Back to Journals » Journal of Pain Research » Volume 14
Correlation Between Intravascular Injection Rate, Pain Intensity, and Degree of Cervical Neural Foraminal Stenosis During a Cervical Transforaminal Epidural Block
Authors Kim J , Kim K, Lee M, Kim S
Received 29 July 2021
Accepted for publication 19 September 2021
Published 24 September 2021 Volume 2021:14 Pages 3017—3023
DOI https://doi.org/10.2147/JPR.S330858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Jiseob Kim,1 Kilhyun Kim,2 MinKyu Lee,2 Saeyoung Kim2
1Department of Anesthesiology and Pain Medicine, School of Medicine, Keimyung University, Daegu, Republic of Korea; 2Department of Anesthesiology and Pain Medicine, School of Medicine, Kyungpook National University, Daegu, Republic of Korea
Correspondence: Saeyoung Kim
Department of Anesthesiology and Pain Medicine, School of Medicine, Kyungpook National University, 130 Dongdeok-ro, Jung-gu, Daegu, 41944, Republic of Korea
Tel +82-53-200-5873
; Fax +82-53-426-2760
Email [email protected]
Purpose: Cervical transforaminal epidural blocks (CTEBs) are useful for the treatment of cervical radicular pain. However, during CTEBs, inadvertent intravascular injection can introduce particulate steroids into the bloodstream, thus leading to serious complications. Moreover, the risk factors associated with intravascular injection during CTEBs have not been identified. Cervical neural foraminal stenosis (CNFS) is a form of neural foraminal narrowing and a common cause of cervical radicular pain. In this study, we aimed to identify whether there is a correlation between the incidence of intravascular injection during CTEB, pain intensity, and the degree of CNFS.
Patients and Methods: A total of 126 patients were recruited. The patients were classified into two subgroups (group M and group S) based on the routine cervical T2-weighted axial magnetic resonance imaging (MRI) findings. Group M (n = 63) consisted of moderate CNFS patients, while group S (n = 63) consisted of severe CNFS patients. The occurrence of intravascular injection during CTEB was established using real-time fluoroscopy. The intravascular injection was determined by the spreading of the contrast medium through the vascular channel during the injection. Additionally, pain intensity was scored using a Numeric Rating Scale (NRS) before the procedure and 1 month after the procedure.
Results: There was no significant difference in the incidence of intravascular injection during CTEB between group M and group S (41.3% vs 39.7%, respectively; p = 0.99) and in the NRS scores before and 1 month after CTEB. However, both groups showed a significant decrease in the NRS scores at 1 month after the procedure compared with that before the procedure.
Conclusion: The degree of CNFS does not affect the incidence of intravascular injection during CTEB. Regardless of whether patients have moderate or severe CNFS, caution should be exercised during CTEB procedures.
Keywords: epidural, cervical vertebrae, complications, pain management, radiculopathy
Introduction
Cervical transforaminal epidural block (CTEB) is a useful treatment option for cervical radicular pain.1–5 Kim et al6 demonstrated that the transforaminal epidural steroid injection can be a beneficial clinical option for managing radicular pain due to cervical foraminal stenosis. In their study, they reported that, out of 53 patients, 37 (69.8%) showed successful treatment outcomes. However, the accidental intravascular injection of particulate steroids during CTEB can cause serious complications, including embolic infarction. During CTEB, an inadvertent injection of particulate steroids to the basilar artery can lead to embolic infarction of the midbrain, pons, cerebellum, thalamus, temporal, and occipital lobes. Additionally, an inadvertent injection of particulate steroids to the vertebral artery can lead to spinal cord, brain stem, and cerebellar strokes.7–9 Inadvertent intravascular injection occurs more frequently during CTEB than with other kinds of spinal transforaminal epidural blocks, with an incidence rate of 19.4–63.4%.10–12 Furthermore, it is important to identify the associated risk factors to avoid complications due to intravascular injection during CTEB.
Cervical neural foraminal stenosis (CNFS) is neural foraminal narrowing caused by facet hypertrophy, degenerative osteophytes, or posterolateral disk herniation.6 It is a common cause of cervical radicular pain. The structure of the neural foramen includes the spinal nerve root, segmental spinal artery, intervertebral veins, and recurrent meningeal nerves. Morikawa et al13 demonstrated that degenerative disk disease with spinal stenosis could influence the diameter of spinal epidural vein. However, until now, there is no study on the correlation between intravascular injection rate and degree of neural foraminal stenosis. We hypothesized that foraminal stenosis could result in compression of the blood vessels based on the degree of foraminal stenosis. Therefore, the degree of CNFS could affect the incidence of intravascular injection during CTEB.
In a previous study, Lee et al14 demonstrated that the degree of CNFS was relatively highly correlated with clinical manifestation. However, Kim et al6 retrospectively assessed pain intensity and treatment outcomes of CTEB based on the degree of CNFS. Furthermore, they demonstrated that CTEB had successful treatment outcomes for CNFS-induced radicular pain. However, the degree of CNFS did not affect pain intensity. Additionally, in the literature, no prospective study to date has investigated the pain intensity before and after CTEB based on the degree of CNFS.
Thus, this study aimed to determine whether there are any correlations between the incidence of intravascular injection during CTEB, pain intensity before and after CTEB, and the degree of CNFS.
Materials and Methods
Participants
A total of 126 CTEB recipients were examined between October 2018 and September 2019. The inclusion criteria for this study included patients over 18 years of age with CNFS, identified by magnetic resonance imaging (MRI), and cervical radicular pain that had persisted following a single cervical interlaminar epidural block. The exclusion criteria included rejection of our participation request, allergy to the contrast media, presence of peripheral neuropathy, spondylolisthesis, or cervical myelopathy, pregnancy, history of cervical spine surgery, and persistent contraindication for a neural blockade, such as coagulopathy or infection at the injection site.
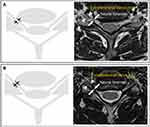
The participants were classified into two subgroups (group M and group S) based on routinely obtained, cervical T2-weighted axial MRI findings, as previously described by Park et al (Figure 1).15 Group M consisted of participants with moderate CNFS (in which the narrowest width of the neural foramen was <80% of the extraforaminal nerve root width but > 50% of the extraforaminal nerve root width at the level of the anterior margin of the superior articular process). Group S consisted of participants with severe CNFS (in which the narrowest width of the neural foramen was ≤ 50% of the extraforaminal nerve root width). A total of 63 participants were included in both group M and group S.
Study Design and Treatment
This was a prospective observational study approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH 2018-06-009-001). The study was conducted in accordance with the tenets of the Declaration of Helsinki. All participants provided written informed consent before enrollment. This study was registered with the ClinicalTrials.gov (NCT04071483).
The CTEB was performed by a physician and observed by three other physicians involved in the study. CTEB was aseptically performed using fluoroscopic guidance using an 8.9 cm, 25 G spinal needle (Hakko Co., Chikuma-shi, Nagano-gen, Japan). Participants were placed in a supine position on a fluoroscopy table, and the fluoroscope (Ziehm Vision, Ziehm Imaging, Nuremberg, Germany) was rotated between 45° and 55° obliquely to the ipsilateral side to allow for visualization of the neural foramen with maximum transverse width. The curved spinal needle was advanced toward the posterior aspect of the intervertebral foramen until the tip reached the superior articular process at the division between the caudal and middle thirds. The needle was advanced 2–3 mm into the foramen, no further than halfway across the facet column, with an anteroposterior fluoroscopic view. Finally, the needle position was confirmed using biplanar fluoroscopy using 2 mL of a nonionic contrast medium (Omnipaque 300, GE Healthcare, Little Chalfont, Buckinghamshire, UK) injected at a rate of 0.5 mL/sec under real-time fluoroscopy (RTF). The intravascular injection was determined based on whether the contrast medium spread through the vascular channel. If the intravascular injection occurred, further injection of local anesthetics and steroids was aborted. Furthermore, we performed cervical epidural percutaneous neuroplasty for these patients. If the intravascular injection did not occur, 3 mL of 0.5% lidocaine in 10 mg of dexamethasone was injected. Finally, only patients with CTEB were enrolled in the pilot study for the further Numeric Rating Scale (NRS) assessment.
Sample Size
In the pilot study, the incidence of intravascular injection in participants with moderate CNFS was 50%. A 50% reduction in the incidence of intravascular injection with severe CNFS was considered clinically meaningful. The sample size was estimated based on the requirement of Type I and II errors being <0.05 and <0.20, respectively. Allowing for a 10% dropout rate, a total of 63 CTEB cases were required for each group.
Statistical Analysis
The data on age, sex, height, weight, site of injection, and injection level were recorded for each participant. Data were analyzed using the Statistical Package for the Social Sciences v. 25.0 (IBM Corporation, Armonk, NY, USA). The continuous variables of age, height, and weight were compared using independent t-tests. The categorical variables of gender, site of injection, injection level, degree of CNFS, and incidence of intravascular injection were compared using chi-square tests. The influence of each factor on intravascular injection was examined by logistic regression analyses, and the adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated. A p-value of <0.05 was considered statistically significant.
Results
A total of 126 participants with a mean age of 52.7 years (range = 28–81 years) were included in the study. Table 1 presents the demographic characteristics of the study participants. No significant differences were observed in demographic or clinical characteristics between the two groups. Table 2 presents the incidence of intravascular injection in each group. No significant difference was observed in the incidence of intravascular injection between the moderate and severe CNFS groups (41.3% vs 39.7%, respectively; p = 0.99). Table 3 shows the adjusted ORs and 95% CIs for each variable’s relation to the intravascular injection rate. The incidence of intravascular injection was not affected by age, gender, height, weight, or site of injection. Furthermore, there were no adverse events in either group.
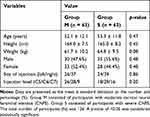 |
Table 1 Demographic and Clinical Characteristics of the Study Participants |
 |
Table 2 Incidence of Intravascular Injection During Cervical Transforaminal Epidural Block (CTEB) According to Cervical Neural Foraminal Stenosis Severity |
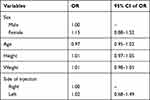 |
Table 3 Odds Ratios (OR) and 95% Confidence Intervals (CI) of the Intravascular Injection Rate Variables |
There was no significant difference in pain intensity scores assessed using the NRS between group M and group S before the procedure (6.14 ± 1.7 vs 6.05 ± 2.0, respectively; p = 0.784) or one month after the procedure (3.58 ± 2.3 vs 3.14 ± 2.2, respectively; p = 0.264). However, both groups showed a significant decrease in the NRS scores at 1 month after the procedure compared with that before the procedure. However, the intergroup changes over time were not significantly different (Figure 2). Furthermore, there were no adverse events in either group.
Discussion
No significant difference in the incidence of intravascular injection during CTEB was observed between the moderate and severe CNFS groups. Additionally, no significant difference in pain intensity was observed between the two groups before the procedure or one month after the procedure.
The CNFS is a disorder characterized by narrowing of the intervertebral foramen. The resulting nerve compression and inflammation frequently cause cervical radicular pain.6 Several methods have been proposed for the grading of CNFS severity.15–17 In this study, the severity of CNFS was assessed using T2-weighted axial MRI scans, as described by Park et al.15 Using this method, a remarkably high correlation has been demonstrated between clinical observation and severity grading. The approach provides reliable and reproducible CNFS diagnoses.14
Our results showed that intravascular injection rates were not correlated with CNFS severity. This result was in contrast to our expectation that the degree of CNFS could affect the incidence of intravascular injection during CTEB. Even though the degree of CNFS was severe, its effect was not significantly different from that in moderate degree stenosis. Therefore, regardless of the extent of the CNFS, care should be taken when conducting CTEB procedures.
To the best of our knowledge, this is the first study to assess the risk factors associated with intravascular injection during CTEB. In addition to the primary finding that the incidence of intravascular injection during CTEB was unaffected by the degree of CNFS, we also established that age, gender, height, weight, and side of injection do not affect the incidence of intravascular injection. This result is consistent with a previous study that assessed intravascular injection during transforaminal epidural blocks at all spinal levels, including the cervical, thoracic, lumbar, and sacral levels. In line with our findings, there were no associations in this research between intravascular injection and age, gender, body mass index, diagnosis, injection level, side of injection, history of spinal surgery at the targeted level, or the number of injections at the target site.10
Several methods have been proposed to reduce the risk of intravascular drug injection during CTEB, including the use of contrast agents with RTF or digital subtraction angiography (DSA),18 the use of blunt rather than sharp needles,19 evaluation of axial T2-weighted MRI data before the procedure,20 and the application of test doses of local anesthetic.21 Injecting the contrast agent with RTF is considered an important method for detecting intravascular injection. DSA has been shown to be more sensitive for detecting vascular uptake.18 However, Jeon et al22 reported that using DSA in cervical transforaminal epidural injection is not beneficial compared to RTF. Therefore, in this study, we used RTF to detect intravascular injection. Furthermore, to prevent intravascular injection when treating cervical stenosis-induced radicular pain with CTEB, we recommend alternative procedures, such as interlaminar epidural injections23 and epidural percutaneous neuroplasty.24
No significant difference in pain intensity based on the degree of CNFS was observed. Therefore, it can be concluded that there is no association between the degree of CNFS and the degree of pain intensity. In a previous study, Stafford et al25 demonstrated that the mechanical compression of a nerve root without inflammation could result in neurological deficits rather than radicular pain. Therefore, inflammation at the nerve root played an important role in initiating processes resulting in radicular pain. The degree of inflammation, not the degree of foraminal stenosis, was thought to be a major factor influencing the intensity of radicular pain.6 We also found that, regardless of the degree of the CNFS, participants’ pain was reduced, and there was no difference in the treatment outcomes of CTEB between the moderate and severe CNFS groups, which is consistent with a previous study by Kim et al.6
There are several limitations to this study. First, we were unable to determine whether intravascular injections occurred via arteries or veins. This has also been an issue in previous studies,10,22,26 which have also been unable to define the vascular contrast spreading patterns as venous or arterial during transforaminal epidural blocks. This is because the patterns are ambiguous, despite the use of RTF or DSA. Second, we assessed each patient`s pain intensity at only one-time point, one month after the procedure; therefore, the long-term effects of CTEB based on the degree of CNFS remain unknown. Finally, although we ran a power analysis based on the effect size observed in a small pilot study to ensure the sample sizes were large enough, the sample size was small and the study was performed in a single center. Therefore, to validate our results, multicenter studies and sufficiently powered replications are needed.
Conclusion
In conclusion, our findings show that the degree of CNFS does not affect the incidence of intravascular injection. Furthermore, good anatomical knowledge of the vertebral vessels is essential to avoid intravascular injection during CTEB. It is also important to use a contrast medium with RTF, to start with a test dose of the local anesthetic, and to use nonparticulate steroids. However, serious complications have been reported even when such precautions have been taken. Therefore, regardless of whether patients have moderate or severe CNFS, it is vital that caution be exercised during CTEB procedures.
Data Sharing Statement
No further data will be shared.
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board of Kyungpook National University Hospital. All participants provided written informed consent before enrollment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas.
All authors took part in drafting, revising or critically reviewing the article.
All authors gave final approval of the version to be published.
All authors have agreed on the journal to which the article has been submitted.
All authors have agreed to be accountable for all aspects of the work.
Funding
There is no funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Costandi SJ, Azer G, Eshraghi Y, et al. Cervical transforaminal epidural steroid injections: diagnostic and therapeutic value. Reg Anesth Pain Med. 2015;40:674–680.
2. Schellhas K, Pollei S, Johnson BA, et al. Selective cervical nerve root blockade: experience with a safe and reliable technique using an anterolateral approach for needle placement. Am J Neuroradiol. 2007;28(10):1909–1914. doi:10.3174/ajnr.A0707
3. Shakir A, Ma V, Mehta B. Prediction of therapeutic response to cervical epidural steroid injection according to distribution of radicular pain. Am J Phys Med Rehabil. 2011;90(11):917–922. doi:10.1097/PHM.0b013e31822de95b
4. Lee JW, Park KW, Chung SK, et al. Cervical transforaminal epidural steroid injection for the management of cervical radiculopathy: a comparative study of particulate versus non-particulate steroids. Skeletal Radiol. 2009;38:1077–1082. doi:10.1007/s00256-009-0735-5
5. Lee SH, Kim KT, Kim DH, Lee BJ, Son ES, Kwack YH. Clinical outcomes of cervical radiculopathy following epidural steroid injection: a prospective study with follow-up for more than 2 years. Spine. 2012;37:1041–1047. doi:10.1097/BRS.0b013e31823b4d1f
6. Kim MS, Lee DG, Chang MC. Outcome of transforaminal epidural steroid injection according to severity of cervical foraminal stenosis. World Neurosurg. 2018;110:e398–e403. doi:10.1016/j.wneu.2017.11.014
7. Ludwig MA, Burns SP. Spinal cord infarction following cervical transforaminal epidural injection: a case report. Spine. 2005;30(10):E266–E268. doi:10.1097/01.brs.0000162401.47054.00
8. Epstein NE. Major risks and complications of cervical epidural steroid injections: an updated review. Surg Neurol Int. 2018;9(1):86. doi:10.4103/sni.sni_85_18
9. Schneider BJ, Maybin S, Sturos E. Safety and complications of cervical epidural steroid injections. Phys Med Rehabil Clin N Am. 2018;29:155–169. doi:10.1016/j.pmr.2017.08.012
10. Nahm FS, Lee CJ, Lee SH, et al. Risk of intravascular injection in transforaminal epidural injections. Anaesthesia. 2010;65:917–921. doi:10.1111/j.1365-2044.2010.06447.x
11. Smuck M, Tang CT, Fuller BJ. Incidence of simultaneous epidural and vascular injection during cervical transforaminal epidural injections. Spine. 2009;34:E751–E755. doi:10.1097/BRS.0b013e3181b043d1
12. Hwang SJ, Han KR, Kim SY, Kim NS, Kim C. Analysis of intravascular flow patterns following cervical transforaminal epidural injection. Korean J Pain. 2009;22:52–57. doi:10.3344/kjp.2009.22.1.52
13. Morikawa M, Sato S, Numaguchi Y, Mihara F, Rothman MI. Spinal epidural venous plexus: its MR enhancement patterns and their clinical significance. Radiat Med. 1996;14:221–227.
14. Lee KH, Park HJ, Lee SY, et al. Comparison of two MR grading systems for correlation between grade of cervical neural foraminal stenosis and clinical manifestations. Br J Radiol. 2016;89:20150971. doi:10.1259/bjr.20150971
15. Park HJ, Kim JH, Lee JW, et al. Clinical correlation of a new and practical magnetic resonance grading system for cervical foraminal stenosis assessment. Acta Radiol. 2015;56:727–732. doi:10.1177/0284185114537929
16. Kim S, Lee JW, Chai JW, et al. A new MRI grading system for cervical foraminal stenosis based on axial T2-weighted images. Korean J Radiol. 2015;16(6):1294–1302. doi:10.3348/kjr.2015.16.6.1294
17. Park HJ, Kim SS, Lee SY, et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol. 2013;86:20120515. doi:10.1259/bjr.20120515
18. Visnjevac O, Kim P, Farid-Davari S, Johnson P, Nader ND. Digital subtraction angiography versus real-time fluoroscopy for detection of intravascular penetration prior to epidural steroid injections: meta-analysis of prospective studies. Pain Physician. 2015;18:29–36.
19. Jeon Y, Lee J, Shin H, Park C, Kim K, Kim S. Comparison of intravascular injection rate between blunt and sharp needles during cervical transforaminal epidural block. Pain Physician. 2019;22:265–270.
20. Beckworth WJ, Sood R, Katzer AF, Wu B. Anomalous location of the vertebral artery in relation to the neural foramen. Implications for cervical transforaminal epidural steroid injections. Pain Med. 2013;14(8):1119–1125. doi:10.1111/pme.12121
21. Karasek M, Bogduk N. Temporary neurologic deficit after cervical transforaminal injection of local anesthetic. Pain Med. 2004;5(2):202–205. doi:10.1111/j.1526-4637.2004.04028.x
22. Jeon Y, Kim S. Detection of intravascular injection during cervical transforaminal epidural injection: a comparison of digital subtraction angiography and real time fluoroscopy. Pain Physician. 2018;21:E181–E186.
23. Manchikanti L, Falco FJ, Diwan S, Hirsch JA, Smith HS. Cervical radicular pain: the role of interlaminar and transforaminal epidural injections. Curr Pain Headache Rep. 2014;18:389. doi:10.1007/s11916-013-0389-9
24. Helm S, Knezevic NN. A review of the role of epidural percutaneous neuroplasty. Pain Manag. 2019;9:53–62. doi:10.2217/pmt-2018-0042
25. Stafford MA, Peng P, Hill DA. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99(4):461–473. doi:10.1093/bja/aem238
26. Kim DW, Han KR, Kim C, Chae YJ. Intravascular flow patterns in transforaminal epidural injections: a comparative study of the cervical and lumbar vertebral segments. Anesth Analg. 2009;109(1):233–239. doi:10.1213/ane.0b013e3181a826db
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.