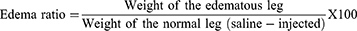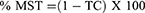Back to Journals » Journal of Inflammation Research » Volume 15
Corosolic Acid Inhibits Secretory Phospholipase A2IIa as an Anti-Inflammatory Function and Exhibits Anti-Tumor Activity in Ehrlich Ascites Carcinoma Bearing Mice
Authors Pundalik S, Hanumappa KR, Giresha AS, Urs D , Rajashekarappa S , Muniyappa N, Jamballi G M, Kuaramkote Shivanna D, S Meti R, Anekere Dasappa Setty S, Thippegowda PB, Krishnappa DK
Received 4 August 2022
Accepted for publication 8 November 2022
Published 31 December 2022 Volume 2022:15 Pages 6905—6921
DOI https://doi.org/10.2147/JIR.S383441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Sophiya Pundalik,1 Krishna Ram Hanumappa,2 Aladahalli S Giresha,3 Deepadarshan Urs,1 Sharath Rajashekarappa,4 Narayanappa Muniyappa,1 Manjunatha Jamballi G,5 Devaraju Kuaramkote Shivanna,6 Rajkumar S Meti,1 Sathisha Anekere Dasappa Setty,7 Prabhakar Bettadathunga Thippegowda,8 Dharmappa Kattepura Krishnappa1
1Inflammation Research Laboratory, Department of Studies and Research in Biochemistry, Mangalore University, Jnana Kaveri Post Graduate Campus, Kodagu, Karnataka, India; 2Nisarga Research and Development Trust (T), Bengaluru, Karnataka, India; 3Department of Biochemistry, School of Science, Jain (Deemed-to-be University), Bangalore, Karnataka, India; 4Department of Food Technology, Davanagere University, Davanagere, Karnataka, India; 5Department of Chemistry FMKMC College Madikeri, Mangalore University Constituent College, Mangalore, Karnataka, India; 6Department of Biochemistry, Karnataka University, Dharwad, Karnataka, India; 7Division of Biochemistry, School of Life Sciences, JSS Academy of Higher Education & Research, SS Nagar, Mysore, Karnataka, India; 8Molecular Biomedicine Laboratory, Postgraduate Department of Studies and Research in Biotechnology, Sahyadri Science College (Autonomous), Kuvempu University, Shivamogga, Karnataka, India
Correspondence: Dharmappa Kattepura Krishnappa, Email [email protected]
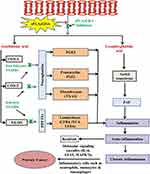
Background: Inflammation is generally connected to tumour progression and development. The secretory phospholipase A2IIa (sPLA2IIa) is an important inflammatory enzyme that catalyse the hydrolysis of membrane phospholipids into arachidonic and lysophosphatidic acid, which are the precursors for production of a lot of pro-inflammatory mediators like prostaglandins, prostacyclins, thromboxanes, leukotrienes and platelet activating factors, which involved in the proliferation, migration, invasion, and metastasis. Therefore, investigating safe and effective sPLA2IIa inhibitors as a therapeutic agent to treat cancer is indeed in need.
Methods: Anti-inflammatory function of corosolic acid was evaluated by docking it with sPLA2IIa enzyme, sPLA2IIa inhibition, calcium and substrate concentration-dependent assays; intrinsic fluorescence and UV-CD analysis; neutralisation of sPLA2IIa induced indirect hemolytic and edema. Evaluated the anticancer activity of corosolic acid by MTT assays and caspase-3 expression; the anti-tumour activity by EAC-induced cell line and interleukin 6 expression.
Results: The corosolic acid inhibits sPLA2IIa activity to 82.21± 2.82%. The inhibition was evaluated by increasing calcium from 2.5 to 15 μM and substrate from 20 to 120 nM, it did not affect the level of inhibition. Corosolic acid altered the intrinsic fluorescence and UV-CD spectra of sPLA2IIa enzyme, indicating the direct interaction. It neutralised sPLA2IIa induced hemolytic activity from 97± 1.23% to 15.75± 1.44% and edema from 171.51± 2.39% to 119.3± 2.6%. Further, as antiproliferative activity, corosolic acid reduced the PC3 cell viability from 99.66± 0.57% to 23± 2.64% and suppressed LPS-induced IL-6 level from 94.35± 2.2% to 34.36± 2.4%. It increased mean survivability time from 30 to 38 days and displayed the drug-like qualities.
Conclusion: All the experimental results have proven the corosolic acid as an anti-inflammatory and anticancer molecule that may further be used to develop it as a drug.
Keywords: corosolic acid, antioxidant, secretory phospholipase A2 IIa inhibition, caspase 3, IL-6, prostate cancer, EAC, ADME-toxicity, Anti-inflammatory
Introduction
Inflammation generally promotes or suppresses the tumour progression.1 Neoplasia initiates the inflammation-related pathway (intrinsic pathway) that leads to the development of the microenvironment and inflammatory reactions (extrinsic pathway) that facilitate cancer development.2 The immune response against cancer development is closely related to the inflammatory reaction.3,4 Also, currently available cancer therapy includes anti-inflammatory drugs like non-steroidal anti-inflammatory drugs (NSAIDs), which have been postulated for anti-cancerous properties.5–7 Hence, modulating inflammation is the principal strategy for cancer prevention and therapy.8
Inflammation is mainly mediated by secretory phospholipase A2IIa (sPLA2IIa) enzymes. It catalyse the membrane phospholipids into arachidonic acid and lysophosphatidate, which further converted into pro-inflammatory mediators like prostaglandins, thromboxanes, leukotrienes, prostacyclins and platelet activation factors9 (Figure 1). The products of the sPLA2IIa enzyme contribute to pathophysiological conditions of inflammatory diseases like sepsis, trauma, asthma,10 atherosclerosis11 and cancers.12,13 The prostaglandins and leukotrienes play a significant role in prostate cancer cell proliferation, migration, invasion, and metastasis.14 They are overexpressed in almost all human prostate cancer specimens.15
Since long ago the NSAIDs have been used to cure inflammatory diseases by targeting lipoxygenases (LOX) or cyclooxygenase (COX-1/2) enzymes. Despite their benefits, these drugs cause serious side effects like gastrointestinal toxicities, renal injuries, and cardiovascular risks.16–18 Furthermore, COX-1/2 or LOX inhibitors are unable to control the production of platelet activating factor (PAF), which intensifies the inflammation.19 Though it looks like a simple approach, a potent and safe inhibitor of sPLA2IIa reduces pro-inflammatory mediators.20,21 For half a century, hundreds of sPLA2IIa inhibitors were invented and developed, few of them trialled at clinical level. But none of them reach the market even though they inhibit sPLA2IIa enzymes in nanograms that might be due to problems related with formulation or cytotoxicity22–24 or it could be bad pharmacodynamics and pharmacokinetic features. Hence, there is a great demand for effective sPLA2IIa inhibitors derived from natural resources to treat inflammatory diseases as well as cancers.
In the current study, an important medicinal plant, Centella asiatica widely used to treat several inflammatory diseases like leprosy, ulcers, psoriasis, and female genitourinary tract diseases25 etc., was considered for the evaluating its antiinflammatory function. Initially, a few pharmacologically important bioactive molecules from C. asiatica like Liquiritigenin, Asiatica acid, 1, 5 Dicaffeoylquinic acid, Campesterol and Corosolic acid (Figure 2) (Table 1) were docked with sPLA2IIa enzyme, among them, corosolic acid showed the greater binding affinity. It also showed a variety of pharmacological activities like anti-diabetic,26 anti-inflammatory,27 anti-obesity activity28,29 and apoptosis in prostate cancer cells.30 But, till date, the corosolic acid has not been evaluated for the anti-inflammatory function by inhibiting sPLA2IIa, an important inflammatory enzyme. Therefore, this study intended to evaluate the potency of corosolic acid for sPLA2IIa inhibition as an anti-inflammatory and anticancer effect by in vitro, in situ and in vivo methods. The oleanolic acid is a triterpenoid and isomer of corosolic acid has proven to be a very good sPLA2IIa inhibitor31 and anticancer molecule32 used as reference control.
 |
Table 1 Molecular Docking of Selective Bioactive Compounds and Their Energy Values Against sPLA2 (1POE) |
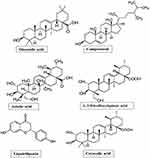 |
Figure 2 Structures of pharmacologically important molecules from Centella asiatica. |
Materials and Methods
Chemicals and Reagents
Sephadex G-25, −50 and −75, CM sephadex C25 Corosolic acid, Oleanolic acid and Lipopolysaccharide (#L2018), Ultima Gold Scintillation Cocktail, Bovine serum albumin (BSA), were procured from Sigma-Aldrich, USA.14C-oleic acid obtained from Perkin Elmer Life Sciences Inc. Boston. Mouse Anti-Human IL-6 (Cat No: 340527) was from B.D Biosciences. Six-well cell culture plates from BioLite - Thermo. The PC3 Cell line was obtained from the national centre for cell science (NCCS), Pune, the cell culture medium (RPMI 1640, #AL028A), Fetal Bovine Serum (#RM10432) and dulbecco’s phosphate buffered saline (D-PBS) (#TL1006) were procured from HiMedia Laboratories, Mumbai, India. Ethylenediaminetetraacetic acid (EDTA) and dimethyl sulphoxide (DMSO) were acquired from SRL (Sisco Research Laboratories), India. The solvents and chemicals utilised in the entire investigation were standard laboratory grade.
Human Sample
Institutional Human Ethical Committee, Mangalore University approved the usage of blood samples (MU/IHEC/2018/1). The blood was collected from healthy volunteers after taking an informed consent letter.
Animals
Swiss albino mice (20–25g) were used for anti-tumour activity and neutralization of edema study. The animals were handled and supervised according to India’s Regulations passed by the laboratory animal welfare act. The approval number of the Institutional Animal Ethical Committee is NGSMIPS/IAEC/NOV-2019/166 (NGSM Institute of Pharmaceutical Science, Mangalore).
sPLA2-IIA Enzyme
The sPLA2IIa was purified according to the method of Kasturi and Gowda 198933 and homogeneity was checked by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) as per the method of Laemmli, 1970.34
In Silico Docking Study
The sPLA2IIa structure was obtained from Protein Data Bank (PDB) (1POE) (https://www.rcsb.org/structure/1POE). Structures of phytoconstituents were obtained from the PubChem database. Details of the active site were obtained from PDB and literature.35,36 The graphical user interface (AutoDock Tools 4.2.6) was used to carry out intermediary steps like energy minimization, grid box formation; protein-inhibitor preparation. The prepared file was saved in PDBQT format by AutoDock using iterated local search global optimizer.37,38 The hydrophobic and H bond interactions between the ligands and receptors of the docked complex were analyzed using the LigPlot tool. After the successful completion of the docking runs, different conformations of the ligands known as binding modes were obtained with their respective binding affinity and the stable one which happens to be the one with the lowest binding affinity was picked and aligned with receptor structure for further analysis.39,40
ADME Toxicity Prediction
Corosolic acid and oleanolic acid MOL files were obtained from the ChemSpider database (http://chemspider.com) and used as a source of data for in silico analysis. The in-silico absorption, distribution, metabolism, excretion, toxicity (ADME-T) properties were estimated using the PreADME-T web-based tool (https://preadmet.bmdrc.kr/adme and https://preadmet.bmdrc.kr/toxicity/).41
Secretory Phospholipase A2 (sPLA2IIa) Assay and Inhibition
The sPLA2IIa activity was determined using 14C-oleic acid labelled E. coli cells (autoclaved) as per the method of Patriarca et al42 For sPLA2IIa inhibition assay, dissolved 10 mg of corosolic acid in 1 mL DMSO and made upto required concentration using Tris-HCl buffer. The sPLA2IIa activity was estimated by treating corosolic acid from 2 to 16 µM concentration. Oleanolic acid is a well-known sPLA2IIa inhibitor and isomer of corosolic acid was used as standard.43 The highest DMSO concentration used in this assay was 0.022%.
Effect of Calcium and Substrate Concentration
The nature of sPLA2IIa inhibition was tested by increasing the concentration of calcium from 2.5 to 15 µM and substrate from 30 nM to 120 nM in presence and absence of IC50 concentration of corosolic acid (The separate assays were carried out for calcium and substrates).
Intrinsic Fluorescence Analysis
Intrinsic fluorescence of sPLA2IIa enzyme with and without corosolic acid was recorded in the Fluorolog-3 spectrofluorometer. The 2.0 mL reaction mixture consists of sPLA2IIa (20µg/mL) and corosolic acid range from 0.02 to 0.1µM was subjected for analysis. Fluorescence spectra were recorded between 300 nm and 380 nm and were empirically corrected using the tryptophan standard.44
Circular Dichroism Analysis
The UltraViolet Circular Dichroism (UV-CD) spectra of sPLA2IIa (30µg/mL) was recorded with and without IC50 concentration of corosolic acid using Jasco J-810 spectropolarimeter between 200 and 240 nm. The response time was 2s and the bandwidth was 1 nm. Total of 10 scans were made into the final spectrum. The spectrum of standard reaction mixture was subtracted to correct the spectra.
Neutralisation of Indirect Hemolytic Activity
The experiment was carried out according to the method of Boman and Kaletta 1957.45 The 1mL of red blood cell (RBC) and 1mL of egg yolk in 8mL of phosphate buffered saline (PBS) was used as substrate. The corosolic acid was pre-incubated with sPLA2IIa at 37°C for 30 minutes, then added 1mL of the substrate and incubated at 37°C for 45 minutes. Added 9 mL of ice-cold PBS to halt the reaction and centrifuged at 1500×g for 20 minutes. The hemolytic activity of sPLA2IIa enzyme in terms of released hemoglobin was measured at 530 nm.
Neutralization of Edema Inducing Activity
The method of Yamakawa et al 197646 altered by Vishwanath et al 198747 was followed. The sPLA2 IIA (5µg) alone or with corosolic acid (range from 0 to 21 µM) in total volume of 20 µL injected into right hind footpad of mice. A 20 µL of saline was injected into the respective left-hind footpad to serve as control. The animals were euthanized after 45 minutes by giving anaesthesia (30 mg/kg of pentobarbital i.p.). The limbs were amputated at the ankle joint and weighed individually.
MTT (3-(4, 5-Dimethylthiazol-2-Yl)-2, 5-Diphenyltetrazolium Bromide) Assay
The protocol of Alley et al48 and the MTT cell proliferation instruction guidelines were used to determine prostate cancer (PC3) cell viability. The cells were grown in T-25 flasks, harvested and plated in a 96-well plate in DMEM at cell density of 10,000 cells per well. After 24h incubation, the cells were treated with different concentrations of corosolic acid. After 24h incubation, MTT reagent was added to get 0.5mg/mL concentration and incubated in the CO2 incubator for 3h. The media containing excess MTT was removed, and formazan crystals were dissolved using 100 µL DMSO. Reading was taken at 570 nm for the purple colour formazan solution and at 630 nm to subtract the background reading. The percentage viability of PC3 cells was calculated.
Determination of Caspase 3 Level by Flow Cytometry
The 3×105 cells/2 mL density were cultured and treated with corosolic acid. The cells were harvested, washed with PBS, fixed with 0.5mL of 2% paraformaldehyde and incubated for 20 minutes. The cells were washed with 0.5% BSA in PBS and permeabilized with 0.1% Triton-X 100 of 0.5% BSA solution. Staining was done with 20µL anti-caspase-3 antibody and incubated for 30 minutes in the dark at room temperature. Unbound antibodies were removed by washing with PBS twice, then 0.5 mL of PBS was added, mixed thoroughly and recorded the fluorescence intensity. Caspase-3 FITC (green) fluorescence was detected in the FL1 detector using a 525 nm bandpass filter.49–51
Anti-Tumor Activity
The anti-tumor activity of the corosolic acid was evaluated by injecting Ehrlich Ascites Carcinoma (EAC) cells into Swiss albino mice.52 In brief, 24 mice of either sex were grouped into 4 sets containing 6 animals in each group. Group 1 received only buffers and served as vehicle control and Group 2 injected 1×106 EAC cells (i. p.) served as disease control; Group 3 animals received corosolic acid (10 mg/kg, p.o). Group 4 was treated with a standard molecule (5 fluorouracil). The inhibitor was prepared by dissolving in Tris HCL buffer (10mM) to the final concentration of 1 μg/μL. Animals were treated after 24 h of tumour induction and continued for the 10 days. Animals were observed for clinical signs and mortality. The weight of the animals was recorded and percentage increase in mean survival time (% MST) was calculated.
Where C= Mean survival time of the control group; T= Mean survival time of the treated group.
Interleukin 6 Expression Studies by Flow Cytometry
IL-6 expression was measured following the manufacturer’s instructions (BD Biosciences PE Mouse Anti-Human IL-6; Cat No: 340527)53 and analysed by FACS – Cell quest pro software.
Statistical Analysis
The obtained test results were given as the mean standard deviation (n=3). IC50 values were calculated by Graph Pad Prism Version 5.0 (La Jolla, USA).
Results and Discussion
The AutoDock4 version (v4.2.6)37 module was used to study the binding pattern of corosolic acid and oleanolic acid with human sPLA2IIa (PDB ID: 1POE organism: Homo sapiens). The His47/Asp48 residues and calcium-binding loop of the sPLA2IIa (1POE) enzyme are necessary for activity.54 The majority of sPLA2IIa inhibitors interact with His47/Asp48 and weaken the Ca2+ association that lowers the enzyme activity.55,56 Some of the sPLA2IIa inhibitors bind to substrate binding pouches that interfere with substrate binding to the active site. The amino acids like Phe-23, Phe-5, Leu-31, and Leu-257 of substrate binding pouch of sPLA2IIa enzyme establish van der Waals interactions with inhibitors. The corosolic acid (PubChem ID 6918774) interacts with active site His47 and Asp48 via hydrogen bonding and van der Waals interaction with Gly-44, Phe-5, Gly-22, Gly-28, Leu-2, Ala-18, Val-30. Similarly, the standard reference oleanolic acid (PubChem ID 10494) interacts with His-47, Asp-48 through hydrogen bonding and van der Waals interaction with Val-30, Cys-44, Tyr-21, Gly-29, Gly-22, Gly-28, Leu-2 and Phe-5 (Figure 3).
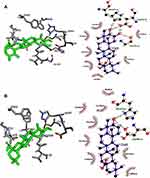 |
Figure 3 Docked images of corosolic acid and oleanolic acid with sPLA2IIa enzyme: Stereo view of corosolic acid (A) and oleanolic acid (B) docked with active site of sPLA2IIa. |
The pharmacokinetic profiling at the initial stages of drug discovery using in silico methods is necessary because it is cheap and timesaving compared to the experimental approach.58 Hence, corosolic acid was subjected to ADME-toxicity profiling to qualify it as a drug candidate. The ligand physical descriptors and pharmaceutically essential properties were analysed using the Pre ADME-T program. The parameters used in ADME-Toxicity prediction are based on Lipinski’s rule of 5; the corosolic acid showed 94.278% intestinal absorption, 99.224% of plasma protein binding and 2.788% of blood-brain barrier (BBB); thus, corosolic acid displayed drug-like qualities (Table 2).
 |
Table 2 Predicted ADME-Toxicology of Corosolic Acid Compared with Standard Oleanolic Acid |
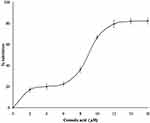
As corosolic acid exhibits better binding energy (negative E value) and displayed drug-like qualities, further subjected to sPLA2IIa enzyme inhibition. The corosolic acid inhibited sPLA2IIa enzyme to 82.21±2.82% at 16 µM concentration in a concentration dependent manner (Figure 4). The IC50 value of corosolic acid was 9.44 ±0.59 μM, whereas standard reference oleanolic acid showed the IC50 value of 6.74±0.71μM.
Some of the inhibitors limit sPLA2IIa activity either by binding to substrate or chelating metal ion, calcium. Hence, the nature of sPLA2IIa inhibition was examined by increasing the calcium concentration from 2.5 to 15 µM in presence and absence of corosolic acid (IC50 concentration), it showed the constant inhibition of 47.90 ± 1.37% over all the ranges of calcium (Figure 5A). Also, estimated sPLA2IIa inhibition by increasing substrate concentrations from 30 to 120 nM in presence and absence of corosolic acid (IC50 concentration), which showed the constant inhibition of 49.63 ± 0.94% over all the ranges of substrates (Figure 5B). The results suggested that sPLA2IIa inhibition does not change due to either increased calcium or substrate concentrations.
Generally, the interaction of inhibitors with the sPLA2IIa enzyme exposes amino acids like phenylalanine, tryptophan and tyrosine to the surrounding media that leads to quenching of intrinsic fluorescence.59 Altering the intrinsic fluorescence indicates structural change in proteins due to substrate or ligand interaction. Increased fluorescence intensity of sPLA2IIa upon binding to corosolic acid indicating the formation of enzyme-inhibitor complex (Figure 6A). Increasing relative intrinsic fluorescence was observed with increasing corosolic acid concentration from 0.02µM to 0.1µM (Figure 6B).
Far UV-CD studies provide the information regarding the changes in sPLA2IIa structure upon binding to an inhibitor, which validates the findings of fluorescence study. The far UV-CD spectrum of sPLA2IIa without corosolic acid gave two characteristic negative bands (peaks) at 210 and 222nm. The addition of corosolic acid (IC50 concentration) reduces peak height and shifts peaks to 217 and 220 nm (Figure 7), which indicates the changes in percentage of secondary structure of sPLA2IIa enzyme. The changes in percentage of the secondary structures of sPLA2IIa in presence of corosolic acid were calculated (Table 3).
 |
Table 3 Secondary Structures of sPLA2IIa Enzyme Before and After Corosolic Acid Treatment |
 |
Figure 7 UV CD spectra of sPLA2IIa enzyme: Recorded UV-CD spectra of sPLA2IIa enzyme alone (red line) and with IC50 concentration of corosolic acid (blue line). |
Further, evaluated the efficacy of corosolic acid for neutralising indirect haemolytic activity of sPLA2IIa. The sPLA2IIa enzyme exhibits 97±1.23% hemolysis. Addition of corosolic acid reduced the sPLA2IIa mediated indirect hemolytic activity to 15.75 ± 1.44% in a dose-dependent manner (Figure 8). Distilled water was used as positive control, which showed 100% hemolysis.
In general, the in vitro studies show positive results but do not show efficiency by in-vivo models that is due to heterogeneity of the environment. Administration of the purified sPLA2IIa enzymes into joints induce inflammatory response with edema (171.51 ± 2.39%), swelling of synovial cells and hyperplasia.60–62 Injection of corosolic acid with sPLA2IIa enzyme (pre-incubated) reduced the inflammatory response (edema) from 171.51±2.39% to 119.3±2.6% with an apparent IC50 value of 7.19 ± 0.34 μM (Figure 9). Conclusively, the in vivo experimental result was in accordance with results of in vitro and in situ experiments.
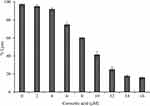
Many sPLA2 inhibitors have been widely explored for cancer prevention and treatment strategies63–65 by targeting different pathways. The studies showed that corosolic acid can modulate a diverse range of cancer signalling pathways like NF-κB, PI3K/Akt, and Wnt/-catenin and apoptosis.66–68 Hence, we hypothesised the current investigation to evaluate the anticancer potency of corosolic acid. Initially treated corosolic acid to PC3-cell line which reduced the viability to 23±2.64% at 30 µM concentration and IC50 value was 9.362 ± 0.56 µM/mL, which was compared to reference molecule oleanolic acid (Figure 10A and B). Oleanolic acid has great structural and functional similarity with corosolic acid and also a known anticancer molecule.69
Caspases play a crucial role in cell apoptosis or programmed cell death.70 Effector caspase 3 is responsible for the actual cleavage of cellular components during apoptosis. Hence, in the present study, caspase-3 level was estimated using FITC rabbit anti-active caspase-3 IgG antibody assay kit (BD Biosciences, catalogue no. 559341). The increase in caspase-3 expression was measured by cell positive FITC (green) fluorescence in the FL1 detector using a 525 nm bandpass filter. The present study showed that cells treated with corosolic acid showed a moderate increase in caspase-3 level (584 MFI) in FITC fluorescence and compared to untreated cells (Figure 11).
 |
Figure 11 Caspase-3 level was estimated in (A) untreated and (B) corosolic acid-treated PC3 cells. |
Animal studies have been of great importance in understanding treatments. Therefore, we have used this EAC-induced mice model to estimate the effect of corosolic acid. Corosolic acid and 5-Fluorouracil (standard molecule) showed a protective effect against EAC-induced mortality. Animals treated with corosolic acid (G3) showed increased MST to 38 days compared to toxic control (G2), which was 30 days, while 5 fluorouracil treatments showed MST upto 40 days (G4) (Figure 12). The toxic control group (G2) injected with EAC showed a significant rise in body weight up to 46.6±4.0g compared to normal control (G1) body weight of 33.3±2.8g, in contrast, corosolic acid-treated groups (G3) showed a reduced body weight of 39.1±3.7g. In addition, toxic control (G2) showed a survival fraction of EAC cells of 56.4±5.9, whereas the corosolic acid-treated group (G3) decreased the survival fraction to 49.1±20.9%, whereas the standard (G4) showed by 50.9±10.48 (Table 4). Thus, corosolic acid showed significant anti-cancer activity against EAC-induced tumour in mice.
 |
Table 4 Antitumor Activity of Corosolic Acid |
 |
Figure 12 Effect of corosolic acid in mice bearing Ehrlich Ascites Carcinoma tumour. |
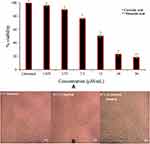
There are reports showing that IL-6 mediates the expression of inflammatory phospholipase A2 enzymes in most chronic inflammatory diseases.71 IL-6 increases the intracellular phospholipid (PL), phosphatidylethanolamine (PE) and sphingomyelin (SM); they act as signalling compounds for either inflammation or apoptosis.72 The elevated level of IL-6 correlates with prostate cancer burden.73,74 Hence, targeting IL-6 would be a novel treatment strategy for various immune-mediated diseases and cancers. Treating LPS (2µg/mL) to the PC3 cells results in the pro-inflammatory marker IL-6 expression upto 99.35%. Treatment of different concentrations of corosolic acid to LPS treated PC3 cells reduced the IL-6 expression from 99.35% to 34.36 ± 2.4% (Figure 13).
 |
Figure 13 Effect of corosolic acid of 3.75 µM (*), 7.5 µM (*), 15µM (*) and 30µM (*) concentration on LPS Induced IL6 expression PC3 cell line (*). |
Conclusion
The sPLA2IIa is an important inflammatory enzyme which plays a very significant role in inflammatory diseases and tumour progression. Investigating effective and safe sPLA2IIa inhibitors is indeed necessary to treat inflammatory diseases and cancers. The current study reports that the corosolic acid has proven as a potential sPLA2IIa inhibitor which neutralised the sPLA2IIa induced indirect haemolytic activity and mouse paw edema in concentration dependent manner, thus proven as an anti-inflammatory molecule. Further, corosolic acid tested for anti-proliferative properties, which reduced the PC3 cell viability, increased expression of caspase 3 level and decreased IL-6 expression. Finally, corosolic acid displayed drug-like qualities by ADME-toxicity analysis. Hence, this molecule may further develop as an anti-inflammatory and anticancer agent for treating the inflammatory diseases and cancers.
Institutional Review Board Statement
The studies on edema and antitumor assays by using Swiss albino mice were conducted according to the guidelines of the Declaration of Indian National Regulations for Animal Research, India and approved by the Institutional Ethical Committee of NGSM Institute of Pharmaceutical Science Mangalore, India (NGSMIPS/IAEC/NOV-2019/166).
Data Sharing Statement
All the data are available within this manuscript. We declare that “The datasets used and/or analysed during the current study available from the corresponding author on reasonable request”.
Informed Consent Statement
All experiments were performed in accordance with AVMA guidelines Animal experiments were performed after obtaining animal ethical approval NGSMIPS/IAEC/NOV-2019/166 (NGSM Institute of Pharmaceutical Science Mangalore) and the human blood sample was collected from healthy volunteers after obtaining ethical approval and an informed consent letter (IHEC-No. MU/IHEC/2018/1).
Acknowledgments
The authors are thankful to the UGC-NFSC fellowship (F1-17.1/2017-18/RGNF-2017-18-SC-KAR-42746/(SA-III/Website) for financial support. We also thank Mangalore University for providing laboratory facilities.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121. doi:10.4103/aam.aam_56_18
2. Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15(2):425–430. doi:10.1158/1078-0432.CCR-08-0149
3. Dranoff G. Immune recognition and tumor protection. Curr Opin Immunol. 2002;2(14):161–164. doi:10.1016/S0952-7915(02)00315-1
4. Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2(4):227–238. doi:10.1038/nri774
5. Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24(1):83–87. doi:10.1002/jso.2930240119
6. Harris RE, Beebe-Donk J, Doss H, Doss DB. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade. Oncol Rep. 2005;13(4):559–583.
7. Rayburn ER, Ezell SJ, Zhang R. Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol. 2009;1(1):29. doi:10.4255/mcpharmacol.09.05
8. Todoric J, Antonucci L, Karin M. Targeting inflammation in cancer prevention and therapy. Cancer Prev Res. 2016;9(12):895–905. doi:10.1158/1940-6207.CAPR-16-0209
9. Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265(29):17381–17384. doi:10.1016/S0021-9258(18)38167-5
10. Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997;155(2):421–425.
11. Menschikowski M, Hagelgans A, Siegert G. Secretory phospholipase A2 of group IIA: is it an offensive or a defensive player during atherosclerosis and other inflammatory diseases? Prostaglandins Other Lipid Mediat. 2006;79(1–2):1–33.
12. Scott KF, Sajinovic M, Hein J, et al. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie. 2010;92(6):601–610. doi:10.1016/j.biochi.2010.03.019
13. Meyer AM, Dwyer-Nield LD, Hurteau GJ, et al. Decreased lung tumorigenesis in mice genetically deficient in cytosolic phospholipase A 2. Carcinogenesis. 2004;25(8):1517–1524. doi:10.1093/carcin/bgh150
14. Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi:10.1038/nrc2809
15. Dong Z, Liu Y, Scott KF, et al. Secretory phospholipase A2-IIa is involved in prostate cancer progression and may potentially serve as a biomarker for prostate cancer. Carcinogenesis. 2010;31(11):1948–1955. doi:10.1093/carcin/bgq188
16. Hatmi M, Samama MM, Elalamy I. Prevention of thrombosis and vascular inflammation: importance of combined cyclooxygenase and 5-lipoxygenase inhibitors. J Mal Vasc. 2006;31(1):4–9. doi:10.1016/S0398-0499(06)76511-8
17. Nasir NN, Sekar M, Fuloria S, et al. Kirenol: a potential natural lead molecule for a new drug design, development, and therapy for inflammation. Molecules. 2022;27(3):734. doi:10.3390/molecules27030734
18. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;1(180):114147. doi:10.1016/j.bcp.2020.114147
19. Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J Clin Invest. 2001;107(12):1491–1495. doi:10.1172/JCI13271
20. Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77(1):495–520. doi:10.1146/annurev.biochem.76.062405.154007
21. Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovasc Drugs Ther. 2009;23(1):49–59. doi:10.1007/s10557-008-6132-9
22. Nicholls SJ, Kastelein JJ, Schwartz GG, et al. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the Vista-16 randomized clinical trial. JAMA. 2014;311(3):252–262. doi:10.1001/jama.2013.282836
23. Zeiher BG, Steingrub J, Laterre PF, Dmitrienko A, Fukiishi Y, Abraham E; EZZI Study Group. LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit Care Med. 2005;33(8):1741–1748. doi:10.1097/01.CCM.0000171540.54520.69
24. Garcia-Pastor P, Randazzo A, Gomez-Paloma L, Alcaraz MJ, Paya M. Effects of petrosaspongiolide M, a novel phospholipase A2 inhibitor, on acute and chronic inflammation. J Pharmacol Exp Ther. 1999;289(1):166–172.
25. Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci. 2010;72(5):546. doi:10.4103/0250-474X.78519
26. Miura T, Ueda N, Yamada K, et al. Antidiabetic effects of corosolic acid in KK-Ay diabetic mice. Biol Pharm Bull. 2006;29(3):585–587. doi:10.1248/bpb.29.585
27. Banno N, Akihisa T, Tokuda H, et al. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci Biotechnol Biochem. 2004;68(1):85–90. doi:10.1271/bbb.68.85
28. Yamaguchi Y, Yamada K, Yoshikawa N, Nakamura K, Haginaka J, Kunitomo M. Corosolic acid prevents oxidative stress, inflammation, and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006;79(26):2474–2479. doi:10.1016/j.lfs.2006.08.007
29. Zong W, Zhao G. Corosolic acid isolation from the leaves of Eriobotrya japonica showing the effects on carbohydrate metabolism and differentiation of 3T3-L1 adipocytes. Asia Pac J Clin Nutr. 2007;16(Suppl 1):346–352.
30. Ma B, Zhang H, Wang Y, et al. Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3. J Exp Clin Cancer Res. 2018;37(1):1–6. doi:10.1186/s13046-018-0889-x
31. Dharmappa KK, Kumar RV, Nataraju A, Mohamed R, Shivaprasad HV, Vishwanath BS. Anti-inflammatory activity of oleanolic acid by inhibition of secretory phospholipase A2. Planta Med. 2009;75(03):211–215. doi:10.1055/s-0028-1088374
32. Niu G, Sun L, Pei Y, et al. Oleanolic acid inhibits colorectal cancer angiogenesis by blocking the VEGFR2 signaling pathway. Anticancer Agents Med Chem. 2018;18(4):583–590. doi:10.2174/1871520617666171020124916
33. Kasturi S, Gowda TV. Purification and characterization of a major phospholipase A2 from Russell’s viper (Vipera russelli) venom. Toxicon. 1989;27(2):229–237. doi:10.1016/0041-0101(89)90136-0
34. Laemmli UK; Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 1970;227(5259):680–685. doi:10.1038/227680a0
35. Tian W, Chen C, Lei X, Zhao J, Liang J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res. 2018;46(W1):W363–7. doi:10.1093/nar/gky473
36. Scott DL, White SP, Browning JL, Rosa JJ, Gelb MH, Sigler PB. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science. 1991;254(5034):1007–1010. doi:10.1126/science.1948070
37. Morris GM, Huey R, Lindstrom W, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi:10.1002/jcc.21256
38. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi:10.1002/jcc.21334
39. Shastri SL, Krishna V, Ravi Kumar S, Pradeepa K. Phytochemical analysis, antibacterial property and molecular docking studies of Mammea suriga Kosterm. World J Pharm Res. 2016;4:331–340.
40. Jinadatta P, Rajshekarappa S, Rao KS, Subbaiah SG, Shastri S. In silico, in vitro: antioxidant and antihepatotoxic activity of gnetol from Gnetum ula Brongn. BioImpacts. 2019;9(4):239. doi:10.15171/bi.2019.29
41. Shastri SL, Krishna V. Jayabaskaran C.In Silico Analysis of ADME-T Properties of Amentoflavone. Bioinform Proteom Opn Acc J. 2017;1(3):000118.
42. Patriarca P, Beckerdite S, Pettis P, Elsbach P. Phospholipid metabolism by phagocytic cells: VII The degradation and utilization of phospholipids of various microbial species by rabbit granulocytes. Biochim Biophys Acta. 1972;280(1):45–56. doi:10.1016/0005-2760(72)90211-1
43. Pastwińska J, Karaś K, Sałkowska A, et al. Identification of corosolic and oleanolic acids as molecules antagonizing the human RORγT nuclear receptor using the calculated fingerprints of the molecular similarity. Int J Mol Sci. 2022;23(3):1906. doi:10.3390/ijms23031906
44. Prigent-Dachary J, Boffa MC, Boisseau MR, Dufourcq J. Snake venom phospholipases A2 A fluorescence study of their binding to phospholipid vesicles correlation with their anticoagulant activities. J Biol Chem. 1980;255(16):7734–7739.
45. Boman HG, Kaletta U. Chromatography of rattlesnake venom A separation of three phosphodiesterases. Biochim Biophys Acta. 1957;Jan(24):619–631. doi:10.1016/0006-3002(57)90256-1
46. Yamakawa M, Nozaki M, Hokama Z. Fractionation of sakishimahabu (Trimeresurus elegans) venom and lethal, hemorrhagic and edema-forming activities of the fractions. Animal Plant Microbial Toxins. 1976;1:97–109.
47. Vishwanath BS, Gowda TV. Interaction of aristolochic acid with Vipera russelli phospholipase A2: its effect on enzymatic and pathological activities. Toxicon. 1987;25(9):929–937. doi:10.1016/0041-0101(87)90155-3
48. Alley MC, Scudiero DA, Monks A, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48(3):589–601.
49. BD Biosciences . Product datasheet - FITC rabbit anti- active caspase-3 IgG Antibody - BD biosciences, catalogue no. 559341. Available from: https://www.bdbiosciences.com/content/dam/bdb/products/global/reagents/flow-cytometry-reagents/research-reagents/single-color-antibodies-ruo/559341_base/pdf/559341.pdf
50. Dai C, Krantz SB. Interferon γ induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. J Am Soc Hematol. 1999;93(10):3309–3316.
51. Amidzadeh Z, Behbahani AB, Erfani N, et al. Assessment of different permeabilization methods of minimizing damage to the adherent cells for detection of intracellular RNA by flow cytometry. Avicenna J Med Biotechnol. 2014;6(1):38.
52. Raihan M, Tareq SM, Brishti A, Alam M, Haque A, Ali M. Evaluation of antitumor activity of leea indica (Burm. f.) Merr. extract against Ehrlich Ascites Carcinoma (EAC) Bearing Mice. Am J Biomed Sci. 2012;4:143–152.
53. BD Biosciences. BD Biosciences PE mouse anti-human IL-6 (Technical Data Sheet, Cat No: 340527). Available from: https://www.bdbiosciences.com/content/dam/bdb/products/global/reagents/flow-cytometry-reagents/clinical-discovery-research/single-color-antibodies-ruo-gmp/340527_base/pdf/23-3468.pdf
54. Mohamed R, Dharmappa KK, Tarannum S, et al. Chemical modification of ascorbic acid and evaluation of its lipophilic derivatives as inhibitors of secretory phospholipase A2 with anti-inflammatory activity. Mol Cell Biochem. 2010;345(1):69–76. doi:10.1007/s11010-010-0561-z
55. Mohamed R, Shivaprasad H, Jameel N, Shekar M, Vishwanath B. Neutralization of local toxicity induced by Vipera russelli phospholipase A2 by lipophilic derivative of ascorbic acid. Curr Top Med Chem. 2011;11(20):2531–2539. doi:10.2174/156802611797633429
56. Dileep KV, Remya C, Cerezo J, Fassihi A, Pérez-Sánchez H, Sadasivan C. Comparative studies on the inhibitory activities of selected benzoic acid derivatives against secretory phospholipase A 2, a key enzyme involved in the inflammatory pathway. Mol Biosyst. 2015;11(7):1973–1979. doi:10.1039/C5MB00073D
57. Gnanaraj C, Sekar M, Fuloria S, et al. In silico molecular docking analysis of karanjin against alzheimer’s and parkinson’s diseases as a potential natural lead molecule for new drug design, development and therapy. Molecules. 2022;27(9):2834. doi:10.3390/molecules27092834
58. Toyama DO, Ferreira MJ, Romoff P, Fávero OA, Gaeta HH, Toyama MH. Effect of chlorogenic acid (5-Caffeoylquinic Acid) Isolated from baccharis oxyodonta on the structure and pharmacological activities of secretory phospholipase A2 from Crotalus durissus terrificus. Biomed Res Int. 2014;2014:1–10. doi:10.1155/2014/726585
59. Bomalaski JS, Lawton P, Browning JL. Human extracellular recombinant phospholipase A2 induces an inflammatory response in rabbit joints. J Immunol. 1991;146(11):3904–3910.
60. Weichman BM, Berkenkopf JW, Marshall LA. Phospholipase A2-induced pleural inflammation in rats. Int J Tissue React. 1989;11(3):129–136.
61. Fawzy AA, Vishwanath BS, Franson RC. Inhibition of human non-pancreatic phospholipases A2 by retinoids and flavonoids. Mech Action Agents Action. 1988;25(3):394–400. doi:10.1007/BF01965048
62. Vecchi L, Araújo TG, Azevedo FV, et al. Phospholipase A2 Drives tumorigenesis and cancer aggressiveness through its interaction with annexin A1. Cells. 2021;10(6):1472. doi:10.3390/cells10061472
63. Javed Z, Khan K, Herrera-Bravo J, et al. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021;21(1):1–2. doi:10.1186/s12935-021-02091-8
64. Lee JY, Kim HS, Song YS. Genistein as a potential anticancer agent against ovarian cancer. J Tradit Complement Med. 2012;2(2):96–104. doi:10.1016/S2225-4110(16)30082-7
65. Kim JH, Kim YH, Song GY, et al. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. 2014;1(67):87–95. doi:10.1016/j.fct.2014.02.019
66. Horlad H, Fujiwara Y, Takemura K, et al. Corosolic acid impairs tumor development and lung metastasis by inhibiting the immunosuppressive activity of myeloid‐derived suppressor cells. Mol Nutr Food Res. 2013;57(6):1046–1054. doi:10.1002/mnfr.201200610
67. Yoo KH, Park J-H, Lee DY, Hwang-Bo J, Baek NI, Chung IS. Corosolic acid exhibits Anti-angiogenic and Anti-lymphangiogenic effects on in vitro endothelial cells and on an in vivo CT-26 colon carcinoma animal model. Phytotherapy Res. 2015;29(5):714–723. doi:10.1002/ptr.5306
68. Qian XP, Zhang XH, Sun LN, et al. Corosolic acid and its structural analogs: a systematic review of their biological activities and underlying mechanism of action. Phytomedicine. 2021;1(91):153696. doi:10.1016/j.phymed.2021.153696
69. Lu Y, Chen GQ. Effector caspases and leukemia. Int J Cell Biol. 2011;2011:1–8. doi:10.1155/2011/738301
70. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin. 2011;30(1):1–4. doi:10.1186/1756-9966-30-87
71. Tazuke Y, Drongowski RA, Teitelbaum DH, Coran AG. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int. 2003;19(5):321–325. doi:10.1007/s00383-003-1003-8
72. Bertsch T, Banks RE, Forbes MA, et al. Phospholipase A2 activity in serum is induced during treatment with recombinant human interleukin-6 in patients with cancer. Ann Clin Biochem. 1996;33(6):565–567. doi:10.1177/000456329603300615
73. Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62(1):S60–5. doi:10.1016/0090-1229(92)90042-M
74. Vainer N, Dehlendorff C, Johansen JS. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget. 2018;9(51):29820. doi:10.18632/oncotarget.25661
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.