Back to Journals » Journal of Pain Research » Volume 16
Correlation Between Postoperative Imaging Parameters and Clinical Outcomes of Percutaneous Endoscopic Transforaminal Decompression for Lumbar Spinal Foraminal and Lateral Recess Stenosis
Authors Wu Q , Yuan S , Zang L , Wang T, Lu X, Wang A, Si F, Fan N, Du P
Received 14 November 2022
Accepted for publication 7 March 2023
Published 31 March 2023 Volume 2023:16 Pages 1149—1157
DOI https://doi.org/10.2147/JPR.S397562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Krishnan Chakravarthy
Qichao Wu,* Shuo Yuan,* Lei Zang, Tianyi Wang, Xuanyu Lu, Aobo Wang, Fangda Si, Ning Fan, Peng Du
Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zang, Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China, Email [email protected]
Objective: To investigate the correlation between postoperative imaging parameters and clinical outcomes in patients with foraminal stenosis (FS) and lateral recess stenosis (LRS) who underwent percutaneous endoscopic transforaminal decompression (PETD).
Methods: The study included 104 eligible patients who underwent PETD, and the mean follow-up time was 2.4 years (range 2.2– 3.6 years). Visual Analog Scale (VAS) scores, Oswestry Disability Index (ODI) scores, and the modified MacNab criteria were used to evaluate the clinical outcomes. The related parameters of the FS and LRS based on computed tomography and magnetic resonance imaging were measured before and after surgery. Correlations between the imaging parameters and clinical outcomes were investigated.
Results: The proportion of excellent and good results following MacNab evaluation was 82.6%. In the treatment of LRS, VAS-back, VAS-leg, and ODI at the 2-year follow-up were negatively correlated with postoperative facet joint length based on computed tomography. In the treatment of FS, the above clinical results were positively correlated with the variation of foraminal width and nerve root-facet distance before and after surgery based on magnetic resonance imaging.
Conclusion: PETD can achieve good clinical outcomes in the treatment of patients with LRS or FS. Postoperative facet joint length was negatively correlated with clinical outcomes of LRS patients. In FS patients, the variation in foraminal width and nerve root-facet distance before and after surgery were positively correlated with their clinical outcomes. These findings may help surgeons optimize treatment strategies and selection of surgical candidates.
Keywords: lumbar spinal stenosis, clinical outcomes, percutaneous endoscopic transforaminal decompression, microsurgery, spinal endoscopy
Introduction
Lumbar spinal stenosis (LSS) is a common spinal disease including central canal, lateral recesses, and intervertebral foramen stenosis. It approximately affects 4–6% of geriatric patients.1–3 The typical symptoms of LSS are neuropathic leg pain, lower back pain, intermittent claudication, and weakness, which can seriously affect the patients’ quality of life.1 Most patients can be treated conservatively in the early stages of the disease by physical therapy and drug therapy.4–6 For persistent symptoms, surgical treatment has proven to be superior to conservative treatment.4,6 Percutaneous endoscopic transforaminal decompression (PETD), a minimally invasive surgical technique, has more advantages than conventional open surgery, but it was reported that 11.4–17.2% of the patients had unsatisfactory clinical outcomes.7–9
The superior articular process (SAP) plays an important role in the operation of PETD. Lee et al10 classified foraminal and lateral recess stenosis (LRS) into three zones: the entry, mid, and exit zones. The classification system has been previously applied in several studies.11,12 Hypertrophy of the SAP is the predominant cause of entry and exit zone stenosis.10 Therefore, in the PETD procedure, surgical intervention of the SAP is crucial and necessary as it can expand the neuroforamen for the establishment of working channel of PETD and further decompression of the entrance zone, as well as decompression of the nerve roots in patients with stenosis in the exit zone. However, there are few studies focusing on the relationship between the anatomical changes caused by PETD procedures and the clinical outcomes that may direct treatments in a more optimal manner.
The objectives of this study are to evaluate the clinical outcomes of PETD in the treatment of FS and LRS and investigate the correlation between postoperative or dynamic changes in the imaging parameters and clinical outcomes of patients.
Materials and Methods
Patient Population
We retrospectively analyzed 104 patients, including 74 patients with LRS and 30 patients with FS, who underwent PETD at Beijing Chaoyang Hospital between 2017 and 2019. According to the evaluation method proposed by Wildermuth,13 there are four levels of FS based on magnetic resonance imaging (MRI): Grade 0 is a normal foramen, with a normal shape and epidural fat (oval); Grade 1 is mild FS, with the fat around the nerve root partially absent but the nerve root still completely surrounded by adipose tissue; Grade 2 is moderate FS, with the fat around the nerve root markedly absent and the nerve root being only partially surrounded by fat; while Grade 3 is significant FS, with complete disappearance of the fat around the nerve root. This study included patients with grades 1, 2, and 3 FS. Patients with FS usually experience increased pain due to the extension of the lumbar spine on one side (Kemp sign). A nerve root block can be utilized to help further determine whether a specific nerve root is causing the symptoms.14 The diagnosis of LRS is based on computed tomography (CT) showing a lateral crypt with a sagittal diameter of less than 3 mm and patients’ symptoms of intermittent claudication with or without radiating pain in the lower extremities.15
The inclusion criteria were as follows: (1) a diagnosis of LRS or FS based on physical examination results, clinical symptoms, and imaging studies. (2) single-level LRS or FS. (3) failure after at least 3 months of conservative treatment. The exclusion criteria were as follows: (1) Patients with LRS combined with FS. (2) a history of lumbar spinal surgery; (3) lumbar spondylolisthesis; (4) symptoms of stenosis caused by disc herniation without degenerative changes of the lumbar spine; (5) scoliosis; (6) spinal trauma, tumor, and systemic or local infection; and (7) incomplete preoperative or postoperative CT and MRI data.
Operative Technique
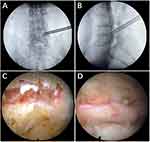
In the PELD procedure, the patient was placed in the prone position, and all procedures were performed under local anesthesia. The entry point of the assumed approach was 10 to 14 cm lateral to the midline of the spine at the affected intervertebral level. The guidewire was inserted into the SAP of the targeted segment through a puncture needle, and the surgical approach was progressively expanded to 7.5 mm with a hollow tapered cannula (Figure 1). Next, a trephine was used to enlarge the aperture under C-arm fluoroscopic guidance. The trephine was removed when it reached the medial border of the pedicle on the anteroposterior view and the posterior border of the vertebral body on the lateral view. A tubular retractor with an outer diameter of 7.5 mm was then placed, and if necessary, the trephine was used again to further enlarge the aperture under endoscopy. The nerve roots were then decompressed dorsally and ventrally under constant irrigation. For patients with LRS, it was especially necessary to remove the osteoproliferation under the nerve root that caused the narrowing of the lateral recess (LR). The herniated disc was resected using a rongeur and could be removed with an endoscope if there was a large amount of disc tissue. The proliferative ligamentum flavum was removed to expose the dura and nerve roots, and the entire nerve root was probed to ensure complete decompression. After irrigation and hemostasis, the surgical wounds were sutured in anatomical order. Representative cases are presented in Figure 2.
Clinical Evaluation
Clinical assessments were conducted preoperatively and at 3 months, 1 year, and 2 years postoperatively. The degree of pain was assessed using the visual analog scale (VAS), from 0 to 100 for the back (VAS-back) and legs (VAS-leg), with higher scores indicating more pain. The Oswestry Disability Index (ODI) was used for the assessment of function on a scale of 0–100%, with higher scores indicating higher levels of disability. Surgical results were defined as excellent, good, fair, and poor according to the modified MacNab criteria.
Imaging Parameters
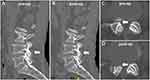
After a thorough review, it was found that most imaging parameters used in the present study had been described in the previous literature,16–19 except for anteroposterior diameter of facet joint length (FJL). All measurement methods are summarized in Figure 3. FJL was measured on the sagittal CT image between the tip of the SAP and the inferior articular process of superior vertebral body (Figure 3A). The parameters measured on CT included foraminal bony canal area (FBCA) (Figure 3B), foraminal width (FW) (Figure 3B), foraminal height (FH) (Figure 3B), disc height (DH) (Figure 3C), segmental lordosis (SL) (Figure 3C), anteroposterior diameter of LR (Figure 3D), and LR angle (Figure 3D). Lumbar lordosis (Figure 3E) was measured on a standardized standing lateral X-ray. Foraminal soft tissue canal area (FSCA) (Figure 3F), nerve root-facet distance (NRFD) (Figure 3G), nerve root-pedicle distance (NRPD) (Figure 3G), nerve root-disc distance (NRDD) on the axial plane (Figure 3G), and nerve root-disc distance (NRDD) on the sagittal plane (Figure 3H) were measured on MRI. All imaging parameters were measured using Mimics Medical 21.0 (Materialise, Leuven, BEL).
Statistical Analysis
All data were analyzed using SPSS 24.0 (IBM, Armonk, NY, USA). Variables were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed data, including clinical outcomes and imaging parameters, were compared using Student’s t-test for paired samples. Pearson’s correlation was used to identify correlations between the imaging parameters and clinical outcomes. Statistical significance was set at P < 0.05.
Results
In total, 104 patients met the inclusion criteria. The mean follow-up time was 2.4 years (range 2.2–3.6 years). Fifty-four of the patients were male (51.9%), and the mean age was 60.9 ± 13.8 years. The most common surgical segment was L4–L5 (67.3%), followed by L5–S1 (25.0%) and L3–L4 (7.7%). There were 74 (71.2%) patients with LRS and 30 (28.8%) with FS. The average duration of the symptoms was 67.6 ± 84.0 weeks, and the mean operative time was 178.0 ± 57.1 minutes. The baseline information is summarized in Table 1.
 |
Table 1 Demographic Data |
The average preoperative VAS scores for lower back and leg pain were 67.9 ± 8.7 and 65.4 ± 9.9, respectively. The preoperative ODI score was 71.3 ± 6.3. All the patients had an average VAS score of 28.4 ± 6.2, 30.7 ± 6.6, and 31.5 ± 6.9 for lower back pain and 27.6 ± 5.9, 30.9 ± 6.6, and 31.1 ± 6.7 for leg pain at the 3-month, 1-year, and 2-year follow-up, respectively. Meanwhile, the average ODI score was 22.7 ± 7.4, 24.7 ± 7.7, and 25.2 ± 7.8 at the 3-month, 1-year, and 2-year follow-up, respectively. Statistical differences were seen in VAS and ODI scores at all time points postoperatively compared to the points preoperatively (Table 2). The proportion of excellent and good results following MacNab evaluation was 82.6% at the 2-year follow-up.
 |
Table 2 VAS Pain Scores and ODI Score |
Statistically significant differences were observed in FBCA (1.19 ± 0.33 vs 1.78 ± 0.41, P < 0.01), FW (4.84 ± 1.97 vs 10.23 ± 3.36, P < 0.01), FJL (14.53 ± 3.00 vs 9.59 ± 3.60, P < 0.01), and anteroposterior diameter of LR (2.61 ± 0.76 vs 6.93 ± 2.36, P < 0.01) based on CT imaging parameters, as well as FSCA (0.77 ± 0.24 vs 1.07 ± 0.40, P < 0.01), NRFD (2.58 ± 2.42 vs 3.81 ± 2.77, P < 0.01), NRFD (sagittal) (2.15 ± 1.95 vs 2.53 ± 1.88, P < 0.05), NRDD (axial) (1.65 ± 1.32 vs 2.50 ± 1.23, P < 0.01), and nerve root-pedicle distance (NRPD) (1.99 ± 1.67 vs 2.41 ± 1.43, P < 0.01) based on MRI parameters (Table 3).
 |
Table 3 Variation of Imaging Parameters Before and After Surgery |
Pearson’s correlation analyses of LRS-related parameters showed that postoperative VAS and ODI scores at different time points had a significantly positive correlation with postoperative FJL (P < 0.05) (Table 4). Meanwhile, in Pearson’s correlation analyses of FS-related parameters, most postoperative VAS and ODI scores at different time points had a significantly negative correlation with the improvement of the NRFD (P < 0.05) and FW (P < 0.05) after the operation (Table 5). There were no significant correlations between clinical outcomes and other imaging parameters.
 |
Table 4 Correlation Between LRS-Related Imaging Parameters and Clinical Outcomes |
 |
Table 5 Correlation Between FS-Related Imaging Parameters and Clinical Outcomes |
Discussion
In this study, we retrospectively analyzed 104 patients (including 74 patients with LRS and 30 patients with FS) who underwent PETD. The clinical outcomes showed that the proportion of excellent and good results following MacNab evaluation was 82.6% with a minimum follow-up period of 2 years. We further analyzed the imaging data and found that the clinical outcomes were related to the postoperative FJL and the variation in FW and NRFD before and after surgery.
Although PETD has been successful in the treatment of LRS and FS, it is reported that 11.4–17.2% of the patients still have unsatisfactory clinical outcomes.7–9 Imaging parameters play an important role in planning surgical procedures; thus, we attempted to analyze the clinical outcomes from an imaging perspective. In our study, we studied the changes in imaging parameters before and after surgery and found significant changes in the CT-based FJL, FW, FBCA, and anteroposterior diameter of LR parameters and the MRI-based FSCA, NRFD, NRDD, and NRPD. It is easy to deduce that the changes of the above parameters are due to the surgical enlargement of the intervertebral foramen and lateral recesses. However, no significant changes were found for DH-, FH-, LR-, LL- and SL-related variables. These negative imaging indicators reflect the absence of spinal instability. PETD does not typically cause spinal instability, as it is that the operation is less damaging to the facet joints and paraspinal muscles than an open approach.
Next, we investigated the correlation between postoperative imaging parameters and clinical outcomes including VAS and ODI score. In the treatment of LRS, we found that the clinical outcomes were negatively correlated with postoperative FJL based on CT. Particularly, the magnitude of FJL depends on the extent of SAP resection. Hypertrophy of the SAP is the most common cause of LRS, and some authors believe that adequate decompression is sufficient.9,10,20,21 Under endoscopy, PETD can achieve selective resection of the SAP and can determine the size of the space around the nerve root. In our study, the reduction in FJL may be due to more resection of the top of SAP, resulting in an increase of space around nerve roots, thus achieving good clinical results. Therefore, more attention should be focused on the management of SAP during surgery. Interestingly, the variation in FJL did not correlate with the clinical outcomes. Some patients have mild hyperplasia of the SAP, and only a small amount of the SAP can be removed during the operation to achieve a good clinical outcome. Therefore, we believe that good clinical outcomes are related to the actual FJL during surgery rather than its variation.
Hypertrophy of the SAP is not only the most common cause of LRS but also plays an important role in the formation of FS, especially affecting the anteroposterior diameter of the foramen.10 In our analysis of the clinical efficacy in patients with intervertebral FS, we found that the outcome was positively correlated with the variation in FW and NRFD before and after surgery. The dimensions of the above two parameters still depend on the extent of the SAP removed during the operation. Also, the increase in FW and NRFD are associated with more resection of SAP, which is beneficial for nerve decompression and an improved surgical outcome. In the process of foraminoplasty, we should pay attention to foraminal enlargement, especially in patients with FS. In addition, Tatsuki et al22 found that the diameter of the intervertebral foramen narrowed by 1 mm/year and that the width of the articular process increased by approximately 0.8 mm/year after foraminoplasty. Bony regeneration of the facet joint after resection was the main reason for FS. Therefore, we believe that adequate decompression is particularly important in foraminal osseous stenosis to reduce restenosis. In terms of stability of the spine, Osman et al23 and Lee et al10 found that 45.5–50% of SAP resections did not increase the range of motion or the neutral zone in any direction and maintained the stability of the spine. This is likely because minimally invasive surgery associated with less damage to the muscles and ligaments attached to the spine. However, if 75% of the SAP was removed, a significant change in rotational movement was observed.24 Nevertheless, further large-scale multicenter studies are needed to determine the SAP retention range, which can help maintain the stability of the spine and meet the greatest degree of decompression.
This study had some limitations. First, in our study, compared to CT, which showed a cross-section thickness of 0.625 mm, MRI showed a higher cross-section thickness of 1.5 mm, which might have reduced the measurement accuracy of MRI-related parameters. Second, the preliminary correlation between the imaging parameters and the clinical outcomes shown in this study requires further biomechanical experiments to determine the specific range and limit the correlation results to that range. Finally, large-scale, multicenter, long-term studies are necessary.
Conclusion
PETD can achieve good clinical outcomes in the treatment of patients with LRS or FS. Postoperative facet joint length was negatively correlated with clinical outcomes of LRS patients. In FS patients, the variation in foraminal width and nerve root-facet distance before and after surgery were positively correlated with their clinical outcomes. These findings may help surgeons optimize treatment strategies and selection of surgical candidates.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
Ethics Statement
This study was approved by the ethics committee of Beijing Chaoyang Hospital, Capital Medical University (Registration number: 2021-KE-478) and the research was performed in accordance with the guidelines of the Declaration of Helsinki. Informed consent for this study was obtained from all patients by both written and verbal.
Acknowledgments
We appreciate all the subjects who participated in the study.
Author Contributions
Conceptualization: Lei Zang and Shuo Yuan; methodology: Shuo Yuan and Qichao Wu; formal analysis and investigation: Tianyi Wang and Xuanyu Lu; writing—original draft preparation: Shuo Yuan; writing—review and editing: Shuo Yuan and Qichao Wu; funding acquisition: Lei Zang; resources: Lei Zang; supervision: Ning Fan, Wenyi Zhu, Lihui Yang, Likun An, Jian Li and Xiaochuan Kong. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fortin M, Lazáry À, Varga PP, Battié MC. Association between paraspinal muscle morphology, clinical symptoms and functional status in patients with lumbar spinal stenosis. Eur Spine J. 2017;26(10):2543–2551. doi:10.1007/s00586-017-5228-y
2. Jacobs WC, Vreeling A, De Kleuver M. Fusion for low-grade adult isthmic spondylolisthesis: a systematic review of the literature. Eur Spine J. 2006;15(4):391–402. doi:10.1007/s00586-005-1021-4
3. Mehdian SH, Arun R. A new three-stage spinal shortening procedure for reduction of severe adolescent isthmic spondylolisthesis: a case series with medium- to long-term follow-up. Spine. 2011;36(11):E705–E711. doi:10.1097/BRS.0b013e3182158c1f
4. Dowling Á, Lewandrowski KU, da Silva FHP, Parra JAA, Portillo DM, Giménez YCP. Patient selection protocols for endoscopic transforaminal, interlaminar, and translaminar decompression of lumbar spinal stenosis. J Spine Surg. 2020;6(Suppl 1):S120–S132. doi:10.21037/jss.2019.11.07
5. Li L, Chang F, Hai Y, et al. Clinical effect evaluation and correlation between preoperative imaging parameters and clinical effect of endoscopic Transforaminal decompression for lumbar spinal stenosis. BMC Musculoskelet Disord. 2020;21(1):68. doi:10.1186/s12891-020-3076-0
6. Hagenmaier HS, Delawi D, Verschoor N, Oner F, van Susante JL. No correlation between slip reduction in low-grade spondylolisthesis or change in neuroforaminal morphology and clinical outcome. BMC Musculoskelet Disord. 2013;14:245. doi:10.1186/1471-2474-14-245
7. Ahn Y, Keum HJ, Lee SG, Lee SW. Transforaminal endoscopic decompression for lumbar lateral recess stenosis: an advanced surgical technique and clinical outcomes. World Neurosurg. 2019;125:e916–e924. doi:10.1016/j.wneu.2019.01.209
8. Lewandrowski KU, Yeung A, De Carvalho P, Yeung A. Minimal clinically important difference in patient-reported outcome measures with the transforaminal endoscopic decompression for lateral recess and foraminal stenosis. Int J Spine Surg. 2020;14(2):254–266. doi:10.14444/7034
9. Tang S, Jin S, Liao X, Huang K, Luo J, Zhu T. Transforaminal percutaneous endoscopic lumbar decompression by using rigid bendable burr for lumbar lateral recess stenosis: technique and clinical outcome. Biomed Res Int. 2018;2018:2601232. doi:10.1155/2018/2601232
10. Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine. 1988;13(3):313–320. doi:10.1097/00007632-198803000-00015
11. Ahn Y, Lee SH, Park WM, Lee HY. Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg. 2003;99(3Suppl):320–323. doi:10.3171/spi.2003.99.3.0320
12. Lewandrowski KU. Successful outcome after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis: the positive predictive value of diagnostic epidural steroid injection. Clin Neurol Neurosurg. 2018;173:38–45. doi:10.1016/j.clineuro.2018.07.015
13. Wildermuth S, Zanetti M, Duewell S, et al. Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology. 1998;207(2):391–398. doi:10.1148/radiology.207.2.9577486
14. Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine. 2000;25(3):389–394. doi:10.1097/00007632-200002010-00022
15. Kirkaldy-Willis WH, Paine KW, Cauchoix J, McIvor G. Lumbar spinal stenosis. Clin Orthop Relat Res. 1974;99:30–50. doi:10.1097/00003086-197403000-00004
16. Choi KC, Shim HK, Park CJ, Lee DC, Park CK. Usefulness of percutaneous endoscopic lumbar foraminoplasty for lumbar disc herniation. World Neurosurg. 2017;106:484–492. doi:10.1016/j.wneu.2017.07.035
17. Hurday Y, Xu B, Guo L, et al. Radiographic measurement for transforaminal percutaneous endoscopic approach (PELD). Eur Spine J. 2017;26(3):635–645. doi:10.1007/s00586-016-4454-z
18. Heo DH, Kim JS. Clinical and radiological outcomes of spinal endoscopic discectomy-assisted oblique lumbar interbody fusion: preliminary results. Neurosurg Focus. 2017;43(2):E13. doi:10.3171/2017.5.Focus17196
19. Steurer J, Roner S, Gnannt R, Hodler J. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12:175. doi:10.1186/1471-2474-12-175
20. Lewandrowski KU. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg. 2014;8:26. doi:10.14444/1026
21. Sairyo K, Chikawa T, Nagamachi A. State-of-the-art transforaminal percutaneous endoscopic lumbar surgery under local anesthesia: discectomy, foraminoplasty, and ventral facetectomy. J Orthop Sci. 2018;23(2):229–236. doi:10.1016/j.jos.2017.10.015
22. Mizouchi T, Watanabe K, Izumi T, et al. Quantitative radiographic analysis of foraminal re-stenosis after posterior cervical foraminotomy with laminoplasty. J Clin Neurosci. 2019;67:99–104. doi:10.1016/j.jocn.2019.06.012
23. Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374(15):1413–1423. doi:10.1056/NEJMoa1513721
24. Natarajan RN, Andersson GB, Patwardhan AG, Andriacchi TP. Study on effect of graded facetectomy on change in lumbar motion segment torsional flexibility using three-dimensional continuum contact representation for facet joints. J Biomech Eng. 1999;121(2):215–221. doi:10.1115/1.2835106
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.