Back to Journals » Cancer Management and Research » Volume 11
Conversion of immunohistochemical markers and breast density are associated with pathological response and prognosis in very young breast cancer patients who fail to achieve a pathological complete response after neoadjuvant chemotherapy
Authors Zhao Y, Wang X, Huang Y, Zhou X, Zhang D
Received 19 December 2018
Accepted for publication 16 May 2019
Published 20 June 2019 Volume 2019:11 Pages 5677—5690
DOI https://doi.org/10.2147/CMAR.S198844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Yue Zhao,1 Xiaolei Wang,2 Yuanxi Huang,1 Xianli Zhou,2 Dongwei Zhang3
1Department of Breast Surgery, The Tumor Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 2Department of In-Patient Ultrasound, The Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 3Department of Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China
Purpose: Patients younger than age 35 that fail to achieve a pathologic complete response (pCR) after Neoadjuvant chemotherapy (NACT) tend to have worse long term outcomes. The purpose of our study was to assess the correlation between the conversion of immunohistochemical (IHC) markers and breast density and investigate their association with pathological response and prognosis.
Methods: We included 119 patients younger than age 35 who failed to achieve a pCR after NACT in this analysis. We evaluated the clinical and pathological response to NACT by the Union for International Cancer control (UICC) and the Miller-Payne grading (MPG) systems, respectively. A breast density assessment was applied via mammography examination at the time of diagnosis. MPG and breast density (BD) have been combined to define a specific classification of three risk levels to evaluate the prognosis of these patients.
Results: The diameter changes of the tumors and lymph nodes were negatively associated with hormone receptor conversion and positively correlated with Ki67 conversion. A significantly large size change was observed in the groups demonstrating conversion from HER-2 (+) to (−). The variation level of IHC markers was related to MPG and BD and was associated with the survival rate of the patients. Patients with a high breast density and low Miller-Payne grading after NACT had a higher risk of distant metastases or local recurrences.
Conclusion: ER, PR and Ki67 conversion are closely related to MPG, while PR and Ki67 conversion are closely related to BD. While ER and PR conversion are independent and significant predictors of disease free survival (DFS) and overall survival (OS), HER-2 and Ki67 conversion are only significant for DFS. This risk factor grouping provides a useful index to evaluate the risk of young women with breast cancer who fail to achieve a pCR.
Keywords: young breast cancer, neoadjuvant chemotherapy, IHC markers, conversion, breast density, survival
Introduction
Breast cancer is correlated with a less favorable prognosis in young women and it is the most frequent cancer in women younger than 40. According to the statistics, patients under age 40 comprise 6.6% of breast cancer cases, 2.4% in patients under 35, and 0.65% in patients under 30.1,2 However, in China, the proportion of women in the age group of under 35 has been reported to be much higher.3 Although patients less than 35 are more likely to receive more intensive therapy, a young age at diagnosis has been shown to be a risk factor for breast cancer recurrence and death.4 Thus, there is a critical need to identify novel actionable targets and develop robust therapies for very young women with breast cancer.
Neoadjuvant chemotherapy is being increasingly used in the management of localized breast cancer as an alternative to adjuvant chemotherapy. There are several arguments for applying NACT. First, by down-staging the tumor, less extensive resections are needed, and breast conservation becomes increasingly feasible.5 Second, micrometastases that may be present are thus treated at the earliest possible time.6 Third, neoadjuvant chemotherapy enables the monitoring of treatment efficacy and makes it possible to identify markers of response to chemotherapy.7
A pCR after NACT has been shown to be a surrogate marker for survival.8 Higher rates of pathological complete responses can be achieved when selecting for certain breast cancer subtypes and treatment regimens.9 Most studies have confirmed that patients who achieve a pCR after NACT are expected to have a significantly more favorable outcome compared with patients with residual disease in the breast and/or axillary lymph nodes (known as non-pCR).10 However, only 10–30% of patients experience a pCR after primary treatment, and women <35 years with a failure to achieve a pCR are clearly associated with worse long-term outcomes.
High percent mammographic density (MD), or the proportion of dense breast tissue on a mammogram, is one of the strongest risk factors for breast cancer.11–13 Higher breast density is significantly associated with a greater proportion of stromal and epithelial tissue and a lower proportion of adipose tissue. For patients <35 years with dense breast tissue, the research shows that mammography is less sensitive for detecting cancer.14 However, studies have shown that the utilization of a screening breast ultrasound in women with dense breast tissue is effective in detecting mammographically occult breast cancer.15,16 Previous studies have shown that NACT can alter the levels of HR, HER-2 and Ki67,17,18 but little information is available on the prognostic and BD impact of their level conversion caused by NACT. The purpose of our study was to assess the correlation between the conversion of IHC markers and breast density and to investigate their effect on pathological response and prognosis in very young (<35) breast cancer patients who fail to achieve pCR after NACT.
Materials and methods
Patients
This study was approved by the Institutional Review Board (IRB) of Harbin Medical University. The records of 4527 patients who were initially diagnosed with breast cancer between 2005 and 2017 were retrieved from the Second and Third Affiliated Hospital, Harbin Medical University. Among these patients, a total of 119 patients who were younger than age 35 and failed to achieve a pathological complete response after NACT were included in this analysis. The patient selection process and pathologic diagnoses are shown in Figure 1. Patients who had any treatment prior to NACT, distant metastatic disease before surgery, bilateral breast cancer, male breast cancer, or inflammatory breast cancer were not eligible for this study.
The patients in our study received a NACT regimen consisting of CEF (cyclophosphamide, epirubicin and 5-fluorouracil), TE (docetaxel and epirubicin), TEC (docetaxel, epirubicin and cyclophosphamide), PC (paclitaxel and carboplatin), TCH (docetaxel, carboplatin and trastuzumab) or other agents for a median of 4 cycles (range, 1–8 cycles). Additional cycles of chemotherapy were administered after the surgery, and a total of six to eight cycles of chemotherapy were completed at the discretion of the treating physician on the basis of the clinical and pathologic evaluations after surgery.
All patients’ diseases were confirmed as invasive carcinoma through core needle biopsy (CNB), and lymph node status was also assessed by CNB before NACT. Mastectomy and axillary lymph node dissection were performed within four weeks of the completion of NACT. The patients who were ER/PR+ received endocrine therapy, and those with more than three positive axillary lymph nodes following NACT were administered radiation therapy. IHC and/or fluorescence in situ hybridization (FISH) assays were used for the detection of ER, PR, HER-2 and Ki-67. Calculation of the conversion of IHC markers was defined as IHC markers after NACT minus before NACT.
Clinical and pathological response to NACT
The Union for International Cancer control (UICC) criteria19 were used in recording the clinical response. A complete clinical response (cCR) was achieved when the original mass became impalpable. A partial response (cPR) represented a 50% or greater reduction in bidimensional tumor measurements. Progressive disease (cPD) was recorded if the bidimensional measurements increased by 20% or more. All others were classified as stable disease (cSD).
Pathological response was evaluated according to the Miller-Payne grading system (MPG).20 This is a five-point scale that focuses on the principal manifestation of the chemotherapeutic effect being a reduction in tumor cellularity. Often, residual tumor cells show specific morphological effects (gross nuclear pleomorphism and cytoplasmic enlargement, cytoplasmic vacuolation). The grading is hinged upon the loss of tumor burden compared with the diagnostic pretreatment core biopsy. The response of MPG to NACT was independently assessed by two pathologists according to the Miller-Payne grading system. The tumors were scored blindly by each pathologist and an agreement by consensus if necessary was achieved. Miller-Payne grading provides a 5-step scale based on tumor cellularity in the excision/mastectomy specimen compared with the pretreatment core biopsy. Grade 1: No reduction in overall cellularity; Grade 2: Minor (<30%) loss of cellularity; Grade 3: Between an estimated 30% and 90% reduction in tumor cells; Grade 4: More than 90% loss of tumor cells; Grade 5: No invasive carcinoma but ductal carcinoma in situ (DCIS) may be present. Patients were excluded from our study if they had an MPG 5 after NACT. Patients with grade 1, 2, 3, or 4 were scored as not having a pCR.
Mammographic breast density assessment
The breast density assessment was performed during the mammography examination at the time of diagnosis. Community radiologists classified breast density as part of routine clinical practice using the four BI-RADS density categories:21 (1) Grade 1: The breasts are almost entirely fatty (<25% glandular). (2) Grade 2: There are scattered areas of fibroglandular density (approximately 25–50% glandular). (3) Grade 3: The breast tissue is heterogeneously dense, which may obscure small masses (approximately 51–75% glandular). (4) Grade 4: The breast tissue is extremely dense, which lowers the sensitivity of mammography (>75% glandular).
Immunohistochemistry
Estrogen receptor (ER)/progesterone receptor (PR) were considered positive if >1% of the cells showed positive staining. Hormonal receptor (HR) positivity was defined as ER and/or PR positive, while patients with negative staining for both ER and PR were considered HR-negative. The IHC staining for HER-2 was scored according to standard criteria as 0, 1+, 2+, or 3+.22 Scores of 0 and 1+ were considered negative, and 3+ was considered HER-2-positive. When a score of 2+ was found, additional fluorescence in situ hybridization (FISH) testing was done to establish the HER-2 gene amplification status. A positive result was defined as an HER-2 gene/chromosome 17 ratio of greater than 2.0. Ki67 expression was divided into two groups: Ki67 index ≥15% (high expression) and Ki67 index <15% (low expression).23
Statistical analysis
The chi-square test was used to evaluate the correlation between the conversion of IHC markers, breast density and clinical-pathologic response to NACT. The possible associations with the conversion of IHC markers and tumor/lymph node size changes were calculated using Pearson correlation coefficient analysis. Disease free survival (DFS) was calculated from the date of surgery to the date of disease relapse (local, regional or distant relapse), the diagnosis of contralateral breast cancer or death from any cause. Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. Survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to test for differences between groups. HazR and their 95% confidence intervals (CIs) were calculated using the Cox regression model. All P-values <0.05 were considered statistically significant. The statistical analysis was carried out using SPSS (version 20.0; SPSS Company, Chicago, IL).
Results
Conversion of IHC markers related to the changes in tumor and lymph node size
A total of 119 patients younger than age 35 that failed to achieve a pathological complete response after NACT were included in our study. Patient and tumor-specific characteristics before NACT are outlined in Table 1. The median age of the patients was 31 years (range: 23–35 years).
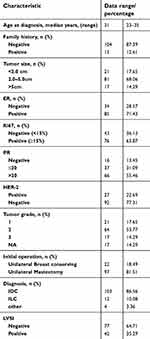 | Table 1 Patient characteristics before NACT |
The initial and residual diameter of the tumor and axillary lymph nodes were measured by ultrasonography, and we calculated the difference before and after NACT. The mean initial and residual diameter of the tumors were 3.36±1.22 cm (range: 1.8–7.5 cm) and 1.37±0.73 cm (range: 0.5–3.1 cm), respectively.
We also calculated the changes of ER, PR and Ki67, and patients with HER-2 status conversions before and after NACT were divided into four groups: (+) to (+), (−) to (−), (+) to (−) and (−) to (+). With regard to HER-2 status, 12 patients (10.08%) presented a discordant HER-2 status; of these individuals, 8 (6.72%) were converted from HER-2 (+) to HER-2 (−) and 4 (3.36%) changed from HER-2 (−) to HER-2 (+). The normality of the ER, PR, Ki67 conversions and tumor/lymph node size changes were verified by the Shapiro Wilk test before the Pearson test, all P>0.05.
The diameter changes of the tumors and lymph nodes were significantly associated with the conversion of IHC markers, all P<0.05 (Figures 2A–F, 3A and B). In this dataset, tumor size change before and after NACT were negatively correlated with hormone receptor (ER and PR) conversion and positively correlated with Ki-67 conversion, and lymph node changes showed the same outcomes as tumor size change in the breast. A significantly high size change was observed in the groups with HER-2 (+) conversion to (−), both in the tumor and lymph nodes.
Conversion of IHC markers, breast density and clinical-pathological response
Patients were excluded if they had an MPG 5 after NACT. A total of 20 (16.81%) patients had MPG 1, 30 (25.21%) MPG 2, 45 (37.81%) MPG 3, and 24 (20.17%) MPG 4. According to the changes of HR and Ki67 markers after NACT, we divided them into three groups: <−10 group, −10 to 10 group, and >10 group. In non-pCR responders, the ER and PR conversion >10 group was positively associated with a reduction in tumor cells, and the Ki67 conversion >10 group was negatively correlated with a loss of tumor cells, all P<0.05 (Figure 4A–C). However, HER-2 conversion was not associated with MPG 1–4 (Figure 4D). In addition, breast density was significantly associated with PR and Ki67 conversion but not with ER and HER-2 conversion. Patients in the PR conversion <−10 group and Ki67 conversion >10 group were correlated with dense breast tissue. The distributions of the relationships are shown in Figure 5A–D.
The clinical response was evaluated according to UICC criteria by ultrasound. Among the total 119 patients, 10 (8.40%) had a CR, 67 (56.30%) a PR, 34 (28.57%) SD, and 8 (6.73%) PD. Among our cohort, BD was significantly associated with a pathological response in non-pCR patients (Figure 6A) but not with a clinical response (Figure 6B).
Factors associated with prognosis
Pathological response and breast density were evaluated to see if they predicted the results of DFS (Figures 7A, 8A) and OS (Figures 7B, 8B) in our cohort, but they all presented values of P>0.05. Therefore, pathological response and breast density in non-pCR patients are not associated with DFS and OS.
To further evaluate the differences in patient survival based on changes in their HR, HER-2 and Ki67 statuses after NACT, Kaplan-Meier and Cox regression analysis of DFS and OS were carried out. The results of the Cox regression analysis are shown in Table 2. In the Kaplan-Meier analysis (Figure 9A–H), ER conversion (P<0.001 and P=0.002) and PR conversion (P=0.013 and P=0.004) were statistically significantly associated with both DFS and OS, but HER-2 conversion (P=0.006) and Ki67 conversion (P=0.004) were only significant for DFS; the same results were found in the Cox regression analysis.
 | Table 2 IHC markers conversion and survival |
Pathological response combined with breast density predicted survival
According to the factors pathological response and breast density, 119 patients were divided into three groups. Group 1’s score is from 2 to 3, group 2’s score is from 4 to 6, and group 3’s score is from 7 to 8. Tumors in the three different groups were calculated as breast density (a grade 1 tumor equals one point, and so on) + pathological response (score: 1 for MPG 4, 2 for MPG 3, 3 MPG 2, and 4 for MPG 1). Groups 1, 2, and 3 were categorized as low risk, medium risk, and high risk, respectively. This classification proposed the important risk factors resulting in significantly different DFS and OS values (all P<0.001). The patients in the high risk group were considered to have the worst DFS and the worst OS in our cohort (Figure 10A and B, and Table 3). We found 22 patients in the high risk group had distant metastases or local recurrences, and 11 of them have died. Hence, this risk grouping is considered a significant independent predictor of DFS and OS.
 | Table 3 Pathological response combined with breast density predict survival |
Discussion
That the expression of IHC markers are altered after NACT has been suggested by several studies24 while others have indicated that they remained stable.25 Few prospective studies have focused on the discordant status of a prognostic value, and IHC markers state conversion have been found to be significantly associated with different outcomes.26 However, the mechanism of the conversion of HR, HER-2 and Ki67 status after NACT is complex. A recent meta-analysis reported that 34% of ER conversion and 17% of PR conversion could be due to technical misclassification, with a corrected discordance of 12.4% for ER and 28.3% for PR.27 On the other hand, tumor heterogeneity could explain a significant proportion of sample mismatches due to the presence of distinct tumor subclones with different expression patterns of ER, PR, HER-2 and Ki67.
We demonstrated that patients showing a conversion from HR (+) to HR (−) in their residual tumors after NACT had a worst outcome (with or without hormonal therapy) compared with other types of HR conversions. However, the conversion of HER-2 status alone did not have a significant impact on the prognosis. In our prospective observational study, we demonstrated that the changes in tumor and node diameter were negatively associated with differences of HR and were positively correlated with Ki67 conversion. This result indicates that the conversion of IHC markers could be used to predict a size change of the tumor and lymph nodes.
Studies have shown that a pCR after NACT is associated with a significantly better outcome in young women with breast cancer compared with those without a pCR.28 Patients younger than age 35 who fail to achieve a pCR after NACT have worse long term outcomes, and there is an urgent need to find a way to save these patients with the worst prognosis. Patients who fail to achieve a pCR should have attention paid to the conversion of their IHC markers. Among patients failing to achieve a pCR, a reduction in HR and an increase of Ki67 predicted a high risk of relapse or death. For some patients in this study, trastuzumab was not given prior to surgery. HER-2+ patients who did not achieve a pCR could still benefit from adjuvant trastuzumab.
Our research highlights the prognostic value of a discordance in IHC expression status before and after NACT using Cox regression and Kaplan-Meier plots. The results showed that patients whose HR level was increased more than 10 points after NACT had significantly better DFS and OS than patients in other groups. Ki67 is known as a cellular proliferation marker, and tumors exhibit relatively more aggressive behavior if they have high expression of Ki67.29 In our study, patients whose Ki67 level was increased more than 10 points and those whose HER-2 (+) converted to (−) after NACT had significantly worse DFS than patients in other groups.
Several studies have shown that the HR status of residual tumors may be altered by decreased circulating levels of hormone, which can be caused by chemotherapy that inhibits ovarian function and adrenal glands.30 It is worth noting that the relationship between imaging and tissue biological markers is not simple, and several previous studies have investigated stromal hormone receptors in relation to breast density; however, none have studied the associations between the change of IHC markers and breast density in very young patients (≤35) who fail to achieve a pCR.
Mottaghy31 found a positive association between PR expression and breast density in breast biopsies from 18 patients at high risk of breast cancer but not for any other stromal markers investigated. Yang and colleagues32 in contrast found no significant association between HR status and mammographic breast density after univariate analysis of 66 patients. Interestingly, we found that the highest values of breast density and pathological response were correlated with changes of PR and Ki67 but not ER and HER-2. Among the analyzed receptors, a PR increase of more than 10 points after NACT was correlated with a greater loss of tumor cells and a lower breast density. In contrast, a Ki67 increase of more than 10 points after NACT was correlated with a lower reduction in tumor cells and a higher breast density.
Attainment of a pCR was associated with a significantly reduced recurrence and improved survival regardless of the baseline clinical stage. Our study found that MPG was not correlated with DFS and OS in patients with the exception of MPG 5. In addition, breast density was not related to survival, which is in accordance with MPG. However, breast density was associated with a pathological response and was unrelated to the clinical response. To our knowledge, this is the first study to examine the added value of incorporating these factors into prognostication, including breast density and Miller-Payne grading with the exception of MPG 5. This combination classification has been divided into three groups, as shown in Table 3 and Figure 10A and B. The patients in the high risk group were found to have the worst DFS and worst OS in our cohort. These results suggest that patients who have high breast density and low Miller-Payne grading after NACT have an increased risk of distant metastases or local recurrences. Hence, this risk grouping is considered a significant independent predictor of DFS and OS in younger than age 35 patients who fail to achieve a pCR.
Conclusions
In summary, patients younger than age 35 who fail to achieve a pCR after NACT have worse long term outcomes. Our study confirms the association between the conversion of IHC markers and the size changes of tumors and lymph nodes. The variation level of IHC markers are related to MPG and BD and are correlated with the survival rate of patients. To our knowledge, this is the first study to examine the added value of incorporating additional factors into prognostication, including breast density and Miller-Payne grading, with the exception of MPG 5. Patients who have a high breast density and low Miller-Payne grading after NACT are at a higher risk of distant metastases or local recurrences, and this might be the main reason for the variation in the level of impact IHC markers have on predicting survival.
Ethics approval and consent to participate
Ethical approval was approved by the Institutional Review Board of Harbin Medical University. Informed consent was obtained from all patients. Patient consent to review their medical records was not required by the research ethics committee as the data collected from electronic medical records were anonymous, confidential, and not linked to the individuals.
Acknowledgments
We would like to extend our sincere gratitude to our departmental chair for their support. Additionally, we would like to give many thanks to our physicians, engineers, and nurses as well as the other staff of the department. This work was supported by the Heilongjiang Science and Technology Planning Project (grants: YS17C22), the Fundamental Research Funds for the Provincial Universities in 2018, Heilongjiang Province of Science and Technology (grant 201716), and the Harbin Science and Technology Bureau (grant 2017RAXQJ067).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi:10.1371/journal.pone.0007695
2. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi:10.1053/j.seminoncol.2009.03.001
3. Meng J, Lang RG, Fan Y, Fu L. Clinicopathological and biological features of breast cancer in young females and their relationship with prognosis. Zhonghua Zhong Liu Za Zhi. 2007;29:284–288.
4. Zhao Y, Dong XQ, Li RG, Song J, Zhang DW. Correlation between clinical-pathological factors and long-term follow-up in young breast cancer patients. Transl Oncol. 2015;8(4):265–272. doi:10.1016/j.tranon.2015.05.001
5. Pelizzari G, Gerratana L, Basile D, et al. Post-neoadjuvant strategies in breast cancer: from risk assessment to treatment escalation. Cancer Treat Rev. 2019;72:7–14. doi:10.1016/j.ctrv.2018.10.014
6. Faneyte IF, Schrama JG, Peterse JL, et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer. 2003;88(3):406–412. doi:10.1038/sj.bjc.6600749
7. Zhao Y, Dong XQ, Li RG, et al. Evaluation of the pathological response and prognosis following neoadjuvant chemotherapy in molecular subtypes of breast cancer. Onco Targets Ther. 2015;8:1511–1521. doi:10.2147/OTT.S83243
8. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi:10.1016/S0140-6736(13)62422-8
9. Rose BS, Winer EP, Mamon HJ. Perils of the pathologic complete response. J Clin Oncol. 2016;34:3959–3962. doi:10.1200/JCO.2016.68.1718
10. Bonnefoi H, Litiere S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014;25:1128–1136. doi:10.1093/annonc/mdu118
11. Autier P, Boniol M, Smans M, Sullivan R, Boyle P. Statistical analysis in Swedish randomized trials on mammography screening and in other randomized trials on cancer screening: a systematic review. J R Soc Med. 2015;108:440–450. doi:10.1177/0141076815593403
12. Wang AT, Vachon CM, Brandt KR, Ghosh K. Breast density and breast cancer risk: a practical review. Mayo Clin Proc. 2014;89:548–557. doi:10.1016/j.mayocp.2013.12.014
13. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical exam, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi:10.1148/radiol.2251011667
14. Berg WA. Current status of supplemental screening in dense breasts. J Clin Oncol. 2016;34:1840–1845. doi:10.1200/JCO.2015.65.8674
15. Weigert JM. The Connecticut experiment: the third installment-4 years of screening women with dense breasts with bilateral ultrasound. Breast J. 2017;23:34–39. doi:10.1111/tbj.12678
16. Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammographically negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol. 2016;34:1882–1890. doi:10.1200/JCO.2015.63.4147
17. Huo CW, Chew G, Hill P, et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015;17:79. doi:10.1186/s13058-015-0592-1
18. van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–430. doi:10.1016/j.ctrv.2010.11.006
19. Bertero L, Massa F, Metovic J, et al. Eighth Edition of the UICC Classification of Malignant Tumours: an overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch. 2018;472(4):519–531. doi:10.1007/s00428-017-2276-y
20. Ogston KN, Miller ID, Payne S. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–327.
21. Wolfe JN. Risk for breast cancer development determined by mammographic parenchymal pattern. Cancer. 1976;37:2486–2492.
22. Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi:10.1200/JCO.2007.14.8197
23. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi:10.1093/jnci/djp082
24. Zhang N, Moran MS, Huo Q, Haffty BG, Yang Q. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest. 2011;29:594–598. doi:10.3109/07357907.2011.621913
25. Kasami M, Uematsu T, Honda M, et al. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast. 2008;17:523–527. doi:10.1016/j.breast.2008.04.002
26. Spring L, Greenup R, Niemierko A, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw. 2017;15(10):1216–1223. doi:10.6004/jnccn.2017.0158
27. Ongaro E, Gerratana L, Cinausero M, et al. Comparison of primary breast cancer and paired metastases: biomarkers discordance influence on outcome and therapy. Future Oncol. 2018;14(9):849–859. doi:10.2217/fon-2017-0384
28. Feeley LP, Mulligan AM, Pinnaduwage D, Bull SB, Andrulis IL. Distinguishing luminal breast cancer subtypes by Ki67, progesterone receptor or TP53 status provides prognostic information. Mod Pathol. 2014;27:554–561. doi:10.1038/modpathol.2013.153
29. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. doi:10.1200/JCO.2005.07.501
30. Cho N, Im SA, Cheon GJ, et al. Integrated 18F-FDG PET/MRI in breast cancer: early prediction of response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45(3):328–339. doi:10.1007/s00259-017-3849-3
31. Mottaghy FM. Is the whole larger than the sum of the parts? Integrated PET/MRI as a tool for response prediction. Eur J Nucl Med Mol Imaging. 2018;45:325–327. doi:10.1007/s00259-017-3908-9
32. Yang WT, Lewis MT, Hess K, et al. Decreased TGF-β signaling and increased COX2 expression in high risk women with increased mammographic breast density. Breast Cancer Res Treat. 2010;119(2):305–314. doi:10.1007/s10549-009-0350-0
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.










