Back to Journals » International Journal of General Medicine » Volume 16
Correlation Between Total Bilirubin, Total Bilirubin/Albumin Ratio with Disease Activity in Patients with Rheumatoid Arthritis
Authors Zhang H , Yang G, Jiang R, Feng D, Li Y, Chen Y, Yuan G
Received 13 October 2022
Accepted for publication 5 December 2022
Published 24 January 2023 Volume 2023:16 Pages 273—280
DOI https://doi.org/10.2147/IJGM.S393273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Hui Zhang,1,* Guizhao Yang,1,* Rongqiong Jiang,1 Dan Feng,1 Yuqin Li,1 Yong Chen,2 Guohua Yuan1
1Institute of Rheumatism and Immunology, the Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China; 2Department of Rheumatism and Immunology, the People’s Hospital of Jianyang City, Jianyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guohua Yuan, Institute of Rheumatism and Immunology, the Affiliated Hospital of North Sichuan Medical College, Nanchong, 637000, People’s Republic of China, Tel +8615983777907, Fax +8608172262301, Email [email protected]
Purpose: Rheumatoid arthritis (RA) is a systemic inflammatory disorder with unknown etiology. Oxidative stress and immune imbalance play a critical role in the pathogenesis of rheumatoid arthritis. Bilirubin has recently been recognized as a potent antioxidant as well as an immunomodulatory agent of physiological importance. The aim of this study was to explore whether increased bilirubin concentrations are correlated with good clinical prognosis of rheumatoid arthritis.
Patients and Methods: In this cross-sectional study, we included 197 healthy individuals and 197 RA patients in the Affiliated Hospital of North Sichuan Medical College from October 2020 to February 2022. The latter were classified into three classes of disease activity according to DAS28-ESR: remission and low (DAS28-ESR< 3.2), moderate (3.2≤DAS28-ESR≤ 5.1), and high (DAS28ESR> 5.1). Based on the clinical and laboratory data, we evaluated the association of bilirubin levels with disease activity in RA using multivariable ordered logistic regression.
Results: The levels of total bilirubin and total bilirubin/albumin ratio were significantly lower (P < 0.001; P < 0.001) in RA patients compared with healthy controls. In RA patients, Spearman’s rank correlation analysis revealed that bilirubin and total bilirubin/albumin ratio were negatively correlated with disease activity and inflammatory marker (C-reactive protein, erythrocyte sedimentation rate, Interleukin-6). In multivariable ordered logistic regression, higher total bilirubin (OR=0.77, 95% CI: 0.67– 0.89, p< 0.001) independently predicted lower disease activity.
Conclusion: Bilirubin levels remain associated with a reduction of disease activity, suggesting that bilirubin may be a protective factor for RA aggravation.
Keywords: rheumatoid arthritis, bilirubin, bilirubin/albumin ratio, diagnostic marker, multivariate analysis
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that involves the joints and extraarticular manifestations, such as rheumatoid nodules, interstitial lung disease, vasculitis, and cardiovascular event.1 RA is more common in females with a female-to-male ratio of 2.5/1, and generally occurs at 40 to 70 years of age.2 With a worldwide population prevalence of 0.5–1%, rheumatoid arthritis places a heavy burden on both individuals and society, which is related to medical costs, functional disability and lost productivity.3 However, considering the safety and effectiveness of drugs, the existing therapies still have certain limitations.
The etiology and pathogenesis of RA is still incompletely understood. It is generally believed that interaction among genetic and environmental factors leads to immune system disorders, which in turn cause a range of inflammatory arthritis changes.4 In addition to a large number of activated cytokines, there are extensive angiogenesis and abundant infiltration of inflammatory cells within the synovium of the joint, such as CD4+T cells, B cells, macrophages and neutrophils.5 Substantial evidence pointed at the key role of oxidative stress played in the pathogenesis of RA.6,7 The curative effect of antioxidant therapy was also seen in experimental animal models of rheumatoid arthritis.8 So far, the main research work has focused on the causes responsible for initiating and maintaining the inflammatory and immune responses, while little attention has been paid to the factors responsible for the regression of inflammation.
Bilirubin, the side product of heme catabolism, is traditionally considered as a harmful, cytotoxic waste product and an indication of hepatobiliary and hematological diseases.9 Whereas, in recent decades, accompanied with the rapid increase of experimental and clinical data of bilirubin, we have gradually realized the important physiological role of bilirubin.10 At physiologic or moderately elevated concentrations, bilirubin has potent antioxidant properties and powerful immunomodulatory ability.11 At high concentrations, indirect bilirubin that exceed the binding capacity of plasma albumin has cytotoxic effects on multiple tissues, especially the nervous system.12 Circulating indirect bilirubin primarily binds to albumin and is excreted through further metabolism in the liver. Obviously, the mutual restriction between bilirubin and albumin regulates the balance between the detrimental cytotoxicity and the beneficial cytoprotection of bilirubin. We also note that albumin-formulated parameters such as albumin-to-fibrinogen ratio and C-reactive protein-to-albumin ratio have emerged as novel biomarkers to predict inflammation.13–15 Concerning that the total bilirubin/albumin ratio has never been mentioned in other literature related to the inflammation of rheumatoid arthritis, the variable was taken into our study.
Although overwhelming clinical evidence has convincingly proved that bilirubin offer protection against a variety of metabolic and inflammatory disease,16–18 the researches of bilirubin on autoimmune disease are still few.19,20 Therefore, we collected clinical and biochemical immunological data from a total of 394 participants, aiming to investigate the association between total bilirubin, total bilirubin/albumin ratio and disease activity in rheumatoid arthritis.
Materials and Methods
Study Participants
From October 2020 to February 2022, a cross-sectional study was designed on 197 RA patients in the outpatient and inpatient department of Rheumatology and Immunology of the Affiliated Hospital of North Sichuan Medical College. All patients fulfilled the 2009 RA criteria of American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR). At the same period, 197 individuals who underwent healthy checkup in the Affiliated Hospital of North Sichuan Medical College were selected as the control group. The patients with RA were matched to healthy controls at a ratio of 1:1 based on age (± 3 years) and sex. A total of 314 RA patients were included, of which 117 were excluded. Exclusion criteria were as follows: elevated liver enzymes (n=33), renal failure (n=9), viral hepatitis (n=20), hepatobiliary and pancreatic surgery (n=15), malignant tumor (n=10), active infection (n=8), other autoimmune diseases (n=22). Eventually, 73 RA patients were remissive and mildly active (DAS28-ESR<3.2), 54 RA patients were moderately active (3.2≤DAS28-ESR≤5.1), 70 RA patients were severely active (DAS28ESR>5.1). The study protocol was reviewed and approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (2022ER458-1) and was performed in accordance with the Declaration of Helsinki. Written informed consents were acquired from all participants before entering the study.
Assessment of Disease Activity
The DAS28 based on erythrocyte sedimentation rate (ESR) has been widely used for evaluating disease activity in patients with rheumatoid arthritis.21 The DAS28-ESR is composed of tender and swollen joint counts, the patient’s measure of general health and erythrocyte sedimentation rate.
Clinical and Laboratory Data
Clinical and laboratory data were documented from the hospital’s electronic medical record system, including age, sex, medical history, disease duration, blood routine and biochemical index, rheumatoid factor antibody spectrum and cytokines. Elbow venous blood was collected from all participants at least 8h fasting, and blood samples were performed in the clinical laboratory of Affiliated Hospital of North Sichuan Medical College. Bilirubin and albumin were measured by automatic biochemical analyzer with the vanadate chemical oxidation and bromocresol green methods, respectively. Cytokines, including interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-17A (IL-17A), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), were detected by flow fluorescence method. Anti-cyclic citrullinated peptide antibody (ACPA) and rheumatoid factor (RF) were determined by chemiluminescence.
Statistical Analysis
Normally distributed variables were represented as means ± standard deviation and compared by independent Student’s t test. Non-normally distributed represented as the median (interquartile range) and compared by Mann–Whitney U-test. Categorical variables were described by numbers (percentages), and chi-square test was performed for comparison. The correlations of bilirubin with clinical and laboratory data were calculated via Spearman’s coefficients. ROC was used to evaluate the ability of total bilirubin and total bilirubin/albumin ratio in identifying disease activity. We used ordered logistic regression to investigate the influence of bilirubin on the disease activity. All statistical tests were two-sided, and P value <0.05 was generally regarded as statistically significant. SPSS 19.0 software (IBM Corp.) was applied for statistical analyses.
Results
The RA group consisted of 35 male and 162 female patients, with a mean age of 50.44 ± 12.75 years. The healthy control group consisted of 35 males and 162 females and the mean age was 50.53 ± 12.82 years. There were no statistically significant differences in sex and age between the two groups. Figure 1 shows that the total bilirubin (10.70 (8.85–14.10) µmol/L, vs 13.70 (11.35–16.25) µmol/L, p<0.001) and total bilirubin/albumin ratio (0.24 (0.20–0.31) µmol/L vs 0.30 (0.25–0.35) µmol/g, p<0.001) in RA patients were noticeably lower when compared with healthy controls.
Table 1 lists the clinical characteristics of 197 RA patients with different disease activity, the potential variables affecting disease activity and the results of univariate analysis. Finally, the following candidate variables were identified: age, use of tofacitinib, use of biological DMARDs, drinking, diabetes, bilirubin, albumin, AST, ALT, ESR, CRP, neutrophils, platelets, Interleukin-6, Interleukin-10, rheumatoid factor, and anti–cyclic citrullinated peptide antibodies (Table 1). Multivariate ordered logistic regression analysis was performed according to these variables. and significant factors identified included use of tofacitinib, use of biological DMARDs, bilirubin, albumin, ESR, CRP, Interleukin-6, rheumatoid factor (Table 2). Notably, for each unit decreased in bilirubin and albumin, rheumatoid arthritis disease activity increased (OR=0.77, 95% CI: 0.67–0.89, p<0.001). In addition, as indicators of inflammation, ESR, CRP and platelets are important experimental indexes in the evaluation of disease activity. The negative correlation between total bilirubin, total bilirubin/albumin ratio and DAS28-ESR, ESR, CRP and platelets in Spearman rank correlation analysis was also validated with the above results (Table 3).
 |
Table 1 Patient Characteristics and Results of Univariate Analysis |
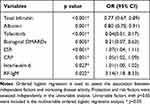 |
Table 2 Results of Multivariate Ordered Logistic Regression Analysis for Variables Affecting Disease Activity |
 |
Table 3 Correlations of Bilirubin and Total Bilirubin/Albumin Ratio with Inflammatory and Immunological Indexes |
To investigate how bilirubin influences disease activity in RA, we analyzed correlations between bilirubin, total bilirubin/albumin ratio, and inflammatory and immunological indices. As shown in Table 3, total bilirubin and total bilirubin/albumin ratio exhibited negative correlations with leukocytes and neutrophils. Moreover, total bilirubin, indirect bilirubin and direct bilirubin were negatively correlated with IL-6, and indirect bilirubin was inversely correlated with rheumatoid factor.
Receiver operating characteristic curve (Figure 2) revealed that higher disease activity (DAS28-ESR>3.2) was more likely to occur with total bilirubin ≤11.65μmol/L (80.7% sensitivity and 74.0% specificity, AUC=0.80) and total bilirubin/albumin ratio ≤0.24μmol/g (64.5% sensitivity and 76.7% specificity, AUC=0.72). We also noted that the AUC value of total bilirubin was higher than the value of total bilirubin/albumin ratio (p<0.001).
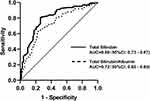 |
Figure 2 Accuracy of total bilirubin and total bilirubin/albumin ratio to predict disease activity in rheumatoid arthritis (DAS28-ESR<3.2 vs DAS28-ESR≥3.2). |
Discussion
In the 2019 EULAR treatment recommendations,22 the treatment goal of RA is defined as complete remission (DAS28-ESR<2.6) or low disease activity (2.6≤DAS28-ESR≤3.2). Therefore, in this cross-sectional study, RA patients were set into three groups with different grades of disease activity. The inverse association between bilirubin and disease activity showed that bilirubin could be used as an indicator to assess the extent of disease activity in RA patients, and increasing bilirubin levels might be an additional therapeutic method in the targeted treatment strategy of RA.
Recently, albumin-to-fibrinogen ratio and C-reactive protein-to-albumin ratio are considered as inflammatory markers to monitor disease activity in patients with RA14 and the use of total bilirubin/albumin ratio for predicting bilirubin encephalopathy in neonates and premature infants has been repeatedly discussed.23,24 Consequently, the concept of total bilirubin/albumin ratio was proposed in this study. Based on the correlation coefficients and the area under the curve (AUC), it is known that total bilirubin/albumin is similarly a reliable index for the assessment of the active degree of rheumatoid arthritis when compared to total bilirubin.
In the past few decades, hyperbilirubinemia has largely been considered to be a sign of poor outcome. Since the first report on bilirubin’s antioxidant capacity was published by Stocker in 1987,25 our traditional viewpoint of bilirubin has radically been broken and studies concentrated on the beneficial effects of bilirubin have emerged one after another. Among them, a mass of epidemiological studies suggested that elevated bilirubin could reduce the mortality and all-cause mortality of chronic diseases such as cardiovascular disease and cancer.26 A case–control study showed that low total bilirubin levels increase the risk of SLE aggravation.27 Sykora et al successfully improved the clinical symptoms, inflammatory indexes and DNA oxidative damage of rats with hyperbilirubinemia induced by unbound bilirubin.28 All these findings are also supported by our research data, and the explanation of how bilirubin plays a protective role in RA is described below.
Elevated markers of oxidative stress have been discovered in rheumatoid arthritis, which is positively correlated with DAS28 score.29 A prospective cohort study showed a negative correlation between intakes of antioxidant foods and the risk of rheumatoid arthritis.30 In addition, patients with rheumatoid arthritis have a 1.5–2-fold increased risk of cardiovascular disease compared to the general population, and oxidative stress is considered the major contributor.31 The above evidence suggest that oxidative stress may be a contributing factor in the pathogenesis of RA. Bilirubin is well known for an effective scavenger of reactive oxygen species (ROS), which prevents the oxidation of protein, lipid and nucleic acid.32 Therefore, oxidative stress was linked with pathological changes of rheumatoid arthritis, indirectly reflecting the possible antioxidation of bilirubin on rheumatoid arthritis.
On the other hand, the increase in ROS production induced by various inflammatory stimuli can mediate NF-κB signaling pathway initiation, downstream inflammatory gene transcription and cytokine secretion, indicating a close link between oxidative stress and inflammatory signals.33 The suppressing effect of bilirubin on inflammation has been seen from the positive therapeutic effect of bilirubin nanoparticles (BRNPs) on mouse asthma model.34 It has also been recorded in a rat model of endotoxemia that administration of bilirubin alleviated liver injury and improved the survival rate of rats.35 In our study, the anti-inflammatory effect of bilirubin was supported by negative correlations between bilirubin and acute reactants (ESR, CRP).
Most recently, a series of animal experiments have proved that bilirubin plays an immunomodulatory role by inhibiting innate and adaptive immune systems. The leukocyte counts (total leukocyte, neutrophil and macrophage), and cytokine concentrations (GM-CSF, MCP-1, IL-6, IL-18) reduced by BRS and BV administration in the rat model of sterile inflammation induced by MSU crystals.36 Experiments conducted in vitro proved that unconjugated bilirubin (≥ 25 μM) exerted toxic effect on splenic T cells, B cells, macrophages and LPS-stimulated CD19+ B cells.37 Nejedla found significantly lower antibody titers in 12 neonates with hyperbilirubinemia who received diphtheria-pertussis-tetanus vaccine when compared to the normal control group.38 Consistent with the above findings, our study found that high concentration of bilirubin caused a reduction of the number of leukocytes and neutrophils, as well as the expression of related antibody and cytokines.
According to the relationship between bilirubin and antibody, we speculate that bilirubin may inhibit B cells, resulting in a decrease of rheumatoid factor production by plasma cells. It is well known that TNF-αand IL-6 are key cytokines implicated in the pathogenesis of rheumatoid arthritis.39 Accordingly, the immunomodulatory effect of bilirubin may be reflected by inhibiting T cell, leading to decreased IL 6 level. However, no similar results were observed in the analysis between bilirubin and TNF-α. The reason for the lack of statistical significance may be the widespread use of biological drugs against tumor necrosis factor in recent decades. Surprisingly, we found an inverse relationship between direct bilirubin and the anti-inflammatory cytokine IL-10, which is contrary to other studies and needs to be further verified by expanding the larger sample sizes or performing animal experiments.
In recent years, a large number of experimental data have shown that bilirubin affects gene transcription system and cell signaling pathway by binding to various cell membranes and nuclear receptors.40 Therefore, it is not difficult to understand the particularly extensive inhibitory effect of bilirubin on almost all immune systems. Correspondingly, a kind of nanomedicine based on bilirubin nanoparticles was applied to animal models such as psoriasis, autoimmune encephalomyelitis, allergic lung inflammation.34,41,42 All of these results point to the possibility that bilirubin may ameliorate the outcome of rheumatoid arthritis and serve as a new treatment for rheumatoid arthritis.
This study still has some limitations. Firstly, owing to the inherent limitations of cross-sectional data, we cannot make a causal inference between bilirubin and rheumatoid arthritis. Prospective cohort studies are needed to prove the causality. Secondly, due to the relatively small sample size of this study, large-scale studies are required to validate our results in the future.
In conclusion, our research shows that patients with rheumatoid arthritis with higher bilirubin are more likely to achieve remission. The total bilirubin, combined with total bilirubin/albumin ratio is valuable for assessing disease activity of RA. Further studies on how bilirubin affects inflammatory signaling pathways to ameliorate rheumatoid arthritis conditions are needed to discover potential therapeutic targets.
Conclusion
This cross-sectional study compared the bilirubin levels of healthy people and patients with rheumatoid arthritis, and the disease activity of patients with rheumatoid arthritis was inversely proportional to the bilirubin levels. Our data emphasize the beneficial effect of bilirubin, but the specific mechanism still requires further study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Conforti A, Di Cola I, Pavlych V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20(2):102735. doi:10.1016/j.autrev.2020.102735
2. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi:10.1016/S0140-6736(01)06075-5
3. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi:10.1016/S0140-6736(16)30173-8
4. Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi:10.1016/S0140-6736(10)60826-4
5. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi:10.1038/nature01661
6. Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6(6):265–278. doi:10.1186/ar1447
7. Kardeş S, Karagülle M, Durak İ, Avcı A, Karagülle MZ. Association of oxidative stress with clinical characteristics in patients with rheumatoid arthritis. Eur J Clin Invest. 2018;48(1). doi:10.1111/eci.12858
8. De Bandt M, Grossin M, Driss F, Pincemail J, Babin-Chevaye C, Pasquier C. Vitamin E uncouples joint destruction and clinical inflammation in a transgenic mouse model of rheumatoid arthritis. Arthritis Rheum. 2002;46(2):522–532. doi:10.1002/art.10085
9. Sullivan JI, Rockey DC. Diagnosis and evaluation of hyperbilirubinemia. Curr Opin Gastroenterol. 2017;33(3):164–170. doi:10.1097/MOG.0000000000000354
10. Gazzin S, Vitek L, Watchko J, Shapiro SM, Tiribelli C. A novel perspective on the biology of bilirubin in health and disease. Trends Mol Med. 2016;22(9):758–768. doi:10.1016/j.molmed.2016.07.004
11. Jangi S, Otterbein L, Robson S. The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int J Biochem Cell Biol. 2013;45(12):2843–2851. doi:10.1016/j.biocel.2013.09.014
12. Wennberg RP. The blood-brain barrier and bilirubin encephalopathy. Cell Mol Neurobiol. 2000;20(1):97–109. doi:10.1023/a:1006900111744
13. Onder ENA, Ertan P. Fibrinogen-to-albumin ratio in familial Mediterranean fever: association with subclinical inflammation. Klin Padiatr. 2021;233(6):292–298. doi:10.1055/a-1610-9745
14. Yang WM, Zhang WH, Ying HQ, et al. Two new inflammatory markers associated with disease activity score-28 in patients with rheumatoid arthritis: albumin to fibrinogen ratio and C-reactive protein to albumin ratio. Int Immunopharmacol. 2018;62:293–298. doi:10.1016/j.intimp.2018.07.007
15. Onder ENA, Cam FS, Ertan P. Relationship between C-reactive protein/albumin ratio and subclinical inflammation in patients with familial Mediterranean fever. Aktuelle Rheumatol. 2021;46(5):479–484. doi:10.1055/a-1403-2309
16. Huang SS, Chan WL, Leu HB, Huang PH, Lin SJ, Chen JW. Serum bilirubin levels predict future development of metabolic syndrome in healthy middle-aged nonsmoking men. Am J Med. 2015;128(10):1138.e35–1138.e1.138E41. doi:10.1016/j.amjmed.2015.04.019
17. Kunutsor SK, Bakker SJL, Gansevoort RT, Chowdhury R, Dullaart RPF. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. 2015;35(3):716–724. doi:10.1161/ATVBAHA.114.304929
18. Leníček M, Ďuricová D, Hradsky O, et al. The relationship between serum bilirubin and Crohn’s disease. Inflamm Bowel Dis. 2014;20(3):481–487. doi:10.1097/01.MIB.0000440817.84251.98
19. Zhang Z, Su Q, Zhang L, Yang Z, Qiu Y, Mo W. Clinical significance of serum bilirubin in primary Sjögren syndrome patients. J Clin Lab Anal. 2020;34(3):e23090. doi:10.1002/jcla.23090
20. Koca TT. Clinical significance of serum bilirubin in Behçet’s disease. J Transl Intern Med. 2018;6(4):185–188. doi:10.2478/jtim-2018-0034
21. van Riel PLCM, Renskers L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(5 Suppl 101):S40–S44.
22. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
23. Wang Y, Sheng G, Shi L, Cheng X. Increased serum total bilirubin-albumin ratio was associated with bilirubin encephalopathy in neonates. Biosci Rep. 2020;40(1):BSR20192152. doi:10.1042/BSR20192152
24. Hulzebos CV, Van Imhoff DE, Bos AF, Ahlfors CE, Verkade HJ, Dijk PH. Usefulness of the bilirubin/albumin ratio for predicting bilirubin-induced neurotoxicity in premature infants. Arch Dis Child Fetal Neonatal Ed. 2008;93(5):F384–F388. doi:10.1136/adc.2007.134056
25. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235(4792):1043–1046. doi:10.1126/science.3029864
26. Temme EHM, Zhang J, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control. 2001;12(10):887–894. doi:10.1023/A:1013794407325
27. Zhang W, Tang Z, Shi Y, et al. Association between gamma-glutamyl transferase, total bilirubin and systemic lupus erythematosus in Chinese women. Front Immunol. 2021;12:682400. doi:10.3389/fimmu.2021.682400
28. Sykora T, Babal P, Mikus-Kuracinova K, et al. Hyperbilirubinemia maintained by chronic supplementation of unconjugated bilirubin improves the clinical course of experimental autoimmune arthritis. Int J Mol Sci. 2021;22(16):8662. doi:10.3390/ijms22168662
29. Oğul Y, Gür F, Cengiz M, Gür B, Sarı RA, Kızıltunç A. Evaluation of oxidant and intracellular anti-oxidant activity in rheumatoid arthritis patients: in vivo and in silico studies. Int Immunopharmacol. 2021;97:107654. doi:10.1016/j.intimp.2021.107654
30. Cerhan JR, Saag KG, Merlino LA, Mikuls TR, Criswell LA. Antioxidant micronutrients and risk of rheumatoid arthritis in a cohort of older women. Am J Epidemiol. 2003;157(4):345–354. doi:10.1093/aje/kwf205
31. Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med. 2017;27(2):136–140. doi:10.1016/j.tcm.2016.07.006
32. Jansen T, Hortmann M, Oelze M, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. 2010;49(2):186–195. doi:10.1016/j.yjmcc.2010.04.011
33. Li Y, Huang B, Ye T, Wang Y, Xia D, Qian J. Physiological concentrations of bilirubin control inflammatory response by inhibiting NF-κB and inflammasome activation. Int Immunopharmacol. 2020;84:106520. doi:10.1016/j.intimp.2020.106520
34. Kim DE, Lee Y, Kim MG, Lee S, Jon S, Lee SH. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials. 2017;140(2017):37–44. doi:10.1016/j.biomaterials.2017.06.014
35. Wang WW, Smith DLH, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology. 2004;40(2):424–433. doi:10.1002/hep.20334
36. Shiels RG, Hewage W, Pennell EN, et al. Biliverdin and bilirubin sulfonate inhibit monosodium urate induced sterile inflammation in the rat. Eur J Pharm Sci. 2020;155:105546. doi:10.1016/j.ejps.2020.105546
37. Khan NM, Poduval TB. Immunomodulatory and immunotoxic effects of bilirubin: molecular mechanisms. J Leukoc Biol. 2011;90(5):997–1015. doi:10.1189/jlb.0211070
38. Rola-Pleszczynski M, Hensen SA, Vincent MM, Bellanti JA. Inhibitory effects of bilirubin on cellular immune responses in man. J Pediatr. 1975;86(5):690–696. doi:10.1016/S0022-3476(75)80352-0
39. McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis-shaping the immunological landscape. Nat Rev Rheumatol. 2016;12(1):63–68. doi:10.1038/nrrheum.2015.171
40. Vítek L. Bilirubin as a signaling molecule. Med Res Rev. 2020;40(4):1335–1351. doi:10.1002/med.21660
41. Keum H, Kim TW, Kim Y, et al. Bilirubin nanomedicine alleviates psoriatic skin inflammation by reducing oxidative stress and suppressing pathogenic signaling. J Control Release. 2020;325:359–369. doi:10.1016/j.jconrel.2020.07.015
42. Kim TW, Kim Y, Jung W, et al. Bilirubin nanomedicine ameliorates the progression of experimental autoimmune encephalomyelitis by modulating dendritic cells. J Control Release. 2021;331:74–84. doi:10.1016/j.jconrel.2021.01.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

