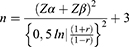Back to Journals » International Journal of General Medicine » Volume 16
Correlation Between Vitamin D Status and HBsAg Antibody Levels in Indonesian Adolescents Immunised Against Hepatitis B
Authors Girsang RT , Rusmil K, Fadlyana E, Kartasasmita CB, Dwi Putra MG , Setiabudiawan B
Received 21 August 2023
Accepted for publication 27 October 2023
Published 8 November 2023 Volume 2023:16 Pages 5183—5192
DOI https://doi.org/10.2147/IJGM.S434290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Rodman Tarigan Girsang, Kusnandi Rusmil, Eddy Fadlyana, Cissy B Kartasasmita, Muhammad Gilang Dwi Putra, Budi Setiabudiawan
Department of Child Health, Universitas Padjadjaran/Dr Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Rodman Tarigan Girsang, Email [email protected]
Introduction: Hepatitis B virus (HBV) infection is a global health problem. Anti-hepatitis B surface antigen (HBsAg) levels increase along with vitamin D levels in adults. However, few studies have examined this relationship in adolescents. Few studies have examined the relationship between vitamin D and HBsAg antibody levels, especially in Indonesia.
Methods: This cross-sectional study examined vitamin D and anti-HBsAg levels before and after hepatitis B immunisation. All subjects blood was taken to check for vitamin D level. This study was part of the Safety and Preliminary of Immunogenicity Following Recombinant Hepatitis B (Bio Farma) Vaccine in Adults and Children Phase I trial.
Results: This study found that 25-hydroxyvitamin D [25(OH)D] status was primarily deficient based on endocrine criteria. The children’s hepatitis B antibody response was mostly < 10 mIU/mL before and ≥ 10 mIU/mL after vaccination. There was a relationship between sex and 25(OH)D status, with median 25(OH)D levels higher in females (18.2 ng/mL) than in males (9.8 ng/mL). However, the relationship between vitamin 25(OH)D status and anti-HBsAg levels pre- and post-vaccination was not significant.
Discussion: However, some research found that vitamin D supplementation after immunisation did not impact vaccine response, several studies have reported that vitamin D can decrease HBV replication through various mechanisms, including reducing viral transcription and interfering with viral protein synthesis.
Conclusion: There was no relationship between 25(OH)D status and anti-HBsAg levels. Further research is needed to elucidate the underlying mechanisms and establish optimal treatment strategies.
Keywords: hepatitis B, vitamin D, adolescents, Anti-HBsAg
Introduction
Hepatitis B is a disease caused by the hepatitis B virus (HBV). Baruch Blumberg first identified the HBV in 1965.1,2 It causes necrosis and inflammation in liver cells. HBV infection is a global health problem. The World Health Organization estimates that as many as 2 billion individuals are infected with HBV, and more than 360 million individuals, or about 6% of the global population, suffer from chronic HBV infection.3,4 These data highlight the need for a special strategy to reduce the incidence of HBV infection, especially in developing countries.1–5
Immunisation programs have been successful in some countries, and screening of blood products is considered one of the efforts to reduce the incidence of HBV infection, which remains high in some HBV-endemic countries, with the incidence of children infected with HBV also relatively high.6 This issue relates to the natural course of HBV, which can develop into chronic and even cause long-term complications such as cirrhosis, hepatitis, or hepatocellular carcinoma.6,7
Indonesia is classified as a medium-to-high-endemicity country. The Basic Health Research (Riskesdas) results in 2007 showed that from 10391 serums examined, the prevalence of HBV seropositivity was 9.4%, meaning that 1 in 10 Indonesian participants had been infected with HBV.6,7 When converted to the total population of Indonesia, the HBV-infected population reaches 23 million individuals. Based on the Riskesdas results in 2013, the number of Indonesians with HBV seropositivity was 7.1, a decrease compared to 2007.6,7 This data changes Indonesia from a country with high HBV endemicity to one with moderate endemicity.7
The risk of being infected with HBV during adolescence is increased by the presence of individuals who are nonresponsive to the HBV vaccine. The incidence of nonresponders ranges from 2–15% in healthy individuals. Nonresponder individuals are susceptible to HBV infection and are at elevated risk of becoming chronically infected.8 Kusnandi et al concluded that immunising adolescents against HBV immunisation with up to three doses can provide immunity in healthy children with hypo/nonresponders.8,9
The quality of the immune response elicited by immunisation depends on several factors: the number of antigen doses, how they were administered, the added adjuvants, nutritional factors, and nutritional status. One of the nutritional factors that play a role is vitamin D.10 Vitamin D is a fat-soluble vitamin that acts like a steroid hormone.9,10 The potential effect of vitamin D on vaccine response is likely mediated by its action on the adenomatous polyposis coli (APC) protein, with the most potent action so far observed with dendritic cells. While the biologically active form of vitamin D, 1.25-dihydroxyvitamin D [1,25(OH)2], is seen to directly blunt B cell function, it also stimulates the effectiveness of vaccines through its effect on the innate immune system.11
Jafarzadeh et al reported that after 20 years of primary HBV vaccination, serum levels of anti-hepatitis B surface antigen (HBsAg) antibodies tend to increase with vitamin D levels.12 In addition, anti-HBs antibody levels also differed significantly between subjects with different vitamin D levels.12 Low vitamin D levels lead to insufficient vitamin D signalling for immune system regulation. Therefore, vitamin D deficiency reduces the quality, quantity, area, and location of the immune response against viral, bacterial, and vaccine antigen infections.12,13
The association between anti-HBs and Vitamin D remains a topic of ongoing discussion, with search results presenting conflicting information. One study suggests that vitamin D status might play a role in the persistence of anti-HBs antibodies and the durability of protection following primary vaccination with a recombinant HB vaccine.12,14 Conversely, another study reveals a significant and inverse correlation between vitamin D levels and HBV-DNA.14,15 However, a separate study contends that vitamin D status has no bearing on the anti-HBs titer in children who received hepatitis B virus vaccination during infancy.16 Some studies even report a lack of connection between vitamin D levels and HBV viral infection.17 In summary, the current evidence is inconclusive, necessitating further research to elucidate the relationship between anti-HBs and Vitamin D.
This study was performed in the context of HBV immunisation being a current part of a government program to prevent HBV transmission. While anti-HBsAg levels increase with vitamin D levels in adults, few studies have examined this relationship in adolescents. Several studies have reported the role of vitamin D in the body’s defence mechanisms. Few studies have examined this relationship between vitamin D and HBV, especially in Indonesia. The aim of this study is to determine the relationship between vitamin D and anti-HBsAg levels in adolescents vaccinated against HBV.
Materials and Methods
Study Design and Population
This cross-sectional study compared vitamin D and anti-HBs levels before and after hepatitis B immunisation and examined the relationship between them. Its subjects were 50 adolescents aged 10–17 years vaccinated against HBV who met all inclusion criteria and none of the exclusion criteria. All subjects blood was taken for vitamin D assessment as part of the Safety and Preliminary of Immunogenicity Following Recombinant Hepatitis B (Bio Farma) Vaccine in Adults and Children Phase I trial.
School selection was conducted using purposive techniques, and subject selection by stratification random sampling using random lists. On the appointed day, an explanation of research activities was given to parents/guardians. For those who have agreed to participate in the study, a schedule for examination and immunisation was determined after they signed a willingness to participate in the research document (informed consent).
The main exclusion criteria included being simultaneously enrolled or scheduled to be enrolled in another experiment; having a direct relative relationship with the research team; having a moderate onset of mild, moderate, or severe illness, especially infectious disease or fever (axillary temperature 37.5°C) within 48 hours before registration; a known history of allergy to any vaccine component (based on anamnesis); a known history of immunodeficiency disorders (HIV infection, leukemia, lymphoma, or malignancy); a history of uncontrolled coagulopathy or blood disorders that are contraindicated for the phlebotomy process; receiving treatment in the previous four weeks that can alter the immune response (intravenous immunoglobulins, blood-derived products, corticosteroid therapy and other immunosuppressants); having any chronic disorder or disease that the research team believed may interfere with the study’s objective assessment of the study; having already been immunised with any vaccine within the previous four weeks and expect to receive another vaccine within four weeks of immunisation; HBsAg positivity; a known history of HBV infection; previous HBV immunisation, proven by written documentation; and planning to move out of the study area before the end of the study period.
A parent/guardian had to provide written consent for children aged 10–11 years. Subjects aged 12–17 years had to provide written consent before a parent/guardian provided informed consent. The informed consent had to be signed by an impartial witness (independent of the researcher and not specified on the list of research contributors) if a legally valid subject representative could not read and sign the form. By signing the consent form, the witness can attest that the information in the consent form and other written information was accurately explained to the parent or their legally acceptable representative and appeared to have been understood. After being informed about the study and providing signed informed consent, all subjects underwent a thorough medical history evaluation, physical exam, chest radiology, and some serology tests.
The investigators checked the inclusion and exclusion criteria. For each recruited subject, the inclusion number was allocated in chronological order. The subject was randomised per treatment group. The doctor strictly followed the list of randomisations provided by Bio Farma. Treatment was allocated according to a randomisation list so that each randomisation number corresponded to only one strictly randomly assigned treatment group. This clinical trial was registered at ClinicalTrials.gov (ID: NCT04188223) and the Indonesian Clinical Research Registry (ID: INA-DPS3T9B).
Procedures
A blood sample was collected in a vacutainer tube. The individual in charge of the blood draw had to verify the subject’s identity and check that the initials on the laboratory request were those of the subject before taking the blood sample. Then, the subject’s initials were written on all labels of the corresponding bands. The label was affixed to the vacutainer immediately before blood sampling. It was vital to obtain a sterile blood sample. Three mL of blood samples were collected at visits V0, V1 and V2. The V0 blood sample was divided into three aliquots. The first aliquot was used for the HBsAg test, the second was used to evaluate pre-immunisation antibody titers (anti-HBsAg test), and the third was used for vitamin D testing. The V1 and V2 blood samples were used for post-immunisation antibody titer evaluation (anti-HBsAg test), and the remaining samples were stored until sent to Prodia Laboratories.
Anti-HBsAg Test
Anti-HBsAg levels were quantified using the ARCHITECT Chemiluminescent Microparticle Immunoassay (CMIA) with the Ausab reagent on an ARCHITECT i1000SR immunoassay analyser. The ARCHITECT Ausab assay is a CMIA for quantitatively determining anti-HBsAg in serum and plasma (dipotassium EDTA, lithium heparin, and sodium heparin) in adults and children and serum from neonates. It is used to quantitatively measure the antibody response after HBV immunisation, to determine immune status against HBV, and for laboratory diagnosis of HBV disease associated with HBV infection in conjunction with other laboratory results and clinical information.
ARCHITECT Ausab calibrators are used to calibrate the ARCHITECT i systems when they are used to quantitatively determine anti-HBsAg using ARCHITECT Ausab reagents. Calibrators have not been used with other anti-HBsAg assays. The ARCHITECT Ausab control was used to monitor the work of the ARCHITECT system when it is used to quantitatively determine anti-HBsAg with ARCHITECT Ausab reagents.
Vitamin D Test
The ARCHITECT 25-OH Total Vitamin D was used to quantitatively determine vitamin D levels in serum and plasma using CMIA technology with a standard working protocol called Chemiflex. First, diluents and paramagnetic anti-vitamin D bind to anti-vitamin D microparticles to form antigen-antibody complexes. After incubation, conjugates containing acridinium vitamin D are added to the mixing reaction and bind to the empty parts of vitamin D. After incubation and leaching, pre-trigger and trigger solutions are added to the mixing reaction. The results of chemiluminescent reactions are measured in relative light units (RLU). The relationship between the amount of 25-hydroxyvitamin D [25(OH)D] in the sample and the RLU was detected using the ARCHITECT optical system. The results are calculated based on previous calibrations. We used endocrine criteria to measure vitamin D levels.
Outcomes
The primary outcome was determining whether there was a relationship between vitamin D and anti-HBsAg levels. The secondary outcomes were determining the vitamin D status of adolescents who received a recombinant HBV vaccine and the anti-HBsAg levels of adolescents before and after receiving a recombinant HBV vaccine.
Sample Size and Statistical Analysis
The sample size was determined based on the purpose of the study, namely to determine the relationship between vitamin D status and anti-HbsAg levels in adolescents who received the recombinant hepatitis B vaccine, by selecting the significance level α = 5%, power test 1-β = 80%. From the results of the study, Jafarzadeh et al obtained the average levels of anti-HB sAg in the group of adolescents with vitamin D levels of <10ng/mL was 16.16 (SD 3.10) ng/mL and in the group of adolescents with vitamin D levels ≥ 10 ng/mL 58.91 (SD 79.43) ng/mL. By using the sample size formula to test the difference between two averages, namely:
Information:
n = sample size per group
S = combined standard deviation
Z α and Z β = deviate values Z obtained from the standard normal distribution table, for α = 5% (Zα = 1.65), and for power tests 80% (Z β = 0.84).
From the sample size formula above, n = 22 per group is obtained.
Establishing the prevalence of low vitamin D levels (<10 ng/mL) in adolescents at 45% requires a sample size of 9 adolescents. Furthermore, from the study of Jafarzadeh et al the relationship between vitamin D levels and anti-HBsAg levels the calculation results are reduced leg r = 0.971 if a sample size formula is used for correlation analysis, namely:
For α, and the same β as above, and r = 0.971 obtained n = 7; and n = 49 corresponds to r = 0.4. So, based on the calculations of the two above, a minimum of 49 teenagers are needed.
The research data were analysed descriptively and analytically. Descriptive analysis included presenting categorical data as numbers and percentages and numerical data as means, standard deviations (SDs), medians, and ranges. Numerical data were tested for normality using the Shapiro–Wilk test and considered normally distributed data if their P-value was >0.05. The correlation between vitamin D status and the anti-HBsAg level was assessed using Pearson’s correlation coefficient if normally distributed and Spearman’s rank correlation coefficient if not normally distributed. Data were compared between categorical variables using the Chi-square test. Data were compared between numerical variables using the unpaired t-test if normally distributed or the Mann–Whitney test if nonnormally distributed. Data were compared between more than two groups using the F-test (analysis of variance) if normally distributed or the Kruskal–Wallis test if nonnormally distributed. Data were processed and analysed using the SPSS for Windows software (version 18). All results with a P < 0.05 were considered statistically significant.
Role of the Funding Source
This trial was funded by PT Bio Farma Indonesia (grant no.: 129K-CT-HepB). PT Bio Farma Indonesia designed the protocol, supervised the study’s conduct, and analysed the study’s results. In collaboration with the other authors, PT Bio Farma Indonesia had a role in data collection, data analysis, data interpretation, report writing, and the decision to submit the manuscript.
Results
This study examined the relationship between vitamin D status and anti-HBsAg levels in 50 adolescents who received a recombinant HBV vaccine. Basic data were recorded for all subjects, including sex, age, and anthropometric height and weight measurements. The subjects’ characteristic (Table 1) shows that there were more females than males and more subjects aged 10–17 years, with an average age of 12.9 years. The value of body mass index indicates that 50% of subjects are classified as normal. Descriptive statistics of the studied variables (Table 2) show subjects’ 25(OH)D and anti-HBsAg levels after complete vaccination. The normality test results for vitamin D and anti-HbSAg levels before and after vaccination had P < 0.05, meaning they are nonnormally distributed. Therefore, the correlation between vitamin D and anti-HBsAg levels was assessed using Spearman’s non-parametric rank correlation coefficient. The interpretation of test results based on endocrine criteria indicated that most subjects had a deficient 25(OH)D status (80%; Table 3). The HBV antibody response was <10 mIU/mL for most subjects before vaccination (74%) and ≥10 mIU/mL for all subjects after vaccination (100%). The 25(OH)D statuses and levels were significantly associated with sex but not age, with females having significantly higher median 25(OH)D levels (18.2 ng/mL) than males (9.8 ng/mL; Table 4). However, 25(OH)D status and anti-HBsAg level were not significantly correlated pre- or post-immunisation (Table 5).
 |
Table 1 Research Subjects’ Characteristics (n = 50) |
 |
Table 2 Subjects’ 25(OH)D and Anti-HBsAg Levels After Complete Vaccination |
 |
Table 3 Subjects’ 25(OH)D Status and Pre- and Post-HBV Immunisation Antibody Responses |
 |
Table 4 Relationship with 25(OH)D Status |
 |
Table 5 Relationship Between 25(OH)D Status and Anti-HBsAg Levels |
Discussion
The liver is the primary organ affected by HBV infection. The HBV is spread by coming into contact with infected blood or bodily fluids such as vaginal secretion and semen. HBV can cause both acute and chronic liver disease, including cirrhosis and liver cancer.18,19 Over 250 million individuals are believed to have chronic HBV infections worldwide.20–22 HBV infection can cause liver damage and diminished liver function.23 Li et al and Wang et al reported that vitamin D regulates liver function, including the modulation of liver enzymes, hepatic inflammation, and fibrosis. Vitamin D may have protective benefits on liver function in the context of HBV infection.23,24
In our study, we found that most children had an insufficient 25(OH)D status (80%) and an HBV antibody response of <10 mIU/mL (74%) before vaccination. However, after vaccination with a recombinant HBV vaccine, all children had an HBV antibody response of ≥10 mIU/mL. Vitamin D has been demonstrated to control both innate and adaptive immune responses.25,26 Vitamin D may alter the immunological response to HBV infection, affecting the equilibrium between viral eradication and immune-mediated liver damage.17,26 Previous studies have shown that vitamin D increases the production of antiviral cytokines and stimulates the differentiation of regulatory T cells, which may help regulate the spread of HBV infection.17
The association between 25(OH)D status and anti-HBs levels was not significant. We found that children vaccinated against HBV as infants had greater anti-HBsAg titers regardless of their vitamin D level. The information currently available in the literature regarding the effect of vitamin D on HBV vaccine response comes from Poland.16 Other studies reported different results. Some studies have shown that a higher vitamin D concentration is associated with a better response to booster HBV immunisation and that vitamin D concentration is an independent significant negative predictor of seroconversion.12,27 However, others have shown no such correlation.28–30 Nevertheless, some noticed that a weaker response to the HBV vaccine was associated with a low vitamin D concentration.12,31
The correlation between vitamin D levels and post-vaccine HBsAg antibody levels remain a subject of contention, with search results yielding conflicting information.27 Some studies propose that inadequate vitamin D levels during the initial vaccination are linked to a diminished response to the hepatitis B vaccine.16 Conversely, others have not identified a significant relationship between vitamin D levels and higher anti-HBs titers in infants who received the hepatitis B vaccine.16,27 In the case of HIV-infected adults, one study discovered no positive connection between vitamin D levels and immune responses to hepatitis B vaccination.30 Nevertheless, there is a solitary study that revealed a positive correlation between vitamin D concentration and IgG avidity percentages in a negative control group.14 Another study discovered a significant and inverse correlation between vitamin D levels and HBV-DNA.15 However, a separate study did not find vitamin D levels to be a significant factor in determining higher anti-HBs titers in infants receiving the hepatitis B vaccine.16
However, they found that vitamin D supplementation after immunisation did not impact vaccine response.27 Several studies have suggested a connection between vitamin D and HBV infection.32 Vitamin D has been shown to have antiviral effects and may be involved in the immune reaction to HBV infection.33 Additionally, a lack of vitamin D has been associated with a higher incidence of HBV infection and worse prognoses for people with chronic hepatitis.24,33 Several studies have reported that vitamin D can decrease HBV replication through various mechanisms, including reducing viral transcription and interfering with viral protein synthesis.24,32–34
Numerous hypotheses about how Vitamin D can affect HBV infection can be made in light of its immunomodulatory qualities and HBV pathogenesis.24,32,33 As a fat-soluble vitamin, vitamin D is essential for maintaining bone health and controlling calcium metabolism.35 However, recent studies have indicated that vitamin D may also have immunomodulatory effects.33,35 Numerous studies have shown that different immune cells contain vitamin D receptors (VDRs), suggesting that this nutrient may have a role in controlling the immune system.35,36
Vitamin D has been shown to reduce viral replication in numerous viral diseases, including hepatitis C.37 It is conceivable that vitamin D could inhibit HBV replication.24,37 Recent studies have shown that vitamin D greatly affects cytokine production and the control of healthy innate and adaptive immune functions in both animals and humans.24,32,33,35–37 The immune system’s response against the antigen will be enhanced by 1.25(OH)2D to produce a potent and long-lasting antibody response to vaccination.27,32,33
Ex vivo experiments on human cells have revealed that vitamin D deficiency and supplementation affect how well an individual’s immune system responds to vaccination.38 The body’s natural and adaptive defence mechanisms depend on vitamin D.35,36,38 Steroids control gene expression, turning on and off the body’s required protein synthesis, whereas vitamins serve as antioxidants or cofactors in enzymatic activities. By interacting with the nuclear VDR, the active form of vitamin D, 1.25(OH)2D, controls >200 genes directly or indirectly.35,36,38 All immune system tissues and cells contain vitamin D in their nuclei. Additionally, vitamin D influences cells in a paracrine or autocrine manner and is affected by the autonomic nervous system. Therefore, vitamin D affects diverse disease processes.9,10,38 Due to the limitations of our laboratory setup, we could not determine memory T-cell levels in this study.
Conclusions
There was no relationship between 25(OH)D status and anti-HBsAg levels. Further research is needed to elucidate the underlying mechanisms and establish optimal treatment strategies. In summary, the existing evidence is inconclusive, and further research is necessary to establish the connection between anti-HBs values and Vitamin D levels.
Institutional Review Board Statement
This study was approved by the Research Ethics Committee of Universitas Padjadjaran (ethical approval no.: 105/UN6.KEP/EC/2022) and the Indonesian Regulatory Authorities. This trial will be conducted according to the latest Edinburgh, Scotland, revision of the Declaration of Helsinki, ICH Good Clinical Practice guidelines, and local regulatory requirements.
The investigator shall be responsible for obtaining approval of the protocol from the Institutional Ethics Committee before starting the trial and approval of all amendments in compliance with local law. The investigator must forward copies of these approvals to PT. Bio Farma Indonesia with the composition (names and qualifications of the members) of the Institutional Ethics Committee. This clinical trial was registered at ClinicalTrials.gov (ID: NCT04188223) and the Indonesian Clinical Research Registry (ID: INA-DPS3T9B).
Data Sharing Statement
The data will be available on the study’s primary site. Please contact the corresponding author for future access.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Acknowledgments
The authors would like to thank all the children and parents who participated in this study, the head of the Bandung District Health Office, the head and staff of Garuda Primary Health Centre in Bandung, and the head and staff of their support. We would also like to express our appreciation for the tremendous support of the Indonesian National AEFI Committee as auditors of the SAEs in this study. We also thank Hadyana Sukandar for his statistical work in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by PT Biofarma Indonesia (grant number: 129K-CT-HepB), and the APC was funded by Universitas Padjadjaran.
Disclosure
There were no conflicts of interest during this study.
References
1. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012;4(3):74–80. doi:10.4254/wjh.v4.i3.74
2. World Health Organisation. Guideline for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015:124.
3. Paganelli M, Stephenne X, Sokal EM. Chronic hepatitis B in children and adolescents. J Hepatol. 2012;57(4):885–896. doi:10.1016/j.jhep.2012.03.036
4. Sokal EM, Paganelli M, Wirth S, et al. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European society of pediatric gastroenterology, hepatology and nutrition. J Hepatol. 2013;59(4):814–829. doi:10.1016/j.jhep.2013.05.016
5. Villar LM, Amado LA, De Almeida AJ, De Paula VS, Lewis-Ximenez LL, Lampe E. Low prevalence of hepatitis B and C virus markers among children and adolescents. Biomed Res Int. 2014;2014:1–5. doi:10.1155/2014/324638
6. Balitbangkes. Situasi dan analisis hepatitis di Indonesia. Pusdatin Kemenkes RI. 2014. p. 1–8.
7. Dewi M, David T, Muljono H, et al. Ethnogeographical structure of hepatitis B virus genotype distribution in Indonesia and discovery of a new subgenotype, B9. Arch Virol. 2011;156(5):855–868. doi:10.1007/s00705-011-0926-y
8. Zhuang GH, Yan H, Wang XL, et al. Hepatitis B revaccination in healthy nonresponder Chinese children: five-year follow-up of immune response and immunologic memory. Vaccine. 2021;24(12):2186–2192. doi:10.1016/j.vaccine.2005.11.004
9. Lee JY, So T-Y, Thackray J. A review on vitamin D deficiency treatment in pediatric patients. J Pediatr Pharmacol Ther. 2021;18(4):277–291.
10. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2021;12(1):1.
11. Lang PO, Aspinall R. Can we translate vitamin D immunomodulating effect on innate and adaptive immunity to vaccine response? Nutrients. 2015;7(3):2044–2060. doi:10.3390/nu7032044
12. Jafarzadeh A, Keshavarz J, Bagheri-Jamebozorgi M, Nemati M, Frootan R, Shokri F. The association of the vitamin D status with the persistence of anti-HBs antibody at 20 years after primary vaccination with recombinant hepatitis B vaccine in infancy. Clin Res Hepatol Gastroenterol. 2021;41(1):66–74. doi:10.1016/j.clinre.2016.06.005
13. Yuzefpolskiy Y, Baumann FM, Penny LA, Studzinski GP, Kalia V, Sarkar S. Vitamin D receptor signals regulate effector and memory CD8 T cell responses to infections in mice. J Nutr. 2014;144(12):2073–2082. doi:10.3945/jn.114.202895
14. Youssry S, Shalaby T, Maher AS, Ghoneim H. Association of hepatitis B vaccine response to vitamin D supplementation and ultraviolet B (UVB) exposure during different time intervals in experimental animals. Immunol Res. 2022;70(4):537–545. doi:10.1007/s12026-022-09287-8
15. Ko WS, Yang YP, Shen FP, et al. The study of correlation between serum vitamin D3 concentrations and HBV DNA levels and immune response in chronic hepatitis patients. Nutrients. 2020;12(4):1114. doi:10.3390/nu12041114
16. Dabrowska-Leonik N, Sawicka-Powierza J, Bernatowska E, et al. Lack of relationship between 25-hydoxyvitamin D concentration and a titer of antibodies to hepatitis B surface antigen in children under 12 years of age. PLoS One. 2022;17(11):e0277473. doi:10.1371/journal.pone.0277473
17. Asghari A, Jafari F, Jameshorani M, et al. Vitamin D role in hepatitis B: focus on immune system and genetics mechanism. Heliyon. 2022;8(11):e11569. doi:10.1016/j.heliyon.2022.e11569
18. Kuo A, Gish R. Chronic hepatitis B infection. Clin Liver Dis. 2021;16(2):347–369. doi:10.1016/j.cld.2012.03.003
19. Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013;10(1):1–25. doi:10.1186/1743-422X-10-239
20. Gish RG, Given BD, Lai CL, et al. Chronic hepatitis B: virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47–58. doi:10.1016/j.antiviral.2015.06.008
21. Barbini L, Tadey L, Fernandez S, Bouzas B, Campos R. Molecular characterization of hepatitis B virus X gene in chronic hepatitis B patients. Virol J. 2012;9(1):1–7. doi:10.1186/1743-422X-9-131
22. Zhang ZH, Wu CC, Chen XW, Li X, Li J, Lu MJ. Genetic variation of hepatitis B virus and its significance for pathogenesis. World J Gastroenterol. 2016;22(1):126–144. doi:10.3748/wjg.v22.i1.126
23. He Q, Huang Y, Zhang L, et al. Association between vitamin D receptor polymorphisms and hepatitis B virus infection susceptibility: a meta-analysis study. Gene. 2018;645:105–112. doi:10.1016/j.gene.2017.12.027
24. Hu YC, Wang WW, Jiang WY, Li CQ, Guo JC, Xun YH. Low vitamin D levels are associated with high viral loads in patients with chronic hepatitis B: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):1. doi:10.1186/s12876-019-1004-2
25. Avdeeva Z, Alpatova N, Lysikova S, Gajderova L, Bondarev V. Analysis of mechanisms of development of immune response in hepatitis b virus infection and ways to improve the effectiveness of vaccination. Immunology. 2021;42(4):403. doi:10.33029/0206-4952-2021-42-3-403-414
26. Khanam A, Chua JV, Kottilil S. Immunopathology of chronic hepatitis B infection: role of innate and adaptive immune response in disease progression. Int J Mol Sci. 2021;22(11):5497. doi:10.3390/ijms22115497
27. Kashi DS, Oliver SJ, Wentz LM, Roberts R, Carswell AT. et al. Vitamin D and the Hepatitis B Vaccine Response: a Prospective Cohort Study and a Randomized, Placebo-Controlled Oral Vitamin D3 and Simulated Sunlight Supplementation Trial in Healthy Adults. Eur J Nutr. 2021;60(1):475–491.
28. Xiong X, Li M, Tong X, et al. Effect of vitamin D deficiency on the hepatitis-B- vaccine immune response in healthy population. Int J Clin Exp Med. 2017;10(7):10852–10856.
29. Jhorawat R, Jain S, Pal A, et al. Effect of vitamin D level on the immunogenicity to hepatitis B vaccination in dialysis patients. Indian J Gastroenterol. 2016;35(1):67–71. doi:10.1007/s12664-016-0621-8
30. Viard JP, Assuied A, Le ́vy Y, et al. No positive association between vitamin D level and immune responses to hepatitis b and streptococcus pneumoniae vacci- nation in HIV-infected adults. PLoS One. 2016;11(12):e0168640. doi:10.1371/journal.pone.0168640
31. Zitt E, Sprenger-ma ̈hr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine. 2012;30(5):931–935. doi:10.1016/j.vaccine.2011.11.086
32. Gotlieb N, Tachlytski I, Lapidot Y, Sultan M, Safran M, Ben-Ari Z. Hepatitis B virus downregulates vitamin D receptor levels in hepatoma cell lines, thereby preventing vitamin D-dependent inhibition of viral transcription and production. Molecul Med. 2018;24(1):1–8. doi:10.1186/s10020-018-0055-0
33. Lin S, Wang W, Shi L, et al. Severe vitamin D deficiency is strongly associated with liver dysfunction and disease severity in hepatitis B virus related cirrhosis and liver failure patients. J Nutr Sci Vitaminol (Tokyo). 2022;68(1):16–22. doi:10.3177/jnsv.68.16
34. Kashi DS, Oliver SJ, Wentz LM, et al. Vitamin D and the hepatitis B vaccine response: a prospective cohort study and a randomized, placebo-controlled oral vitamin D 3 and simulated sunlight supplementation trial in healthy adults. Eur J Nutr. 2021;60:475–491. doi:10.1007/s00394-020-02261-w
35. Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The role of vitamin D and vitamin D binding protein in chronic liver Diseases. Int J Mol Sci. 2022;23(18):10705. doi:10.3390/ijms231810705
36. Wang R, Zhu X, Zhang X, Liu H, Ji Y-L, Chen Y-H. Association of vitamin D and polymorphisms of its receptor with antiviral therapy in pregnant women with hepatitis B. World J Gastroenterol. 2023;29(19):3003. doi:10.3748/wjg.v29.i19.3003
37. Tsounis EP, Tourkochristou E, Sapsani A, et al. The role of vitamin D receptor polymorphisms in the course of chronic hepatitis C infection. Ann Gastroenterol. 2022;35(2):203. doi:10.20524/aog.2022.0697
38. Teymoori‐Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29(2):e2032. doi:10.1002/rmv.2032
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.