Back to Journals » International Journal of General Medicine » Volume 14
Correlation Between Higher Cumulative Dose of Cisplatin for Concurrent Chemoradiation and Acute Kidney Disease Incidence Among Nasopharyngeal Carcinoma Patients: A Comparative Study
Authors Rachman A , Shatri H , Salamat R
Received 19 October 2021
Accepted for publication 7 December 2021
Published 31 December 2021 Volume 2021:14 Pages 10527—10539
DOI https://doi.org/10.2147/IJGM.S343644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Andhika Rachman,1 Hamzah Shatri,2 Ruben Salamat3
1Division of Hematology and Medical Oncology, Department of Internal Medicine, Faculty of Medicine, University of Indonesia – Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; 2Division of Psychosomatic and Palliative Care, Department of Internal Medicine, Faculty of Medicine, University of Indonesia – Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; 3Department of Internal Medicine, Faculty of Medicine, University of Indonesia – Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia
Correspondence: Andhika Rachman Tel +62 813-9862-0570
Email [email protected]
Introduction: Nasopharyngeal carcinoma (NPC) is the most malignant cancer in the head and neck area. According to the stage, the management of NPC includes radiation, chemotherapy, or a combination of both. The standard agent for radiosensitizing chemotherapy is cisplatin. Among the several effects of cisplatin administration, nephrotoxicity raises the most concern, especially in high doses. Acute kidney disease (AKD) is a condition in which an acute kidney injury occurs at > 7 days but < 90 days. This study aimed to assess whether there is a significant difference in the incidence of AKD between NPC patients who received a cumulative dose of cisplatin up to (≤) 200 mg/m2 and patients who received more than (>) 200 mg/m2.
Methods: This is a cohort retrospective study conducted in the radiotherapy unit of Cipto Mangunkusumo General Hospital. Medical records of 540 patients from January 2014 to December 2018 were collected and sorted. After sorting, 120 of the records were analyzed.
Results: The analysis showed that 38.4% of patients who received > 200 mg/m2 cumulative dose of cisplatin experienced AKD, whereas 38.3% of the patients who received ≤ 200 mg/m2 cumulative dose of cisplatin experienced AKD.
Conclusion: This study found that in patients with locally advanced NPC who received cisplatin chemoradiation, there was no significant difference in the incidence of AKD, recovery of renal function, or progression of chronic kidney disease between patients receiving a cumulative dose of cisplatin ≤ 200 mg/m2 and those receiving > 200 mg/m2.
Keywords: cisplatin, nasopharyngeal cancer, acute kidney disease, chemoradiation
Background
Nasopharyngeal carcinoma (NPC) emerges in the nasopharynx, the area above the throat and behind the nose, showing mild or ultrastructural microscopic squamous differentiation.1 Nasopharyngeal carcinoma (NPC) is the most malignant tumor in the head and neck region. Based on GLOBOCAN 2018, worldwide, there are 129,079 new cases every year and 72,987 deaths due to NPC.2 Indonesia is the country with the second-highest prevalence of NPC, after China. Compared to the NPC incidence (17,992/year), the mortality rate (11,204/year) is relatively high in Indonesia.2 This is because 80% of patients diagnosed with NPC have entered the locally advanced stage.3
NPC is a complex disease caused by the interaction of genetic factors, chronic infection such as Epstein–Barr virus (EBV), and environmental factors. A definite diagnosis is carried out using endoscopic biopsy of the primary nasopharyngeal tumor.4 The management of NPC includes radiation, chemotherapy, or a combination of both, according to the stage. Chemotherapy can be administered as neoadjuvant (induction), concurrent (concurrent with radiotherapy), or adjuvant (post-definitive) therapy.5 Combination of chemoradiation as a radiosensitizer is mainly administered to patients with T2–T4 and N1–N3 NPC. Chemotherapy as a radiosensitizer is administered six times using platinum-based preparations of 30–40 mg/m2, once a week, 2.5–3 hours before radiation. The standard agent used for concurrent chemoradiation is cisplatin, which has been shown to improve survival, tumor control, and metastasis.4
Several concurrent chemoradiation protocols have been used worldwide, including cisplatin 100 mg/m2 administered every 3 weeks in the USA,6 cisplatin 20 mg/m2 per day for 4 days administered at weeks 1 and 5 in Taiwan,7 and cisplatin 40 mg/m2 per week in Hong Kong.8 The center in the current study, Cipto Mangunkusumo General Hospital/RSUPN Dr. Cipto Mangunkusumo (RSCM), adopts a concurrent chemoradiation protocol with cisplatin 40 mg/m2 per week. The optimal cumulative cisplatin dose for NPC has not acquired global or national consensus. One study showed that patients with >200 mg/m2 cumulative cisplatin dose have better 5-year overall survival than those who receive 200 mg/m2,9 while other studies had different results.10–12 Our center’s chemotherapy protocol states that the minimum cumulative cisplatin dose for NPC is 200 mg/m2.
According to the National Comprehensive Cancer Network (NCCN) guideline, the primary treatment choice for patients with locally advanced NPC is cisplatin-based concurrent chemoradiation.13 Cisplatin is a broad-spectrum anticancer therapy.14 It is used in the treatment of various types of cancer, such as head and neck cancer, soft tissue sarcomas, small and non-small lung cancer, breast cancer, cervical cancer, gastric cancer, bone cancers, testicular cancer, ovarian cancer, bladder cancer, Hodgkin and non-Hodgkin lymphoma, neuroblastoma, melanoma, and mesothelioma.15
The main target of platinum agents is the DNA inside the nucleus. These drugs inhibit transcription and replication and also result in apoptosis. The most common cisplatin toxicities are nephrotoxicity, neurotoxicity, and ototoxicity. Among these side effects, nephrotoxicity is the most frequent adverse effect, which can limit subsequent use of this agent. A direct manifestation of nephrotoxicity is acute kidney injury (AKI), which can progress to acute kidney disease (AKD).16–18
AKI, AKD, and chronic kidney disease (CKD) lie on a spectrum of impaired kidney function, where AKI is a sudden disruption/decrease in kidney function within <7 days, CKD is a decrease in kidney function occurring in >90 days, and AKD is a condition in which AKI stage 1 or more occurs >7 days after initiating AKI but <90 days.17,19 The degree of AKI, depending on the number of chemotherapy lines and the dose, can be reversible or irreversible. Data from the M.D. Anderson Cancer Center in the USA showed that that AKI occurred in 45% of all cancer patients during the first 2 days and 55% subsequently.20 Meanwhile, in Indonesia, Prasaja et al21 found that AKI occurred in 34% of all cancer patients receiving cisplatin.
AKI, which is marked by increased serum creatinine and blood urea nitrogen, can occur within a few days after cisplatin administration.22,23 A study by Hayes et al24 found that the increase in serum creatinine occurs on days 6–7 and remains high for 3–4 weeks after cisplatin administration. AKI can be partially reversible, progressive, or permanent, especially with repeated cisplatin administration.25–28
Based on a literature search conducted by the authors, it turns out that no studies have examined the relationship between AKD incidence and cumulative dose of cisplatin in NPC patients who received concurrent chemoradiation. The existing studies primarily looked at AKI incidence (not AKD), using different doses and protocols from those used in RSCM.29–32 This study aimed to confirm whether the incidence of AKD was significantly different between the cumulative doses of ≤200 mg/m2 and >200 mg/m2. It is hoped that this study will serve as a guide for determining the optimal cumulative dose of cisplatin to produce beneficial antitumor effects with tolerable toxicity.
Methods
Study Participants
This retrospective cohort study was conducted in the radiotherapy unit of Cipto Mangunkusumo General Hospital. The target population of this study was adult patients with NPC. Data were collected from patients’ medical records. The inclusion criteria were NPC patients 18–65 years of age, who received concurrent chemoradiation therapy with cisplatin 30–40 mg/m2 weekly, with a performance status score of at least 80 using the Karnofsky scale or at least 1 using the Eastern Cooperative Oncology Group (ECOG) scale, and a glomerular filtration rate (GFR) >60 mL/min/1.73 m2. The staging of NPC is based on the International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) staging system. Adverse effects are defined according to the Common Terminology Criteria for Adverse Events (CTCAE). The exclusion criteria were incomplete records, uncontrolled hypertension with a diastolic blood pressure of ≥100 mmHg, urinary tract obstruction, diabetes, congestive heart failure, CKD, severe infections/sepsis, receiving furosemide, pregnant women, patients who had received cisplatin in the past/before their current treatment, patients with a history of hospitalization during chemoradiation, and patients with a history of hemodialysis.
Data from January 2014 to December 2018 were collected in July to August 2019. Data analysis was carried out using SPSS version 23.0. Bivariate analysis was conducted using a 2×2 table to determine the odds ratio (OR) for the probability of AKD between patients receiving cisplatin with a cumulative dose of >200 mg/m2 and those with a cumulative dose of ≤200 mg/m2.
Acute Kidney Injury and Acute Kidney Disease
The diagnostic criteria for AKI consist of an increase in serum creatinine by ≥0.3 mg/dL within 48 hours or increased serum creatinine to ≥1.5 times baseline, occurring within 7 days, or a decrease in urine output to <0.5 mL/kg/hour for 6 hours.33 AKI is then divided into stages 1, 2, and 3 based on the serum creatinine increase or urine output. AKD is a condition in which AKI stage 1 or more persists for more than 7 days but less than 90 days.33 GFR was calculated using the CKD-EPI formula, which consists of four variables: gender, race, age, and serum creatinine.
AKD is typically divided into stages 0–3. Compared to the baseline creatinine level, stage 1 AKD is defined as an increase in serum creatinine level to 1.5–1.9 times, stage 2 AKD is defined as an increase in serum creatinine level to 2.0–2.9 times, and stage 3 AKD is defined as an increase in serum creatinine level to ≥3 times, all of which persist for 7–90 days after the initial AKI event.19,34 Stage 0, also known as subacute AKD, is further divided into A, B, C, and B/C, depending on specific clinical findings. “A” is defined as no sign of injury and not fitting “B” or “C”. “B” is defined as evident prolonged kidney injury with several biomarkers indicating loss of renal reserve. “C” is defined as an increase in serum creatinine <1.5 times baseline level that does not return to the baseline value.19,34
In this study, serum creatinine levels were evaluated periodically, whereby the initial serum creatinine measurement was collected before cisplatin-based chemotherapy was initiated. Subsequent serum creatinine measurements were taken after each chemotherapy cycle between 1 week post-chemotherapy and the day before the next cycle. For the last cycle, creatinine measurement was taken between 1 week and 90 days after chemotherapy.
Premedication and Hydration Protocols
Standard premedication for cisplatin chemotherapy consists of 50 mg of intravenous ranitidine, 8 mg of intravenous ondansetron, 10 mg of intravenous diphenhydramine, and 5–10 mg of intravenous dexamethasone. Patients were also administered 1–2 L of intravenous saline hydration before chemotherapy administration. Normal saline hydration was then continued at 100 mL/hour until 3 days after chemotherapy. Hydration protocols for subjects in this study did not include mannitol.
Concurrent Chemoradiation
The chemotherapeutic agent used as radiosensitizers in RSCM is 40 mg/m2 cisplatin once a week (on Mondays), 2.5–3 hours before radiation. Chemotherapy may be administered until week 7. Radiation therapy was delivered using two-dimensional intensity-modulated radiation therapy (IMRT) with a radiation dose of 70–70.2 Gy (1.8–2.0 Gy/fraction) every Monday to Friday for 7 weeks.
Statistical Analysis
The collected data were analyzed using SPSS version 23.0. The primary characteristics of the subjects are presented in Table 1. Mean and standard deviation evaluations were performed on quantitative data. Bivariate analysis using a 2×2 table was conducted to evaluate the odds ratio (OR) of AKD between the subjects receiving a cumulative dose of cisplatin >200 mg/m2 and those receiving ≤200 mg/m2. Chi-squared analysis was performed to determine the p-value. For categorical variables, p values were also determined using chi-squared analysis. Multivariate analysis and logistic regression were conducted on variables with p<0.250.
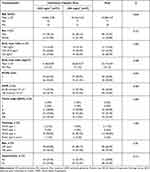 |
Table 1 Characteristics of the Study Subjects |
Ethical Approval
This study has been approved by the Ethical Committee of the Faculty of Medicine, University of Indonesia (ethical approval number KET-718/UN2.F1/ETIK/PPM.00.02/2019). It was decided by the committee that individual patient consent was not required as long as confidentiality could be maintained. This waiver was granted because this study did not apply any intervention, and complete anonymity is maintained. Patient confidentiality is protected and maintained in compliance with the Declaration of Helsinki.
Results
In this study, 120 subjects were eligible for data analysis. Out of 120 subjects, 89 were male (74.2%), and the subjects’ age ranged from 18 to 71 years old (mean: 45.08 years old). About 33% were <40 years old, while 66.7% were over 40. Most patients were in stage IVA (43.3%), with the most common histopathological classification being WHO type 3 (95.8%). The subjects’ ECOG status was mainly in a fair condition (ECOG 1=70%). The characteristics of the subjects are shown in Table 1.
All subjects had good renal function before chemotherapy. Patients who received a cumulative dose of cisplatin >200 mg/m2 had a mean pre-chemotherapy GFR of 103.58 mL/min/1.73 m2 and 86.35 mL/min/1.73 m2 post-chemotherapy. Those who received a cumulative dose of ≤200 mg/m2 had a mean pre-chemotherapy GFR of 99.92 mL/min/1.73 m2 and 84.19 mL/min/1.73 m2 post-chemotherapy. Hydration was more aggressive in those who received cisplatin >200 mg/m2 compared to those who received cisplatin ≤200 mg/m2 (median total hydration volume 9000 mL vs 7500 mL, respectively). Table 2 shows the clinical characteristics of the subjects’ pre- and post-chemoradiation therapy.
 |
Table 2 Clinical Characteristics of the Subjects, Before and After Chemoradiation |
It can be seen that the decrease in renal function (seen from the mean estimated GFR [eGFR]) is more apparent in those who received >200 mg/m2 cisplatin cumulative dose. The mean eGFR of both groups for each cycle of chemotherapy administered can be seen in Figures 1 and 2. The figures show a slight improvement in eGFR in the fifth cycle for both groups (Figures 1 and 2).
 |
Figure 1 Mean eGFR for each chemoradiation cycle in subjects receiving >200 mg/m2 cumulative dose of cisplatin. |
 |
Figure 2 Mean eGFR for each chemoradiation cycle in subjects receiving ≤200 mg/m2 cumulative dose of cisplatin. |
Effect of Cumulative Cisplatin Dose on AKD
Of the subjects receiving cisplatin >200 mg/m2, 28 patients (38.4%) experienced AKD, while among subjects receiving ≤200 mg/m2 cisplatin, 18 experienced AKD (38.3%). The mean cumulative dose in the first group was 200 mg/m2. In the higher dose group, the mean cumulative cisplatin dose was 246.31 mg/m2. The occurrence of AKD post-chemotherapy can be seen in Table 3. Among those who experienced AKD, most of the subjects had grade 1 AKD. The proportions of AKD grades of the subjects can also be seen in Table 3.
 |
Table 3 Occurrence of AKD After Completion of Chemotherapy Cycle |
Potential Confounding Factors and Their Correlation with AKD Post-Chemoradiation
Some notable confounding factors in this study were age, sex, grade 2 mucositis, hypertension, initial GFR, vomiting, and ECOG performance status. The correlation of these factors with AKD can be seen in Table 4.
 |
Table 4 Potential Confounding Variables |
Multivariate Analysis
Variables with a p-value <0.25 were analyzed further for multivariate analysis: grade 2 mucositis, vomiting, and sex. The results can be seen in Table 5. Here, it is seen that grade 2 mucositis, vomiting, and sex are not confounding factors for a cumulative dose of cisplatin with the occurrence of AKD because the change in OR is less than 10%.
 |
Table 5 Crude RR and Adjusted RR with 95% CI of Cumulative Dose Towards AKD Post-Chemotherapy with Gradual Addition of Confounding Factors |
Analysis of Progression and Subject Recovery from AKD Post-Chemotherapy
Subjects who had AKD were observed to see whether there were further progressions or recovery. The proportions of progressions and recovery from AKD can be observed in Table 6. Furthermore, it can be seen that of all the subjects with AKD who received >200 mg/m2 cumulative dose of cisplatin, three (10.7%) experienced recovery, while of those who received ≤200 mg/m2 only one (5.6%) achieved recovery. Recovery in the following months can be seen in Table 6.
 |
Table 6 Subjects Who Experienced Recovery After AKD |
Of the subjects from the group that received >200 mg/m2 cumulative dose of cisplatin who experienced AKD, 10 subjects (35.7%) progressed to CKD, while seven (38.9%) progressed to CKD from the other group (Table 7).
 |
Table 7 Proportions of Subjects with AKD Who Experienced Progression Towards CKD |
Discussion
In this study, the mean age of the patients with NPC was 45.08 years. The mean ages of subjects in the groups with a cumulative dose of cisplatin >200 mg/m2 and ≤200 mg/m2 were relatively the same. These characteristics are similar to those in another study conducted in Indonesia by Prasaja et al.21 In contrast, in studies in other countries the subjects were older, as reported by Faig et al in the USA32 and Driessen et al in the Netherlands,17 where the mean ages (± standard deviation) were 54±10.8 years and 55.3±7.6 years, respectively.
In this study, there were more male patients than female patients, with a ratio of 7:3. There was a higher proportion of men than women in both groups. According to the data from GLOBOCAN 2021, head and neck cancer is more common in men than in women, with a ratio of 2–3:1.35 A large number of men experience head and neck cancer cases; this is associated with the microendocrine milieu, which is rich in the hormone testosterone.36
Most of the subjects in this study had stage IVA cancer (43.3%). This is in accordance with a study by Faiza et al37 in Western Indonesia, where most subjects were stage IVA and IVB (31.82% and 31.82%, respectively). The most common histopathological result in our study was WHO type 3 (95.8%). This is similar to a study by Adham et al,38 which was also conducted in Indonesia. There were no significant differences in the histopathological type of cancer between patients receiving cisplatin ≤200 mg/m2 and those receiving >200 mg/m2.38
Most of the patients had good performance and nutritional status. These data are important because poor nutritional status may influence the result of the serum creatinine examination, affecting the eGFR values. Moreover, as many as 25.8% of the patients had hypertension. Hypertension is a known factor for kidney disease. Other comorbidities, such as diabetes mellitus, heart disease, CKD, and severe infections, have been excluded. There was no significant difference in hypertension between the two groups.
In this study, all patients had good kidney function before the administration of chemotherapy. Mean baseline eGFR was comparable with the results of the studies by Driessen et al17 and Faig et al.32
Mucositis affected the fluid intake of the patients in this study. Almost all patients in this study had mucositis, mostly grade 1. However, mucositis in this study was less frequent and less severe than that in the study by Faig et al,32 where all of the patients had mucositis, which varied from grade 1 to grade 4. This may be because the cisplatin dose in Faig’s study was higher, ie, 100 mg/m2 per cycle. It is known that cisplatin is cytotoxic to all cells, so that the greater the cisplatin dose, the greater the damage, including mucositis. Apart from mucositis, 27.5% of the patients in this study experienced vomiting.
Kidney disorder induced by cisplatin chemotherapy is characterized by several histological changes, including acute focal necrosis of the proximal convoluted tubules and collecting ducts, dilatation of the collecting tubules, and formation of urinary casts. Cisplatin has a low molecular weight and hence it is freely filtered in the glomerulus and almost entirely excreted in the urine. This drug will penetrate tubular cells and reach high concentrations in proximal tubular cells in the inner renal cortex and outer medulla. High-dose cisplatin can cause injury to the distal tubule and the collecting duct.21
Weekly cisplatin is a very effective chemotherapy regimen, but the risk of nephrotoxicity limits its administration. Several mechanisms are responsible for renal dysfunction following cisplatin use. These mechanisms are tubular epithelial cell toxicity, vasoconstriction in the renal microvasculature, and the proinflammatory effects of cisplatin. Exposure of tubular cells to cisplatin is followed by accumulation of the toxic compound in the tubular fluid. These toxic compounds diffuse into tubular cells, which are highly permeable, leading to tubular cell injury and cell death. This tubular epithelial cell toxicity involves various cytotoxic signaling pathways, namely p53, MAPK, and SOR. Cisplatin also causes renal vascular injury, ischemia, cell death, decreased renal blood flow, and decreased GFR. The proinflammatory effects of cisplatin are mainly mediated by the production of tumor necrosis factor (TNF)-alpha, which triggers a series of inflammatory responses.22,29
The results showed that the total incidence of AKD in subjects who received chemoradiation with cisplatin >200 mg/m2 was 38.4%, slightly higher than in the study by Patimarattananan et al in Thailand,39 which reported an AKD incidence of 27.9%. This difference is thought to be attributed to prophylactic feeding being administered to all patients in the study by Patimarattananan et al,39 which was not provided in this study. Grade 1 AKD was more commonly discovered among subjects who received a cumulative cisplatin dose of >200 mg/m2. The incidence of AKD in the group of subjects who received a cumulative dose of cisplatin >200 mg/m2 was slightly higher than in those who received ≤200 mg/m2 of cisplatin (38.4% vs 38.3%). Still, there was no statistically significant difference (p=0.995).
In this study, the measurement of renal function was performed in each chemotherapy cycle because several studies have shown variable responses to cisplatin exposure in terms of renal function. One of the studies regarding kidney recovery was conducted by Mizushima et al40 in rats, where the creatinine value was lower in the rats that received a second cisplatin injection than the creatinine value after receiving the first injection. After the third and fourth injections, the serum creatinine value gradually increased. According to Mizushima et al, this could be due to the host defense response, where some enzymes in the renal epithelium can reduce the renal toxicity of cisplatin.40 The study by Tezcan et al, in humans, produced similar results, where the GFR was higher after receiving the third chemotherapy session compared to the first and second chemotherapy sessions.41 Another renal recovery survey was conducted by Sharp and Siskind, who compared the nephrotoxic effect in mice receiving a single high dose of cisplatin versus mice receiving low and repeated doses. This study found that creatinine was more significant in mice receiving cisplatin in a single high dose. This was due to the increased necrosis caused by increased cleavage caspase-3 in mice that received cisplatin in high doses. Meanwhile, mice that received low and repeated doses tended to develop fibrosis. Inflammatory events predominantly occur at repeated low doses rather than at single high doses. This inflammation will continue in repair activities, which then develop into adaptive or maladaptive repair. Adaptive repair will result in kidney recovery, while the maladaptive repair will progress to fibrosis and lead to CKD.42
There is limited published research available regarding AKD. It is known that the greater the cisplatin dose per cycle, the higher the incidence of AKI, as obtained in a study by Morgan et al.43 Furthermore, a study on AKI by Prasaja et al stated that the higher the cumulative dose, the greater the incidence of AKI.21 The same results were obtained by Hwan Moon et al.44 However, our study obtained different results, showing no significant difference between the cumulative dose of ≤200 m2 and >200 mg/m2, which is in line with the results of Patimarattananan et al.39 It is stated that high doses of cisplatin will cause destruction of tubular cells directly through the necrosis pathway. In contrast, small doses of cisplatin will cause cells to experience cell death through the apoptotic pathway.42 In the necrosis pathway, cells will experience death within a few hours, while cells will experience death within a few days in the apoptotic pathway. Hence, cells may still have time to undergo repair in small-dose administrations. The eGFR of the subjects in this study tended to decrease, similarly to Prasaja’s study.21 However, differently from Prasaja’s study,21 both groups showed improvement in the eGFR within a cycle in this study. This may indicate that there is kidney recovery in patients who have AKD.
These results showed that there were significant differences in the incidence of AKD based on sex, grade 2 mucositis, and the incidence of vomiting. However, after adjusting for confounding factors in the multivariate analysis, sex, grade 2 mucositis, and vomiting were no longer confounders.
There was no significant difference in the incidence of AKD based on age. AKD was less common in subjects aged >65 years, consistent with the study by Hrushesky et al.45 This may be due to several factors, such as a decrease in the ability to concentrate urine and short cisplatin exposure time due to low accumulation of cisplatin in the kidney tubules in old age.45 In addition, an animal study by Sharp and Siskind42 found no significant difference between old and young mice in terms of renal function.
This study found that the incidence of AKD was higher in patients who had hypertension, but this difference was not significant (38.7% vs 38.2%, p=0.960), similarly to the study by Mizuno et al.46
In this study, AKD was more common in patients with an initial eGFR of 60–90 mL/min/1.73 m2 than in those with eGFR >90 mL/min/1.73 m2, but this difference was not statistically significant (46.7% vs 31.3%, p=0.278). This result is different from the studies by Loh et al47 and Faig et al,32 where patients with high GFR were at higher risk of developing AKI. The lack of statistical significance may be due to the limited number of subjects in our study.
There was a significant difference between the incidence of AKD in subjects with and those without grade 2 mucositis. Although, theoretically, solid food intake is preserved in patients with grade 2 mucositis, the presence of painful ulcers may impair their ability to ingest adequate fluid. This result is consistent with the results of a study by Andronesi et al,48 which found that mucositis increases the risk of AKI.
Moreover, this study also detected no significant difference in the incidence of AKD between the cumulative cisplatin doses of ≤200 mg/m2 and >200 mg/m2. However, a slightly higher incidence of AKD was seen in the group receiving cisplatin >200 mg/m2. This may be because the group with a cumulative dose of cisplatin >200 mg/m2 had more mucositis (89% vs 84%), which has been proven to increase the incidence of AKD in this study.
Of the subjects with AKD who received a cumulative dose of cisplatin >200 mg/m2, three (10.7%) recovered, while of the subjects who received a cumulative dose of cisplatin ≤200 mg/m2, only one subject (5.6%) recovered. This result is different from the study by Patimarattananan et al,39 where no one experienced recovery of renal function. The difference arises because subjects in Patimarattananan’s study received a higher cumulative cisplatin dose, up to 300 mg/m2. Another study, by Latcha et al, study also reported that none of the subjects experienced recovery; however, in that study the total cumulative cisplatin dose varied widely, with the highest dose reaching >700 mg/m2.18
In terms of progressivity of AKD to CKD, there was no significant difference in the number of subjects who experienced progressivity in both groups in this study (38.9% vs 35.7%, p=0.879). This finding suggests that in those who experienced AKD, the cisplatin cumulative dose does not affect the progression to CKD.
After an episode of AKI, the kidneys can repair their structures back to normal and return to function even though there appears to be severe damage. Recovery of the kidney is then initiated by repopulation of the damaged tubules by regenerating cells.30 After much debate as to whether the cells involved in this regenerative process are endogenous tubular epithelial cells, mature renal stem cells, or bone marrow-derived stem cells, there is now increasing evidence that repopulation is primarily dependent on endogenous tubular cells, which are still viable with stem cells derived from bone marrow by a paracrine pathway through the secretion of growth factors.31 In the kidney, adaptive repair can take place, where the condition of the kidney can return to normal or become maladaptive, which leads to the occurrence of CKD.30,31 Our findings showed that there were no significant differences in terms of recovery of renal function and progression from AKD to CKD in patients receiving cisplatin cumulative doses of ≤200 mg/m2 versus >200 mg/m2.
From the above results, considerations can be added to the policy regarding the dosing of cisplatin in chemoradiation. In NPC patients, the choice of administering cisplatin dose >200 mg/m2 is not associated with significant increase of the incidence of AKD. Results from studies by Loong et al9 and Gundog et al49 show that overall survival is better at doses >200 mg/m2. From the results of those studies, administration of cisplatin with a cumulative dose >200 mg/m2 produces better outcomes. Another study, by Peng et al,50 compared the effect of different cumulative cisplatin doses (≥240 mg/m2 vs <240 mg/m2) on the long-term survival of patients with NPC receiving concurrent chemotherapy. This study stated that a cumulative cisplatin dose ≥240 mg/m2 is an independent prognostic factor for disease-free progression in NPC patients receiving cisplatin concurrent chemoradiation.50
One limitation of administering high-dose cisplatin is the risk of nephrotoxicity. Our study showed that cisplatin can be administered safely at the recommended dose suggested by the guideline, without significant renal adverse effects compared to lower doses.
Beside the drug cisplatin itself, factors that affect the decline in kidney function after chemotherapy include fluid intake at home and excess fluid loss due to vomiting, which is one of the side effects of cisplatin chemotherapy. This can occur up to days 6–7 after cisplatin chemotherapy (delayed emesis), even though all subjects had received pre-chemotherapy antiemetic premedication followed by oral antiemetic drugs post-chemotherapy. Unfortunately, this study did not obtain data on the incidence of emesis at home or data on patient diuresis at home up to days 6–7 after chemotherapy owing to technical limitations in data collection.
Another limitation of this study is the retrospective design, which took data from medical records. The weaknesses of collecting data from medical records are that there are some incomplete data and there is a possibility of information bias.
Conclusion
This study found that in patients with locally advanced NPC who received cisplatin chemoradiation, there was no significant difference in the incidence of AKD, recovery of renal function, or progression of CKD between patients receiving a cumulative dose of cisplatin ≤200 mg/m2 and those receiving >200 mg/m2.
Ethical Approval
Ethical clearance was granted by the Ethics Committee of the Faculty of Medicine, University of Indonesia (ethical approval number: KET-718/UN2.F1/ETIK/PPM.00.02/2019). The ethics committee waived the requirement for individual patient consent because this study did not involve any intervention. Furthermore, full anonymity and confidentiality are maintained in compliance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank Dr. Dimas Priantono and Dr. Bayu Bijaksana Rumondor for their valuable contributions in preparing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kentjono WA. Perkembangan terkini penatalaksanaan karsinoma nasofaring (Current updates on the treatment of nasopharyngeal carcinoma). Maj Kedokteran Trop Indones; 2003.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Fles R, Wildeman MA, Sulistiono B, Haryana SM, Tan IB. Knowledge of general practitioners about nasopharyngeal cancer at the Puskesmas in Yogyakarta, Indonesia. BMC Med Educ. 2010;10. doi:10.1186/1472-6920-10-81
4. Dasari S, Bernard Tchounwou P. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
5. Reiss KA, Hilary Calvert A, O’Dwyer PJ. Platinum analogs. In: Devita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. Lippincott Williams & Wilkins; 2018.
6. Al-Sarraf M, LeBlanc M, Giri PGS, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi:10.1200/JCO.1998.16.4.1310
7. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi:10.1200/JCO.2003.06.158
8. Chan ATC, Leung SF, Ngan RKC, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97(7):536–539. doi:10.1093/jnci/dji084
9. Loong HH, Ma BBY, Leung SF, et al. Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):300–304. doi:10.1016/j.radonc.2011.12.022
10. Lv JW, Qi ZY, Zhou GQ, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy. Cancer Sci. 2018;109(3):751–763. doi:10.1111/cas.13474
11. Peng H, Chen L, Zhang Y, et al. Prognostic value of the cumulative cisplatin dose during concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a prospective Phase III Clinical Trial. Oncologist. 2016;21(11):1369–1376. doi:10.1634/theoncologist.2016-0105
12. Danchaivijitr P, Ngamphaiboon N, Jiarpinitnun C, Sirachainan E, Pattaranutaporn P, Setakornnukul J. An optimal cumulative dose of cisplatin in chemoradiotherapy as a definitive treatment for non-metastatic nasopharyngeal carcinoma: a retrospective multicenter study. Ann Oncol. 2016;27(Supplement 6):vi344. doi:10.1093/annonc/mdw376.50
13. National Comprehensive Cancer Network. NCCN guidelines Version 2.2020 head and neck cancers. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®); 2020.
14. Dabholkar M, Bostick-bruton F, Weber C, Bohr VA, Egwuagu C, Reed E. ERCC1 and ERCC2 expression in malignant tissues from ovarian cancer patients. J Natl Cancer Inst. 1992;84(19):1512–1517. doi:10.1093/jnci/84.19.1512
15. Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi:10.3390/cancers3011351
16. Vincent IS, Okusa MD. Biology of renal recovery: molecules, mechanisms, and pathways. Nephron Clin Pract. 2014;127:10–14. doi:10.1159/000363714
17. Driessen CML, Uijen MJM, Van Der Graaf WTA, et al. Degree of nephrotoxicity after intermediate- or high-dose cisplatin-based chemoradiotherapy in patients with locally advanced head and neck cancer. Head Neck. 2016;38:E1575–E1581. doi:10.1002/hed.24281
18. Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016;11:1173–1179. doi:10.2215/CJN.08070715
19. Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi:10.1038/nrneph.2017.2
20. Salahudeen AK, Doshi SM, Pawar T, Nowshad G, Lahoti A, Shah P. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol. 2013;8(3):347–354. doi:10.2215/CJN.03530412
21. Prasaja Y, Sutandyo N, Andrajati R. Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pacific J Cancer Prev. 2015;16(3):1117–1122. doi:10.7314/APJCP.2015.16.3.1117
22. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi:10.1038/sj.ki.5002786
23. Peres LA Lbert B, da Cunha ADA. Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol ‘Orgão of Soc Bras e Latino-Americana Nefrol. 2013;35(4):332–340.
24. Hayes DM, Cvitkovic E, Golbey RB, Scheiner E, Helson L, Krakoff IH. High dose Cis‐platinum diammine dichloride. Amelioration of renal toxicity by mannitol diuresis. Cancer. 1977;39:1372–1381. doi:10.1002/1097-0142(197704)39:4<1372::AID-CNCR2820390404>3.0.CO;2-J
25. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–2518. doi:10.3390/toxins2112490
26. Ruggiero A, Rizzo D, Trombatore G, Maurizi P, Riccardi R. The ability of mannitol to decrease cisplatin-induced nephrotoxicity in children: real or not? Cancer Chemother Pharmacol. 2016;77(1):19–26. doi:10.1007/s00280-015-2913-6
27. Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy special interest group on cancer care. Cancer Chemother Pharmacol. 2008;61:903–909. doi:10.1007/s00280-008-0711-0
28. Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237:219–227. doi:10.1016/j.toxlet.2015.06.012
29. Wei W, Huang Z, Li S, et al. Pretreatment Epstein-Barr virus DNA load and cumulative cisplatin dose intensity affect long-term outcome of nasopharyngeal carcinoma treated with concurrent chemotherapy: experience of an institute in an endemic area. Oncol Res Treat. 2014;37(3):88–95. doi:10.1159/000360178
30. Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855–866. doi:10.1007/s00134-017-4809-x
31. Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276. doi:10.1038/nrneph.2015.3
32. Faig J, Haughton M, Taylor RC, et al. Retrospective analysis of cisplatin nephrotoxicity in patients with head and neck cancer receiving outpatient treatment with concurrent high-dose cisplatin and radiotherapy. Am J Clin Oncol Cancer Clin Trials. 2018;41:432.
33. Kellum JA, Lameire N, Aspelin P, et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–38.
34. Hsu CK, Wu IW, Chen YT, et al. Acute kidney disease stage predicts outcome of patients on extracorporeal membrane oxygenation support. PLoS One. 2020;15(4):1–11. doi:10.1371/journal.pone.0231505
35. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
36. Nainani P, Nagpal N, Agrawal M, Paliwal A. Sex hormones in gender-specific risk for head and neck cancer: a review. J Int Soc Prev Community Dent. 2014;4:1. doi:10.4103/2231-0762.144557
37. Faiza S, Rahman S, Asri A. Karakteristik klinis dan patologis karsinoma nasofaring di bagian THT-KL RSUP Dr.M.Djamil Padang (Clinical and pathological characteristics of nasopharingeal carcinoma in the otorhinolaryngology department of Dr. M. Djamil Hospital, Padang). J Kesehat Andalas. 2016;5(1):90–96.
38. Adham M, Kurniawan AN, Muhtadi AI, et al. Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chin J Cancer. 2012;31:185–196. doi:10.5732/cjc.011.10328
39. Patimarattananan T, Nongnuch A, Pattaranutaporn P, Unwanatham N, Jiarpinitnun C, Ngamphaiboon N. Risk and impact of delayed renal impairment in patients with locally advanced head and neck squamous cell carcinoma receiving chemoradiotherapy with cisplatin. Support Care Cancer. 2021;29(2):877–887. doi:10.1007/s00520-020-05566-y
40. Mizushima Y, Nagahama H, Yokoyama A, Morikage T, Yano S. Studies on nephrotoxicity following a single and repeated administration of cis-Diamminedichloroplatinum (CDDP) in rats. Tohoku J Exp Med. 1987;151:129–135. doi:10.1620/tjem.151.129
41. Tezcan S, Izzettin FV, Sancar M, Yumuk PF, Turhal S. Nephrotoxicity evaluation in outpatients treated with cisplatin-based chemotherapy using a short hydration method. Pharmacol Pharm. 2013;04:296–302. doi:10.4236/pp.2013.43043
42. Sharp CN, Siskind LJ. Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol. 2017;313:F835–F841. doi:10.1152/ajprenal.00285.2017
43. Morgan KP, Snavely AC, Wind LS, et al. Rates of renal toxicity in cancer patients receiving cisplatin with and without mannitol. Ann Pharmacother. 2014;48(7):863–869. doi:10.1177/1060028014533303
44. Moon HH, Seo KW, Yoon KY, Shin YM, Choi KH, Lee SH. Prediction of nephrotoxicity induced by cisplatin combination chemotherapy in gastric cancer patients. World J Gastroenterol. 2011;17(30):3510-3517. doi:10.3748/wjg.v17.i30.3510
45. Hrushesky WJM, Shimp W, Kennedy BJ. Lack of age-dependent cisplatin nephrotoxicity. Am J Med. 1984;76:579–584. doi:10.1016/0002-9343(84)90280-8
46. Mizuno T, Ishikawa K, Sato W, et al. The risk factors of severe acute kidney injury induced by cisplatin. Oncol. 2013;85(6):364–369. doi:10.1159/000356587
47. Loh JM, Tran AL, Ji L, et al. Baseline glomerular filtration rate and cisplatin- induced renal toxicity in urothelial cancer patients. Clin Genitourin Cancer. 2018;16(1):90–98.e1. doi:10.1016/j.clgc.2017.08.016
48. Andronesi AG, Tanase AD, Sorohan BM, et al. Incidence and risk factors for acute kidney injury following autologous stem cell transplantation for multiple myeloma. Cancer Med. 2019;8(6):3278–3285. doi:10.1002/cam4.2187
49. Gundog M, Basaran H, Bozkurt O, Eroglu C. A comparison of cisplatin cumulative dose and cisplatin schedule in patients treated with concurrent chemo-radiotherapy in nasopharyngeal carcinoma. Braz J Otorhinolaryngol. 2020;86(6):676–686. doi:10.1016/j.bjorl.2019.04.008
50. Peng H, Chen L, Li WF, et al. The cumulative cisplatin dose affects the long-term survival outcomes of patients with nasopharyngeal carcinoma receiving concurrent chemoradiotherapy. Sci Rep. 2016;6:1–8. doi:10.1038/s41598-016-0001-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
