Back to Journals » Research and Reports in Urology » Volume 14
Correlation Between Long-Term Acetylsalicylic Acid Use and Prostate Cancer Screening with PSA. Should We Reduce the PSA Cut-off for Patients in Chronic Therapy? A Multicenter Study
Authors Mantica G, Chierigo F, Cassim F, Ambrosini F, Tappero S, Malinaric R , Parodi S , Benelli A, Dotta F, Ennas M, Beverini M , Vaccaro C, Smelzo S, Guano G, Mariano F, Paola C, Granelli G, Varca V, Introini C, Dioguardi S, Simonato A , Gregori A, Gaboardi F, Terrone C, Van der Merwe A
Received 20 June 2022
Accepted for publication 14 October 2022
Published 21 October 2022 Volume 2022:14 Pages 369—377
DOI https://doi.org/10.2147/RRU.S377510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Guglielmo Mantica,1 Francesco Chierigo,1,2 Farzana Cassim,3 Francesca Ambrosini,1,2 Stefano Tappero,1,2 Rafaela Malinaric,1,2 Stefano Parodi,1,2 Andrea Benelli,4 Federico Dotta,4 Marco Ennas,4 Martina Beverini,1,2 Chiara Vaccaro,5 Salvatore Smelzo,6 Giovanni Guano,1,2 Federico Mariano,1,2 Calogero Paola,1,2 Giorgia Granelli,1,2 Virginia Varca,5 Carlo Introini,4 Salvatore Dioguardi,7 Alchiede Simonato,7 Andrea Gregori,8 Franco Gaboardi,6 Carlo Terrone,1,2 André Van der Merwe3
1IRCCS Ospedale Policlinico San Martino, U.O. Urologia, Genova, Italy; 2Department of Surgical and Diagnostic Integrated Sciences (DISC), University of Genova, Genova, Italy; 3Department of Urology, Tygerberg Academic Hospital, Stellenbosch University, Cape Town, South Africa; 4Department of Urology, Galliera Hospital, Genoa, Italy; 5Department of Urology, ASST Rhodense, G. Salvini Hospital, Milan, Italy; 6Department of Urology, San Raffaele Turro Hospital, Milan, Italy; 7Department of Surgical, Oncological, and Oral Sciences, Section of Urology, University of Palermo, Palermo, Italy; 8Urology Unit, Ospedale Sacco, Milan, Italy
Correspondence: Guglielmo Mantica, IRCCS Ospedale Policlinico San Martino, Largo Rosanna Benzi, 10, Genoa, 16132, Italy, Tel +390105552815, Email [email protected]
Purpose: To evaluate the prostate cancer (PCa) detection rate in men with chronic use of Aspirin and to compare it with the detection rate of non-users.
Patients and Methods: Prospectively maintained database regarding patients undergoing prostate biopsy over the last 10 years in five institutions. Patients were divided into two groups according to their exposure to Aspirin. We relied on multivariable linear and logistic regression models to test whether Aspirin administration was associated with lower PSA values at prostate biopsy, higher PCa diagnosis, and higher Gleason Grade Grouping (GGG) at biopsy.
Results: Were identified 1059 patients, of whom 803 (76%) did not take Aspirin vs 256 (24%) were taking it. In multivariable log-linear regression analysis, Aspirin administration was associated with lower PSA levels (OR 0.83, 95% CI 0.71– 0.97, p = 0.01), after controlling for age, prostate volume, smoking history, associated inflammation at prostate biopsy, presence of PCa at biopsy, and GGG. In multivariable logistic regression analysis, Aspirin administration was not found to be a predictor of PCa at prostate biopsy (OR 1.40, 95% CI 0.82– 2.40, p = 0.21) after controlling for age, PSA, smoking history, prostate volume, findings at digital rectal examination and the number of biopsy cores. In patients with PCa at prostate biopsy (n = 516), Aspirin administration was found to predict higher GGG (OR 2.24, 95% CI 1.01– 4.87, p = 0.04).
Conclusion: Aspirin administration was found to be a predictor of more aggressive GGG. These findings suggest that a lower PSA threshold should be considered in patients taking Aspirin, as, despite low PSA levels, they might harbour aggressive PCa.
Keywords: prostate-specific antigen, aspirin, inflammation, prostate cancer, prostate biopsy
Introduction
Prostate cancer (PCa) is the most common cancer diagnosed in men accounting alone for 26% of diagnoses in 2021 in the United States, according to population-based cancer incidence data collected by the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) program.1,2 Europe had an all-age incidence ASR of 63 per100000males (473 334 estimated new cases in the year 2020).3
The role of chronic inflammation in carcinogenesis has been widely studied, and it is estimated that it may contribute to the development of up to 20% of all adult cancers.4
In recent years, considerable attention has been paid to how the presence of chronic inflammation, which is found in nearly 70% of autopsied prostates,5 may influence the development of PCa.6–10
In addition, chronic inflammation is known to increase prostate-specific antigen (PSA) levels.6 The increase in PSA level in PCa patients may be inevitably due to PCa itself and partly due to the prostate volume and the associated inflammatory processes.
Considering all these aspects, several studies on anti-inflammatory drugs have been carried out to investigate the potential effect of reduction on lowering both PSA levels and the risk of PCa development.11–16
A recent meta-analysis and population-based case-control studies examined the efficacy of NSAIDs and Acetylsalicylic acid (Aspirin) in preventing PCa: they found a significant 5–29% reduction in relative PCa risk compared with non-users.17–20
On the other hand, the Nashville Men’s Health Study suggests that PSA levels are significantly lower in Aspirin users with latent cancer. The results suggest that Aspirin may interfere with the detection of PCa, highlighting a possible detection bias.11
The aim of our study is to evaluate the PCa detection rate in men with chronic use of Aspirin and to compare it with the detection rate non-users. Aspirin users are expected to have a significantly higher PCa rate, because of the reduction in the proportion of PSA that would be elevated due to inflammation.
Materials and Methods
Study Population and Study Design
This study was approved in 2018 by the South African Health Research Ethics Committee (HREC) (Stellenbosch Health Research Ethics Committee; protocol N18/02/023 - Project ID: 6408).
Patients submitted to prostate biopsy in the past 10 years were consecutively recruited at five different institutions (IRCCS Policlinico San Martino Genova, Italy; San Raffaele Turro Hospital, Milan, Italy; Hospital Garbagnate Milanese, Italy; Ospedale Galliera, Genova, Italy; and Tygerberg Hospital, Cape Town, South Africa) (Flow chart A).
The recommendation for prostate biopsy was made by urologists based on clinical suspicion of PCa (PSA levels, digital rectal examinations, US or MRI findings). Data from our Institution’s prospectively updated databases were retrospectively analyzed including age, race, PSA history, previous prostate surgery, personal habits (smoking and alcohol intake), family history of PCa, DRE, type of biopsy performed, mpMRI performed, and prostate size. Prostate volume was determined based on the transrectal ultrasound (TRUS) before biopsy or MRI findings. We considered systematic prostate biopsies performed by either a transperineal or transrectal approach.21
Transperineal prostate biopsies were performed using either the “fan technique” (FT) or the Free Hand technique (FH).22
Moreover, we included MRI ultrasound fusion biopsies performed with a transrectal approach at all institutions. In these cases, combined targeted and systematic biopsies were performed according to the current EAU guidelines.
The pathology results were reported according to the 2014 International Society of Urological Pathology (ISUP) classification.23
All the prostate biopsy specimens were performed by the same urologist at each institution and the specimens were analyzed by dedicated uro-pathologists in each pathology department. We recorded the number of specimens, the number of positive specimens, the percentage (%) of positive samples, associated inflammation, histology, and the Gleason Score.
Patients’ medical history was collected focusing on long-term Acetylsalicylic Acid, years of use, and dose. We included patients with at least three months of Aspirin therapy, at a dose of 100 mg per day. Aspirin therapy was not discontinued in all 5 institutions before biopsy.
Exclusion criteria were PSA levels above 100 ng/dL, unknown histopathological report at prostate biopsy, immunosuppressive medications or long-term corticosteroids, Aspirin dosages other than 100 mg, unknown duration or dosage of Aspirin therapy, and men with baseline PCa. Patients taking other NAIDS or on 5-alpha-reductase inhibitors (finasteride, dutasteride) were not included. We also excluded prostate biopsies performed by inexperienced urologists (at least 100 procedures).
Endpoints
The primary endpoint was to determine whether there was a higher detection rate in men on chronic therapy with acetylsalicylic acid, compared with non-users.
As a secondary endpoint, we evaluated whether long-term users of acetylsalicylic acid users who were diagnosed with PCa were affected by more aggressive disease in terms of Gleason Grade Group.23
Statistical Analysis
Patients were divided into two groups according to their exposure to Aspirin (group A: Aspirin users; group B: non-users).
Descriptive statistics were used to examine the differences in clinic-pathological characteristics between the two groups with frequencies and proportions reported for categorical variables. Means, medians, and interquartile ranges (IQR) were reported for continuously coded variables. The Wilcoxon rank-sum test and the Kruskal–Wallis rank-sum test examined the statistical significance of difference between the means and distributions of the 2 groups (Aspirin users vs Aspirin non-users). Pearson’s Chi-squared test and Fisher’s exact test were used to test whether or not there was a significant association between the variables.
We relied on multivariable log-linear and logistic regression models to test whether Aspirin administration was associated with lower PSA levels at prostate biopsy, higher prostate cancer diagnosis, and higher Gleason Grade Grouping (GGG) at biopsy.
All tests were two-sided with a level of significance set at p < 0.05. R software environment for statistical computing and graphics (version 3.4.3) was used for all analyses.
Results
We identified 1059 patients (Figure 1), of whom 803 (76%) were not taking Aspirin and 256 (24%) were taking Aspirin. Aspirin users were significantly older (median age 72 years vs 67 years), were smokers (44 vs 15%), had lower median PSA levels (8 ng/mL vs 7 ng/mL), and had displayed prostatic inflammation at prostate biopsy (124 (48%) vs 273 (34%)) (all p < 0.001). The median (interquartile range (IQR)) duration of Aspirin therapy was 25 (12–35) months.
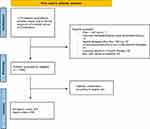 |
Figure 1 Patients’ selection flow chart. |
The majority of participants were Caucasian (985 patients (93%)), followed by Black (52 patients (4,9%)), Hispanic/Latino (20 patients (1,9%)) and American Indian (2 cases (<1%)). No Asian cases were collected. Among group A, 54 (5.1%) transrectal fusion biopsies, 119 (11.24%) systematic transperineal biopsies, and 83 (7.84%) systematic transrectal prostate biopsies were performed. Similarly, among group B 143 (13.5%) transrectal fusion biopsies, 390 (49.4%) systematic transperineal biopsies and 270 (47%) systematic transrectal prostate biopsies were carried out (p = 0.5).
Conversely, there were no statistically significant or clinically meaningful differences between Aspirin users vs Aspirin non-users in terms of prostate volume (57 mL vs 57 mL, p = 0.4), histology [cancer (124 (48%) and 273 (34%)) vs non-cancer (132 (52%) and 530 (66%)), p = 0.6] and higher GGG [4–5 (39 (15%) and 95 (12%)) vs 1-2-3 (89 (35%) and 293 (36%)), p = 0.4] (Table 1). In multivariable log-linear regression analysis, Aspirin administration was associated with lower PSA levels (OR 0.83, 95% CI 0.71–0.97, p = 0.01), after controlling for age, prostate volume, smoking history, associated inflammation at prostate biopsy, presence of PCa at biopsy, and GGG (Table 2). In multivariable logistic regression analysis, Aspirin administration was not found to be a predictor of PCa at prostate biopsy (OR 1.40, 95% CI 0.82–2.40, p = 0.21) after controlling for age, PSA, smoking history, prostate volume, findings at digital rectal examination (DRE) and the number of biopsy cores (Table 3). After controlling for the same variables, in patients with PCa at prostate biopsy (n = 516), Aspirin administration was found to predict higher GGG (OR 2.24, 95% CI 1.01–4.87, p = 0.04) (Table 4).
 |
Table 1 Descriptive Characteristics of 1059 Patients Who Underwent Prostate Biopsy in the Last 10 Years at 5 Different Institutions, Divided into Aspirin Users and Aspirin Non-Users |
 |
Table 2 Regression Models to Test Whether Aspirin Administration Was Associated with Lower PSA Levels at Prostate Biopsy |
 |
Table 3 Regression Models to Test Whether Aspirin Administration Was Associated with Higher Prostate Cancer Diagnosis |
 |
Table 4 Regression Models to Test Whether Aspirin Administration Was Associated with Higher Gleason Grade Group (GGG) at Biopsy |
Discussion
We hypothesized a significantly higher detection rate of PCa in Aspirin users, due to the potential PSA lowering effect associated with Aspirin’s anti-inflammatory properties. Our analysis resulted in many remarkable evaluations.
First, we confirmed that Aspirin users who underwent prostate biopsy had lower PSA levels. Our findings are consistent with previous observations addressed in a post hoc analysis of the REDUCE cohort conducted by Vidal et al. After adjusting for age, race, and DRE, relative to nonusers, both Aspirin use and Aspirin and NSAIDs use were significantly associated with lower PSA compared with non-users (β= - 0.18; β=- 0.22, respectively, p < 0.006).24 The same conclusion was reached in recent research by Praise et al who analyzed the impact of Aspirin use on PSA levels and PCa development in a prospective screening study cohort of 4314 men from the Swiss section of the European Randomized Study of Screening for Prostate Cancer (ERSPC).16 They found that PSA values were significantly lower in ASA users at both baseline (1.6 vs 1.8 ng/mL, p = 0.007) and follow-up visits (1.75 vs 2.1 ng/mL, p < 0.001).
Second, according to our results, Aspirin administration does not appear to be associated with a higher likelihood of PCa at prostate biopsy.
There is still considerable concern regarding the use of Aspirin and/or NSAIDs and the overall risk of PCa. Some studies available in literature found no association between Aspirin intake and PCa risk.12,25,26 On the contrary, several studies supported the PCa risk reduction among Aspirin users. According to a recent meta-analysis by Wang et al which examined whether Aspirin intake can reduce or increase cancer risk in general, the use of Aspirin resulted in a risk reduction of colorectal cancer (RR = 0.85, 95% CI: 0.78–0.92), gastric cancer (RR = 0.67, 95% CI: 0.52–0.87), breast cancer (RR = 0.93, 95% CI: 0.87–0.99) and prostate cancer (RR = 0.92, 95% CI: 0.86–0.98). Regular Aspirin use was estimated to have a significant reduction of 7% prostate cancer risk, with no significant association with PCa mortality. The meta-analysis included 2 randomized clinical trials (RCTs) and 29 cohort studies on PCa incidence and mortality related to the use of NSAIDs.27 Several biological mechanisms have been described by which Aspirin may exert its anticancer effects, including its well-known anti-cyclooxigenase-2 (COX-2) activity.28
COX-2 plays a critical role in the human inflammatory response and its persistent effects may promote cellular changes and, ultimately, cancer development. In contrast, a meta-analysis involving more than 100,000 PCa cases worldwide highlighted that the association between NSAID use and PCa risk varies by geographic region, with some European case-control studies finding an increased risk of PCa overall.15 Although most of the available studies support a favorable protective effect of the Aspirin intake on PCa risk, further large-scale cohort studies and prospective clinical trials are needed to validate these findings.
Finally, we observed that Aspirin administration, in patients with PCa at prostate biopsy, was found to be a predictor of more aggressive Gleason Grade Group. Literature on this topic is controversial and no clear conclusion can be drawn. The studies by Bosetti et al and Olivan et al are in line with our findings both reporting an increase in high-grade PCa among Aspirin Users.13,29 On the contrary, further evidence points in different directions. According to Vidal et al's study, the use of aspirin and/or NSAIDs was significantly associated with decreased total (OR = 0.87, P = 0.030) and high-grade (OR = 0.80, P = 0.040), but not with low-grade, PCa risk (OR = 0.90, P = 0.15).24
Moreover, Nordström et al examining the effect of Aspirin, Statin, or Antidiabetic medications on PCa in a population-based cohort of 185,667 patients, found a borderline significant association was found between the risk of PCa and Aspirin use (OR 1.12; 95% CI: 0.99–1.25; p = 0.06). This association was not significant for high-grade cancer (OR 1.03; 95% CI: 0.89–1.17; p = 0.72).30
Our study is not devoid of limitations. We included patients who regularly took aspirin, but we did not have complete information on the drug recommendation. Due to the retrospective nature of the research, the analysis is prone to selection, detection, or recall bias. The difference in age may be justified considering that the incidence of diseases requiring Aspirin generally increases with age.
We pointed out that the two groups were quite unbalanced for some variables including age. Nevertheless, multivariable adjustment for age was performed in all analyses.
In the multivariable linear and logistic regression models, we did not adjust for other medication such as metformin and statins, which could be potential confounding factors, because of the missing data in the databases. This limitation may impair the strength of the results. Anyway, we believed that our research could be a starting point for further studies.
Furthermore, our analysis focused on patients on Aspirin 100 mg therapy. It is likely that in these patients, Aspirin acts more like anti-platelets than an anti-inflammatory drug. However, it was demonstrated that low-dose aspirin has been shown to have the ability to inhibit innate immune responses.31 However, we believe that future studies should focus on other NSAIDs to further validate our findings.
Moreover, the data were collected from multi-institutional experiences. Of course, the multi-centric nature of the cohort could be considered as a limitation; however, in order to evaluate the generalizability of the results, the heterogeneity of baseline characteristics might be desirable.
Finally, the lack of a centralised pathology review could be considered an additional limitation. However, in each participating institution, pathology specimens are reviewed by dedicated, expert uro-pathologists.
Conclusions
Our study suggests that Aspirin administration may be associated with lower levels of PSA in patients undergoing prostate biopsy. Aspirin administration does not seem to be associated with a higher probability of PCa at prostate biopsy. However, among patients with PCa at prostate biopsy, Aspirin administration was found to be a predictor of more aggressive Gleason Grade Grouping.
Further studies are needed in order to obtain stronger evidence.
Ethics and Consent Statements
Informed consent was obtained from all individual participants included in the study.
Approval of the research protocol by an Institutional Reviewer Board: available, protocol N18/02/023.
Acknowledgments
The authors gratefully acknowledge the multidisciplinary teams of our Institute for their expertise and assistance throughout all aspects of our study. An abstract related to this study has been presented during the AUA22 annual meeting (New Orleans – 13/16 May 2022), https://www.auajournals.org/doi/10.1097/JU.0000000000002537.
Funding
The authors did not receive funding support from any organization for the submitted work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose in this work.
References
1. Mezei T, Bőde I, Tenke P, et al. The correlation between platelet count and survival in prostate cancer. Res Rep Urol. 2022;14:193–202. doi:10.2147/RRU.S359715
2. Söderdahl F, Xu LD, Bring J, Häggman M. A novel risk score (P-score) based on a three-gene signature, for estimating the risk of prostate cancer-specific mortality. Res Rep Urol. 2022;14:203–217. doi:10.2147/RRU.S358169
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
4. Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60(1):199–215. doi:10.1111/j.1365-2559.2011.04033.x
5. Moreira DM, Nickel JC, Gerber L, et al. Baseline prostate inflammation is associated with a reduced risk of prostate cancer in men undergoing repeat prostate biopsy: results from the REDUCE study. Cancer. 2014;120(2):190–196. doi:10.1002/cncr.28349
6. Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60(1):78–83. doi:10.1016/s0090-4295(02)01637-0
7. Gurel B, Lucia MS, Thompson IM, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23(5):847–856. doi:10.1158/1055-9965.EPI-13-1126
8. Jiang J, Li J, Yunxia Z, Zhu H, Liu J, Pumill C. The role of prostatitis in prostate cancer: meta-analysis. PLoS One. 2013;8(12):e85179. doi:10.1371/journal.pone.0085179
9. Cheng I, Witte JS, Jacobsen SJ, et al. Prostatitis, sexually transmitted diseases, and prostate cancer: the California men’s health study. PLoS One. 2010;5(1):e8736. doi:10.1371/journal.pone.0008736
10. Delongchamps NB, de la Roza G, Chandan V, et al. Evaluation of prostatitis in autopsied prostates--is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179(5):1736–1740. doi:10.1016/j.juro.2008.01.034
11. Fowke JH, Motley SS, Smith JA, et al. Association of nonsteroidal anti-inflammatory drugs, prostate specific antigen and prostate volume. J Urol. 2009;181(5):2064–2070. doi:10.1016/j.juro.2009.01.031
12. Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst. 2012;104(16):1208–1217. doi:10.1093/jnci/djs318
13. Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23(6):1403–1415. doi:10.1093/jnci/djs318
14. De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. doi:10.1038/nrc2090
15. Liu Y, Chen J-Q, Xie L, et al. Effect of aspirin and other non-steroidal anti-inflammatory drugs on prostate cancer incidence and mortality: a systematic review and meta-analysis. BMC Med. 2014;12:55. doi:10.1186/1741-7015-12-55
16. Prause LW, Manka L, Millan C, et al. Influence of regular aspirin intake on PSA values, prostate cancer incidence and overall survival in a prospective screening trial (ERSPC Aarau). World J Urol. 2020;38(10):2485–2491. doi:10.1007/s00345-019-03054-5
17. Jafari S, Etminan M, Afshar K. Nonsteroidal anti-inflammatory drugs and prostate cancer: a systematic review of the literature and meta-analysis. Can Urol Assoc J J Assoc Urol Can. 2009;3(4):323–330. doi:10.5489/cuaj.1129
18. Salinas CA, Kwon EM, FitzGerald LM, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol. 2010;172(5):578–590. doi:10.1093/aje/kwq175
19. Leitzmann MF, Stampfer MJ, Ma J, et al. Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1108–1111.
20. Smith CJ, Dorsey TH, Tang W, Jordan SV, Loffredo CA, Ambs S. Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American Men. Cancer Epidemiol Biomarkers Prev. 2017;26(6):845–853. doi:10.1158/1055-9965.EPI-16-1027
21. Xue J, Qin Z, Cai H, et al. Comparison between transrectal and transperineal prostate biopsy for detection of prostate cancer: a meta-analysis and trial sequential analysis. Oncotarget. 2017;8(14):23322–23336. doi:10.18632/oncotarget.15056
22. Mantica G, Pacchetti A, Aimar R, et al. Developing a five-step training model for transperineal prostate biopsies in a naïve residents’ group: a prospective observational randomised study of two different techniques. World J Urol. 2019;37(9):1845–1850. doi:10.1007/s00345-018-2599-6
23. Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–252. doi:10.1097/PAS.0000000000000530
24. Vidal AC, Howard LE, Moreira DM, Castro-Santamaria R, Andriole GL, Freedland SJ. Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study. Clin Cancer Res off J Am Assoc Cancer Res. 2015;21(4):756–762. doi:10.1158/1078-0432.CCR-14-2235
25. Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988–2006. Int J Cancer. 2011;128(10):2444–2452. doi:10.1002/ijc.25811
26. Platz EA, Rohrmann S, Pearson JD, et al. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore longitudinal study of aging. Cancer Epidemiol Biomarkers Prev. 2005;14(2):390–396. doi:10.1158/1055-9965.EPI-04-0532
27. Wang L, Zhang R, Yu L, et al. Aspirin use and common cancer risk: a meta-analysis of cohort studies and randomized controlled trials. Front Oncol. 2021;11:690219. doi:10.3389/fonc.2021.690219
28. Joshi SN, Murphy EA, Olaniyi P, Bryant RJ. The multiple effects of aspirin in prostate cancer patients. Cancer Treat Res Commun. 2021;26:100267. doi:10.1016/j.ctarc.2020.100267
29. Olivan M, Rigau M, Colás E, et al. Simultaneous treatment with statins and aspirin reduces the risk of prostate cancer detection and tumorigenic properties in prostate cancer cell lines. BioMed Res Int. 2015;2015:1–11. doi:10.1155/2015/762178
30. Nordström T, Clements M, Karlsson R, Adolfsson J, Grönberg H. The risk of prostate cancer for men on aspirin, statin or antidiabetic medications. Eur J Cancer Oxf Engl. 2015;51(6):725–733. doi:10.1016/j.ejca.2015.02.003
31. Morris T, Stables M, Hobbs A, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol Baltim Md. 2009;183(3):2089–2096. doi:10.4049/jimmunol.0900477.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
