcome.
Data extraction and synthesis
All references retrieved were initially grouped under the respective search engine. Duplicates were removed, and titles were screened for eligibility. After removal of irrelevant studies, regrouping was done according to the nature of the study, ie, case series, clinical trial, guideline, etc. Relevant demographic, scientific, and clinical information was recorded in a separate data extraction sheet (Supplement). Later, the level of corrected potassium was calculated by subtracting 0.6 mmol/L from the reported potassium level against each 0.1 decreases in arterial pH from 7.4.15
Two of the authors (AU and TMK) independently reviewed the titles and abstracts of all identified studies to determine the articles that were suitable for further consideration. Disagreements were resolved by discussion among AU and TMK. A standardized form was used to extract the data from selected studies which included authors, year of publication, country of conduct of the study, region of the country, study objectives, sample size, the duration for study, study design and nature, and level of reported potassium and pH (Supplement). The extracted data was subsequently reviewed independently by IR and AB, whereas verification of extraction was done by TMK, NM, and MMB. Disagreements encountered in data synthesis, if any, were resolved by discussion between AU and TMK, and IR and AB. The value of the pH-adjusted corrected potassium level was later added to the respective study.
Quality assessment
The quality of randomized clinical trials was assessed using the Cochrane risk of bias tool,28 while observational studies were assessed by using the Newcastle Ottawa Scale for cross-sectional and cohort studies.29,30 For Cochrane risk of bias, bias that was likely to affect the results seriously was graded as high risk, bias that was unlikely to affect results seriously was graded as low risk, and bias that was likely to raise doubts regarding results was graded as unclear bias.28
The Newcastle Ottawa scale for cross-sectional studies was scored as: “selection (up to 4 points), comparability (up to 2 points), and outcome (up to 3 points)”.29 A similar standard was used for the Newcastle Ottawa scale for cohort studies as well.30 Overall, the study quality was labeled as poor (score ≤3), fair (score 4–6), and good (score ≥7). Quality assessment was solely done by AU and independently reviewed by IR, AB, and TMK. Lastly, TMK and SLWH resolved disagreements from quality assessment through discussion.
Results
Study selection
The search identified 5,083 studies after removing duplicates (5,229) and title screening (2,968) (Figure 1). Of these, 2,115 studies were selected for abstract screening. A total of 2,039 studies were excluded as they did not have relevant data related to DKA (502), were reviews (440), were published in a language other than English (205), or either had a patient pool of pregnant or pediatric patients (228). The review is concluded on 47 studies.9,16,21,31–74
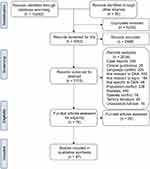 |
Figure 1 PRISMA flow chart of inclusion and exclusion of studies in systematic review.Note: Adapted from PRISMA.25Abbreviation: DKA, diabetic ketoacidosis. |
Characteristics of included full-text retrievals
Of the total 47 research articles, 249,21,33,39,45,47–49,51–53,56,58–60,62–64,68–71,73,74 were cross-sectional case series mainly describing retrospective data on either prevalence or simple characteristics of DKA patients upon admission (Table 1). Two of the studies38,65 were cohorts of DKA patients further divided on the basis of treatment intervention given at a given time and retrospectively evaluated for the efficacy or safety of the said treatment intervention. Seven studies37,40,41,43,55,57,67 were clinical trials that prospectively measured such effects; however, only five of these studies40,43,55,57,67 were randomized control trials. Only 37 of the studies concurrently reported the value of pH and potassium,16,34,35,37,40–44,46–50,52–74 six provided only CV outcomes,9,31–33,36,45 and only four21,38,39,51 discussed potassium, pH, and CV outcomes concurrently (Table 1).
 |  |  |  |
Table 1 Basic characteristics of included studies |
 |
Figure 2 Cochrane Risk of Bias for included clinical trials. |
Quality of the included studies
In terms of quality of the studies, 22 studies31–39,41,42,44,46,49,52–54,56,58,59,61,71 scored poor, 18 studies9,16,21,45,47,48,50,51,60,62,63,66,68–70,72–74 scored fair, and two studies64,65 scored good on NOS. Among studies scoring 5, four 43,55,62,72 were scored on the basis of comparability by controlling confounding factors between the groups as well. Similarly, of the studies that scored 3 and 4 on NOS, three studies33,58,61 from score 3, and two studies47,57 from score 4 were also scored for controlling confounding factors between the groups (Table 1 footnote). Of four studies that established comparability between respondents and non-respondents, one each scored 440 and 657 on NOS and two scored 7.64,67 Notably, there was only one study that defined the sample size in terms of calculating on the basis of prevalence of DKA.69
The Cochrane Risk of Bias tool was used to assess five studies.40,43,55,57,67 None of the studies was completely blinded for participants (Figure 2). In the context of blinding of outcomes, only two of the studies55,67 predefined outcomes for assessment of endpoints. Among these studies, the least reporting bias was observed in the study by Ersöz et al,55 while the rest of the studies were found with a moderate risk of bias.40,43,57,67
Data analysis: potassium, pH, and pH-adjusted corrected potassium
Concurrently, the level of potassium and pH was only available in 41 studies16,21,34,35,37–44,46–74 (Table 2). The least actual value of potassium was noted at 3.4 mmol/L,56 while the maximum was 5.8 mmol/L.43 On average, the potassium level was 5.8 mmol/L. In a similar context, the least observed pH was 6.9341 and the highest 7.28,44 with an average pH being 7.13. The level of potassium after adjusting against pH ranged from 2.4–4.34 (Table 2), with an average of 3.56 mmol/L. The corresponding values for actual potassium level and pH of mentioned values was 3.4 mmol/L with a pH of 7.19,56 and 5.6 with a pH of 7.14.40 Thirteen included studies38,39,41,42,51,53,60,64,65,67,68,70 calculated the value of potassium to be equal or below 3.5 mmol/L when the patients sought medical advice; out of these, only one study56 reported the average actual potassium level to be lower than 3.5 mmol/L. Upon distributing the countries on the basis of their region, it was notable that the least potassium level was seen in mid-Eastern countries (4.4 mmol/L); the minimum pH was observed in the far-Western region (7.11); and the maximum number of patient data originated from mid-Western countries (patient number = 1,995). Nevertheless, a meta-analysis was not performed as quantitative data was not sufficient for further analysis.
 |
Table 2 Measure and pH adjusted potassium level in the included studies grouped on the basis of region |
Cardiovascular outcomes
Of a total of 10 studies9,31–33,36,38,39,45,51 discussing CV outcomes, only four21,38,39,51 reported potassium and pH, and discussed CV outcomes concurrently (Table 3). Two31,45 of the six remaining studies neither provided pH nor potassium level in the report. The maximum number of patients reported with CV disorder in any single study was 17, where abnormal ECG was mentioned with recorded CV abnormality.32 In the context of insulin, one death was reported secondary to an increase in insulin.36 Interestingly, one study9 evaluated the extent of hypokalemia in DKA and reported three deaths to myocardial infarction (MI); however, none of the deaths were attributed to hypokalemia. Only one study33 mentioned the difference between the potassium level of alive and deceased DKA patients.
 |
Table 3 Cardiovascular observations reported in the studies fulfilling secondary objective |
Discussion
This is the first systematic review which aims to explore CV events in patients of DKA in correlation with admission serum potassium vs pH-adjusted corrected potassium level. Moreover, it is also an attempt to signify the importance of pH-adjusted corrected potassium level in the diagnosis and management of DKA episode when weighed against the risk of CV events during an episode. In this regard, 45 studies reported admission potassium level of DKA patients and the pH-adjusted corrected potassium level was calculable for 41 studies only. However, none of the studies reported a pH-adjusted corrected potassium level and, hence, it was self-calculated to find the difference between the two values. Similarly, as for secondary outcome, 10 studies focused on the secondary outcome of a CV event in DKA, while none of the studies correlated CV outcomes with either reported or pH-adjusted corrected potassium level.
The reported admission potassium level in current review was 4.91 mmol/L. Contrary to significant loss of potassium, DKA patients usually present with normal or increased potassium level, which is an established phenomenon and, hence, the observed value seems to be well within the normal range.9,15 Moreover, most of the clinical guidelines do not suggest that potassium supplementation shall be started once the value of serum potassium is below 4.5 mmol/L.4–7 Similarly, the mean pH-adjusted corrected potassium level observed in the current review was within the normal range of potassium level as well (3.56 mmol/L), although this value is borderline-low. However, such normo- or hyper-kalemia was deceiving in nature as there were 13 studies38,39,41,42,51,53,56,60,64,65,67,68,70 which had a corrected potassium level below 3.5 mmol/L. Further findings of the current review were in line with an aforementioned hypothesis where, in five of the studies,38,41,56,60,64 pH-adjusted corrected potassium was as low to produce an adverse CV outcome resultant of hypokalemia.11,15 When these studies were analyzed for any reported CV event in correlation of potassium, only three of the studies38,39,51 reported such events; the results were, however, not in specificity to hypokalemia. The incidence of hypokalemia was very frequent in the study reported by Keller et al,38 where 23% of DKA cases experienced a low potassium level. However, it is not mentioned whether the extent of hypokalemic episodes was associated with any of the four cases of early CV adverse outcomes or not; the authors also confirmed that these fatalities were established cases of chronic CV disease. In another study with below-normal corrected potassium level, Asplin et al did not provide any hint to the extent of hypokalemia.39 Death was attributed to cardiac arrest 3 hours post-admission due to pulmonary embolism; however, the laboratory data of this patient seem insignificant in correlation to pH-adjusted corrected potassium. Similarly, the mean level of corrected potassium was calculated to be 3.2 mmol/L in the study by Jabbar et al.51 Strikingly, the mortality rate was 21%, out of which five deaths were attributed to myocardial infarction (MI). No further description of post-mortem reports was provided and, hence, the link of potassium with these events remains unknown. Beside the findings above, none of the studies reported any findings related to the pH-adjusted corrected potassium level.
None of the studies reported any findings related to the pH-adjusted corrected potassium level and only one reported CV outcomes of DKA with respect to potassium.32 It is understandable that an initial report that focused on clerking the files of DKA patients after the discovery of insulin did not focus on the potassium at all, despite adverse CV events being the reason for eight deaths in uncomplicated DKA.31 This is because it was Holler8 who described the effect of insulin on potassium during the treatment of DKA, which later became a part of established protocols. The attempt by Cohen et al32 reported a detailed description of DKA admissions. The authors did report on the measured potassium level at the time of admission and also concluded for a case that hypokalemia might have been a reason for the death of a patient from septicemia. Nevertheless, any correlation between potassium level and pH was not used at the time of diagnosis.
Treatment of DKA consists of three modalities and all of these directly affect serum potassium level, ie, 1) volume resuscitation causes dilution of serum potassium concentration, 2) correction of hyperglycemia via insulin helps cellular re-entry of potassium, and 3) correction of acidosis utilizing bicarbonate causes renal clearance of potassium.4–7 Bicarbonate is a least recommended therapeutic intervention, and a recent systematic review in this regard also did not find benefits of bicarbonate in the resolution of DKA.75 Moreover, significance of potassium supplementation is much required crucial when bicarbonate is given with insulin. However, in the current review, there were four studies that evaluated bicarbonate in the therapy of DKA; one each by Assal et al,34 Duhon et al,64 Keller et al,38 and Lutterman et al.41 Apart from the pH-adjusted corrected potassium level for a study conducted by Assal et al34 (3.86 mmol/L), the rest of the three studies had a very low level of corrected potassium and was calculated to be 2.9, 2.78, and 2.6 mmol/L, respectively (Table 2). Such a low level of actual potassium put the question of interest on CV profiles of patients of these studies. Notably, only Keller et al38 reported an indescribable lower CVP which was translated as a complication of severe acidaemia and use of bicarbonate; both of these factors independently cause cardiac suppression and potassium excretion via diuresis. Neither any finding related to CV impact of DKA nor corrected potassium level has been reported in the rest of the studies. This finding of the current systematic review once again highlights the importance of adjustment of potassium with respect to acidosis so that the impact of serum potassium on CV outcomes can be dealt with in a way that further improves patient related outcomes of DKA patients.
The extent of non-adherence to the treatment protocol for the management of DKA is also a reason for hypokalemic events.76 Abela et al56 reported that potassium was given to the patients at a later stage of DKA resolution, with the main reasons being lack of clear instructions or secondary to not following the DKA management protocol to analyze the blood for potassium concentration; importantly, the volume of potassium resuscitation was higher and aggressive when initiated. In a similar report, a study conducted by Dhatariya et al71 in the UK found that hypokalemia was observed in most of the patients. Additionally, non-adherence to the potassium management protocol was the highest component of deviation from treatment guidelines and observed in 20% of the cases. Moreover, as high as 67% of patients observed a value of potassium level below 4.0 mmol/L at least once during the course of hospitalization. The authors emphasized the fact that potassium replenishment, and not replacement, shall be done aggressively and insulin shall be used to maneuver the potassium level between 4.0–5.5 mmol/L. Singh et al48 also reported similar findings when they audited the implementation of DKA treatment guidelines in the UK. In the context of adherence with the potassium resuscitation, only 30% of the cases satisfied the recommended standard of administration of potassium chloride of 70 mmol. Furthermore, 31% of the cases were found to experience hypokalemic episode incidence which was even higher when compared with events of hypoglycemia (14%). In terms of adherence to DKA treatment guidelines, nearly the same results were reported by Solá et al54 when only 20% of the cases satisfied the potassium resuscitation criterion which resulted in a high incidence of iatrogenic hypokalemia. In another study, Wong et al9 identify multiple entities during analyzing factors associated with non-adherence of physicians with guidelines and the event of hypokalemia. Among these were a failure to prevent, to monitor, to diagnose, and to treat hypokalemia appropriately. The study also reported that delay in initiating potassium resuscitation, inadequate amount of potassium supplementation, infrequent monitoring of potassium level, under-recognition on the hypokalemic episode, and failure to withhold insulin were the key errors which may cause an adverse event. Although the well established guidelines strongly give recommendations regarding potassium monitoring and supplementation in DKA, none of these guidelines suggest using the pH-adjusted corrected potassium level to initiate potassium resuscitation or adjust the treatment plan.4–7 And on top of it, count to carry out the recommendations for potassium resuscitation remains very low, regardless of time, whether it is observed in the distant9,48,54,56,71 or near past.76 Even further, despite all the included studies in the current review that were either depicting important parameters of DKA or experimenting with its management to improve patient oriented outcomes, not a single study demonstrated the impact of potassium and acidosis on cardiovascular outcomes in DKA patients, which remains an area to explore further.
Conclusion
Potassium has an interesting outlook during the course of DKA. Despite being an intracellular ion and most affected entity throughout the development and resolution of DKA, we find that it is given the least consideration in the literature encompassing DKA. Given the extent of the current review, none of the studies in our cohort mentioned potassium in correlation with pH. It is also worthy to look into under-utilization of pH to adjust and correct the level of potassium during diagnosis and management of DKA, despite DKA patients from almost 27% of the studies experienced hypokalemia. Furthermore, most of the clinical guidelines highlight the significance of potassium supplementation during the management of DKA. However, the current review also suggests that the implementation for such supplementation is not carried out effectively. This remains a strong recommendation for healthcare professionals given that the cardiovascular outcomes are not monitored in the context of potassium and acidosis. Moreover, the features of hypokalemia are extensively observable upon an incident; the reports do not encompass potassium, pH and CV disorders simultaneously, which remains an unexplored area for reputable diabetic associations worldwide.
Strengths and limitations
Current systematic review tends to include most of the relevant scientific literature, which also encompassed the literature considered weak in terms of authenticity, or being lower in the grading of scientific criteria; hence, the results, although genuine, may not reflect light on the exact extent of the problem. Moreover, more than one third of the literature included had low quality assessment score, further weakening the suggestion provided via this systematic review. In a similar context, unavailability of the quantitative data did not allow for meta-analysis where only one of the study fulfilled three objectives concurrently. Other than the literature quality, calculation of pH from venous and arterial blood may significantly be different for which most studies do not report the origin of sample. Similarly, although serum analysis is used mostly in a hospital setting to retrieve renal profile values, unless declared, it was not clear whether potassium was calculated using serum or plasma samples. Moreover, due to no funding of the project, the authors were not able to include non-English studies secondary to the cost of translation.
Disclosure
The authors report no conflicts of interest in regard to this work.
References
1. Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic ketoacidosis and hyperosmolar state. In: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KG, editors. International Textbook of Diabetes Mellitus. Fourth edition. Hoboken: John Wiley & Sons, Ltd.; 2015:799-814. doi:10.1002/9781118387658.ch54.
2. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016;65(4):507–521. doi:10.1016/j.metabol.2015.12.007
3. Lebovitz HE. Diabetic ketoacidosis. Lancet. 1995;345(8952):767–772. doi:10.1016/s0140-6736(95)90645-2
4. Keller U. Diabetic ketoacidosis: current views on pathogenesis and treatment. Diabetologia. 1986;29(2):71–77.
5. National Collaborating Centre for Women's and Children's Health (UK). Diabetes (type 1 and type 2) in children and young people: diagnosis and management. London: National Institute for Health and Care Excellence (UK); 2015. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26334077. Accessed: March 18, 2016.
6. Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(12):2739–2748. doi:10.2337/dc06-9916
7. Savage MW, Dhatariya KK, Kilvert A, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabetic Med. 2011;28(5):508–515. doi:10.1111/j.1464-5491.2011.03246.x
8. Holler JW. Potassium deficiency occurring during the treatment of diabetic acidosis. J Am Med Assoc. 1946;131(15):1186–1189.
9. Wong B, Cheng A, Yu C, Goguen J. Examining the “Killer K” of diabetic ketoacidosis at a Tertiary Care Hospital: an exploratory study. Can J Diabetes. 2016;40(3):204–209. doi:10.1016/j.jcjd.2015.10.002
10. Gennari FJ. Hypokalemia. N Engl J Med. 1998;339(7):451–458. doi:10.1056/NEJM199808133390707
11. Gandhi M, Suvarna TT. Cardiovascular complications in diabetic ketoacidosis. Int J Diab Dev Countries. 1995;15:132–133.
12. Kraut JA, Madias NE. Treatment of acute metabolic acidosis: a pathophysiologic approach. Nature Rev Nephrol. 2012;8(10):589–601. doi:10.1038/nrneph.2012.186
13. Schaefer TJ, Wolford RW. Disorders of potassium. Emerg Med Clin North Am. 2005;23(3):
14. Chiasson JL, Aris-Jilwan N, Belanger R, et al. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. CMAJ. 2003;168(7):859–866.
15. Shargel L. Comprehensive Pharmacy Review for NAPLEX. Baltimore, MD: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
16. Adrogue HJ, Lederer ED, Suki WN, Eknoyan G. Determinants of plasma potassium levels in diabetic ketoacidosis. Medicine (Baltimore). 1986;65(3):163–172. doi:10.1097/00005792-198605000-00004
17. IDF. Diabetes Atlas. International Diabetes Federation,
18. Umpierrez GE, Khajavi M, Kitabchi AE. Review: diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Am J Med Sci. 1996;311(5):225–233.
19. Sica DA, Struthers AD, Cushman WC, Wood M, Banas JS
20. Kjeldsen K. Hypokalemia and sudden cardiac death. Exp Clin Cardiol. 2010;15(4):e96–99.
21. Talebi S, Ghobadi F, Cacacho A, et al. Looking at diabetic ketoacidosis through electrocardiogram window! Am J Emerg Med. 2016;34(2):263–265. doi:10.1016/j.ajem.2015.10.032
22. Soler NG, Bennett MA, Fitzgerald MG, Malins JM. Electrocardiogram as guide to potassium replacement in diabetic ketoacidosis. Diabetes. 1974;23(7):610–615. doi:10.2337/diab.23.7.610
23. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014;2(10):488–496. doi:10.12998/wjcc.v2.i10.488
24. Abdo AS, Geraci SA. Significance of elevated cardiac troponin I in patients with diabetic ketoacidosis. J Miss State Med Assoc. 2013;54(5):127–130.
25. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W264. doi:10.7326/0003-4819-151-4-200908180-00135
26. Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Gonzalez-Padilla DA. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Database Syst Rev. 2016;1:CD011281.
27. Tran TTT, Pease A, Wood AJ, et al. Review of evidence for adult diabetic ketoacidosis management protocols. Front Endocrinol (Lausanne). 2017;8:106. doi:10.3389/fendo.2017.00106
28. Higgins JAD. Chapter 8: assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. 2008. Avaliable from: http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm.
29. Liu Y, He Y, Li T, et al. Risk of primary liver cancer associated with gallstones and cholecystectomy: a meta-analysis. PLoS One. 2014;9(10):e109733. doi:10.1371/journal.pone.0109733
30. Wells G, Shea B, O’Connell D, et al. Newcastle-Ottawa quality assessment scale cohort studies. 2014. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 21, 2018.
31. Baker TW. A clinical survey of one hundred and eight consecutive cases of diabetic coma. Arch Intern Med. 1936;58(3):373–406. doi:10.1001/archinte.1936.00170130002001
32. Cohen AS, Vance VK, Runyan JW
33. Beigelman PM. Severe diabetic ketoacidosis (diabetic “coma”). 482 episodes in 257 patients; experience of three years. Diabetes. 1971;20(7):490–500. doi:10.2337/diab.20.7.490
34. Assal JP, Aoki TT, Manzano FM, Kozak GP. Metabolic effects of sodium bicarbonate in management of diabetic ketoacidosis. Diabetes. 1974;23(5):405–411. doi:10.2337/diab.23.5.405
35. Genuth SM. Constant intravenous insulin infusion in diabetic ketoacidosis. Jama. 1973;223(12):1348–1351.
36. Page MM, Pyke DA, Watkins PJ, et al. Treatment of Diabetic Coma with Continuous Low-dose Infusion of Insulin. Br Med J. 1974;2(5921):687–690. doi:10.1136/bmj.2.5921.687
37. Semple PF, White C, Manderson WG. Continuous intravenous infusion of small doses of insulin in treatment of diabetic ketoacidosis. Br Med J. 1974;2(5921):694–698. doi:10.1136/bmj.2.5921.694
38. Keller U, Berger W, Ritz R, Truog P. Course and prognosis of 86 episodes of diabetic coma. A five year experience with a uniform schedule of treatment. Diabetologia. 1975;11(2):93–100.
39. Asplin CM. Experience in a district general hospital using a low dose insulin regimen for diabetic coma. Bristol Med Chir J. 1976;91(339–340):5–9.
40. Kitabchi AE, Ayyagari V, Guerra SM. The efficacy of low-dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med. 1976;84(6):633–638. doi:10.7326/0003-4819-84-6-633
41. Lutterman JA, Adriaansen AAJ, van ‘t Laar A. Treatment of severe diabetic ketoacidosis - A comparative study of two methods. Diabetologia. 1979;17(1):17–21.
42. Pfeifer MA, Samols E, Wolter CF, Winkler CF. Low-dose versus high-dose insulin therapy for diabetic ketoacidosis. South Med J. 1979;72(2):149–154. doi:10.1097/00007611-197902000-00012
43. Sacks HS, Shahshahani M, Kitabchi AE, Fisher JN, Young RT. Similar responsiveness of diabetic ketoacidosis to low-dose insulin by intramuscular injection and albumin-free infusion. Ann Intern Med. 1979;90(1):36–42. doi:10.7326/0003-4819-90-1-36
44. Owen OE, Licht JH, Sapir DG. Renal function and effects of partial rehydration during diabetic ketoacidosis. Diabetes. 1981;30(6):510–518. doi:10.2337/diab.30.6.510
45. Basu A, Close CF, Jenkins D, Krentz AJ, Nattrass M, Wright AD. Persisting mortality in diabetic ketoacidosis. Diabetic Med. 1993;10(3):282–284.
46. Rajasoorya C, Wong SF, Chew LS. Diabetic ketoacidosis–a study of 33 episodes. Singapore Med J. 1993;34(5):381–384.
47. Chu CH, Lee JK, Lam HC, Lu CC The occurrence of diabetic ketoacidosis in type 2 diabetic adults; 1997. Avaliable from: http://wwwtsimorgtw/journal/jour10-6/P10_230PDF.
48. Singh RK, Perros P, Frier BM. Hospital management of diabetic ketoacidosis: are clinical guidelines implemented effectively? Diabetic Med. 1997;14(6):482–486. doi:10.1002/(SICI)1096-9136(199706)14:6<482::AID-DIA371>3.0.CO;2-A
49. Wagner A, Risse A, Brill H, et al. Therapy of severe diabetic ketoacidosis: zero-mortality under very-low-dose insulin application. Diabetes Care. 1999;22(5):674–677. doi:10.2337/diacare.22.5.674
50. Umpierrez G, Freire AX. Abdominal pain in patients with hyperglycemic crises. J Crit Care. 2002;17(1):63–67.
51. Jabbar A, Farooqui K, Habib A, Islam N, Haque N, Akhter J. Clinical characteristics and outcomes of diabetic ketoacidosis in Pakistani adults with Type 2 diabetes mellitus. Diabetic Med. 2004;21(8):920–923. doi:10.1111/j.1464-5491.2004.01249.x
52. Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: clinical and biochemical differences. Arch Intern Med. 2004;164(17):1925–1931. doi:10.1001/archinte.164.17.1925
53. Lin SF, Lin JD, Huang YY. Diabetic ketoacidosis: comparisons of patient characteristics, clinical presentations and outcomes today and 20 years ago. Chang Gung Med J. 2005;28(1):24–30.
54. Solá E, Garzón S, García-Torres S, Cubells P, Morillas C, Hernández-Mijares A. Management of diabetic ketoacidosis in a teaching hospital. Acta Diabetol. 2006;43(4):127–130. doi:10.1007/s00592-006-0227-1
55. Ersöz HÖ, Ukinc K, Köse M, et al. Subcutaneous lispro and intravenous regular insulin treatments are equally effective and safe for the treatment of mild and moderate diabetic ketoacidosis in adult patients. Int J Clin Pract. 2006;60(4):429–433. doi:10.1111/j.1368-5031.2006.00786.x
56. Abela AG, Magri CJ, Debono M, Calleja N, Vassallo J, Azzopardi J. An audit of the management of diabetic ketoacidosis at St. Luke’s Hospital. Malta Med J. 2008;20(2):16–21.
57. Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a priming dose of insulin necessary in a low-dose insulin protocol for the treatment of diabetic ketoacidosis? Diabetes Care. 2008;31(11):2081–2085. doi:10.2337/dc08-0509
58. Huri HZ, Foong GTK, Pendek R, Widodo RT. Different characteristics of diabetic ketoacidosis between type 1 and type 2 diabetes patients in Malaysia. Asian Biomed. 2009;3(2):201–205.
59. Lopes AD, Maciel AT, Park M. Evolutive physicochemical characterization of diabetic ketoacidosis in adult patients admitted to the intensive care unit. J Crit Care. 2011;26(3):303–310. doi:10.1016/j.jcrc.2010.08.013
60. Robles FC, Neto DL, Dias FG, et al. Diabetic ketoacidosis: difference between potassium determined by blood gas analysis versus plasma measurement. Arq Bras Endocrinol Metabol. 2011;55(4):256–259.
61. Al-Rubeaan KA, Aftab SA, Alotaibi MS, Alghamdi AA, Rafiullah MR. Clinico-laboratory characteristics of diabetic keto acidosis in adults in a tertiary hospital in Saudi Arabia. Eur Rev Med Pharmacol Sci. 2011;15(10):1202–1206.
62. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138–145. doi:10.1016/j.jcrc.2012.01.007
63. Weinert LS, Scheffel RS, Severo MD, et al. Precipitating factors of diabetic ketoacidosis at a public hospital in a middle-income country. Diabetes Res Clin Pract. 2012;96(1):29–34. doi:10.1016/j.diabres.2011.11.006
64. Duhon B, Attridge RL, Franco-Martinez AC, Maxwell PR, Hughes DW. Intravenous sodium bicarbonate therapy in severely acidotic diabetic ketoacidosis. Ann Pharmacother. 2013;47(7–8):970–975. doi:10.1345/aph.1S014
65. Azevedo LCP, Choi H, Simmonds K, Davidow J, Bagshaw SM. Incidence and long-term outcomes of critically ill adult patients with moderate-to-severe diabetic ketoacidosis: retrospective matched cohort study. J Crit Care. 2014;29(6):971–977. doi:10.1016/j.jcrc.2014.07.034
66. Seth P, Kaur H, Kaur M. Clinical profile of diabetic ketoacidosis: a prospective study in a Tertiary Care Hospital. J Clin Diagn Res. 2015;9(6):OC01–OC04. doi:10.7860/JCDR/2015/8586.5995
67. Houshyar J, Bahrami A, Aliasgarzadeh A. Effectiveness of insulin glargine on recovery of patients with diabetic ketoacidosis: a randomized controlled trial. J Clin Diagn Res. 2015;9(5):Oc01–Oc05. doi:10.7860/JCDR/2015/12005.5883
68. Usman A, Syed Sulaiman SA, Khan AH, Adnan AS. Profiles of diabetic ketoacidosis in multiethnic diabetic population of Malaysia. Trop J Pharm Res. 2015;14(1):179–185. doi:10.4314/tjpr.v14i1.25
69. Guisado-Vasco P, Cano-Megías M, Carrasco-de la Fuente M, Corres-González J, Matei AM, González-Albarrán O. Clinical features, mortality, hospital admission, and length of stay of a cohort of adult patients with diabetic ketoacidosis attending the emergency room of a tertiary hospital in Spain. Endocrinologia Y Nutricion. 2015;62(6):277–284. doi:10.1016/j.endonu.2015.02.003
70. Navarro-Díaz FJ, Amillo M, Rosales M, Panadero A, Ena J. Opportunities to improve hospital emergency care of patients with diabetic ketoacidosis. Emerg. 2015;27(1):39–42.
71. Dhatariya KK, Nunney I, Higgins K, Sampson MJ, Iceton G. National survey of the management of Diabetic Ketoacidosis (DKA) in the UK in 2014. Diabetic Med. 2016;33(2):252–260. doi:10.1111/dme.12875
72. Kakusa M, Kamanga B, Ngalamika O, Nyirenda S. Comatose and noncomatose adult diabetic ketoacidosis patients at the University Teaching Hospital, Zambia: clinical profiles, risk factors, and mortality outcomes. Indian J Endocrinol Metab. 2016;20(2):199–205. doi:10.4103/2230-8210.176358
73. Kamata Y, Takano K, Kishihara E, Watanabe M, Ichikawa R, Shichiri M. Distinct clinical characteristics and therapeutic modalities for diabetic ketoacidosis in type 1 and type 2 diabetes mellitus. J Diabetes Complications. 2017;31(2):468–472. doi:10.1016/j.jdiacomp.2016.06.023
74. Balili CAV, Gomez MHS. Efficacy and safety of subcutaneous insulin analogue versus intravenous insulin infusion among patients with mild to moderate diabetic ketoacidosis at the university of Santo Tomas hospital. Phillippine J Internal Med. 2017;55:1.
75. Chua HR, Schneider A, Bellomo R. Bicarbonate in diabetic ketoacidosis - a systematic review. Ann Intensive Care. 2011;1(1):23. doi:10.1186/2110-5820-1-23
76. Cieluch A, Uruska A, Falkowski B, et al. Nonadherence to potassium replacement protocol leads to prolonged management of diabetic ketoacidosis. Pol Arch Internal Med. 2018;128(7–8):416–420. doi:10.20452/pamw.4293
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
