Back to Journals » Pragmatic and Observational Research » Volume 8
Conventional culture versus nucleic acid amplification tests for screening of urethral Neisseria gonorrhea infection among asymptomatic men who have sex with men
Authors Budkaew J , Chumworathayi B , Pientong C, Ekalaksananan T
Received 17 March 2017
Accepted for publication 21 July 2017
Published 1 September 2017 Volume 2017:8 Pages 167—173
DOI https://doi.org/10.2147/POR.S137377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. David Price
Jiratha Budkaew,1 Bandit Chumworathayi,2,3 Chamsai Pientong,3,4 Tipaya Ekalaksananan3,4
1Department of Social Medicine, Khon Kaen Center Hospital, Khon Kaen, Thailand; 2Department of Obstetrics and Gynecology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 3HPV and EBV and Carcinogenesis Research Group, Khon Kaen University, Khon Kaen, Thailand 4Department of Microbiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Background: Many methods are used to detect urethral Neisseria gonorrhea (NG) infection among asymptomatic men who have sex with men (MSM). The objective of this study was to define the performance of conventional culture compared to real-time polymerase chain reaction (PCR) for diagnosis of asymptomatic urethral gonorrhea among MSM.
Methods: In this cross-sectional study, 147 clinical specimens for NG testing from asymptomatic participants were evaluated. MSM >18 years old who consented to undergo urethral swab and collection of urine samples from two clinics (one was the sexually transmitted diseases (STDs) mobile clinic and the second was the antiretroviral clinic) located in Khon Kaen, Thailand, were recruited. For conventional culture, 147 swab specimens from urethra were analyzed. For real-time PCR, the same samples and collected urine (147 urethral swab and 62 urine) were evaluated.
Results: Participants were predominately older aged (mean age: 28.79 years, range: 18–54), asymptomatic (99.3%), and engaged in sex with multiple partners (63% had at least two partners and 36% had at least three partners during the previous 3 months). Twenty-five MSM (17%) had history of STD, mainly human immunodeficiency virus infection. Of the 147 specimens, 42 were positive for NG detected by real-time PCR (prevalence: 28.6%, 95% confidence interval [CI]: 24.8%–32.4%), while none of the 147 MSM were positive for NG detected by conventional culture (prevalence: 0.0%, 95% CI: 0.0%–7.3%). These findings indicated that conventional culture had low sensitivity but high specificity (0.0% and 100%, respectively). We could not demonstrate that many of the factors that were identified in other studies were associated to increased (or decreased) risk of urethral gonococcal infection in our population.
Conclusion: In asymptomatic MSM, nucleic acid amplification tests are more appropriate for screening of urethral NG infection than conventional culture. However, the culture method is necessary for monitoring emerging antimicrobial resistance and to inform gonorrhea treatment guidelines.
Keywords: asymptomatic, Neisseria gonorrhea, urethral gonorrhea, men who have sex with men
Introduction
Neisseria gonorrhea (NG) is one of several important pathogenic Neisseria species causing gonorrhea, a major sexually transmitted infection (STI), globally.1 The gold standard for detecting NG is a conventional culture with a range of sensitivity 50%–95% and specificity 80%–95% depending on the sites tested and the criterion used as a standard.2 This technique cannot only isolate the organism but also allows assessment of antimicrobial susceptibility testing. However, using conventional culture requires well-trained staff, and the result tests are likely to lead to false-negative attributable to poor specimen storage, transport problems, or growth inhibition by components of selective media. Other techniques currently used including microscopy and tests that detect gonococcal antigen or nucleic acid. Nucleic acid amplification tests (NAATs) show higher sensitivity (ranging 90%–99%)3 and can be used on noninvasive samples such as urine. However, NAATs can cross-react with other Neisseria species, are expensive, require highly trained technicians, and considerable investment in equipment.
Recently, several N. gonorrhea strains resistant to antibiotics have emerged. Thus, isolation of the organism in culture and antimicrobial susceptibility testing are essential. A variety of methods are used to confirm the identification of N. gonorrhea, including biochemical testing, serological testing, colorimetric testing, and nucleic acid methods. More than one system may be required to confirm identification.4 The detection of this organism by the polymerase chain reaction (PCR) is now recognized as a sensitive and specific method for diagnosing N. gonorrhea infection. Real-time PCR assay has the additional advantage of reducing the detection time of regular PCR. Gonorrhea is a major cause of urethritis in men and cervicitis in women.5 Extragenital infections of pharynx and rectum are prevalent in certain groups, such as men who have sex with men (MSM). Globally, the increase in gonorrhea rates during 2011–2012 was observed among both men and women (266.1 million cases). Males accounted for 53% (141 million) of the new cases. The prevalence rate of urethral gonorrhea among males by culture technique was 8.97%, while by NAATs it was found to be considerably higher (16.4%).6 In MSM, gonorrhea may increase the risk of contracting human immunodeficiency virus (HIV) through receptive anal intercourse and insertive anal intercourse. Also, the prevalence of this infection varied by anatomic sites (oral, anal, and urethra) and, importantly, by detection methods. A minority of MSM with gonorrhea (20%–30%) are infected at multiple sites. Asymptomatic gonorrhea is common in MSM and may lead to a reservoir which can lead to higher rates of transmission, especially as this population has a higher propensity for multiple partners.7 Indeed, the overall prevalence of gonorrhea among MSM is 13.8%. The most common site of NG infection in males is the urogenital tract. Men with this infection may experience dysuria or burning sensation during urination with penile discharge. In countries such as Thailand, the prevalence of urethral NG among MSM is thought to be considerably higher than what is reported in the literature.8 The main reasons of this phenomenon might be underdetection and underreporting in asymptomatic cases and using a low-sensitivity detection method. In the present study, we screened for urethral gonorrhea among MSM by using two techniques, a conventional culture and real-time PCR in Khon Kaen, Northeastern Thai, MSM population. The aim of this study was to compare the performance of the conventional culture and real-time PCR methods for the diagnosis of urethral gonorrhea among MSM in Khon Kaen, Thailand.
Material and methods
Study population
In August 2014, a prospective study of MSM considered to be at high risk for gonorrhea infection was initiated in a research clinic. MSM aged >18 years were eligible if they met any of the following criteria: reported having receptive anal intercourse and insertive anal intercourse in their lifetime, and did not take antibiotics in previous 2 weeks.
Recruitment
MSM were recruited from two walk-in clinics. The first clinic was a sexually transmitted diseases (STDs) mobile clinic, and the second, an antiretroviral clinic. Both clinics are located in Khon Kaen, northeast Thailand. In addition, identification and recruitment data of MSM were collected by the first author who approached individuals via personal networks and at social venues. Recruitment activities encompassed a region of Khon Kaen municipality area. Meetings were held with local M-REACH teams to enlist support for the ongoing research and to prevent misunderstanding in the study population. Verbal informed consent was obtained from all study participants, and the study was approved by the Ethics Committee of Human Research, Khon Kaen University and Ethics Committee of Human Research, Khon Kaen Hospital. In our settings, the institutional review boards at both Khon Kaen University and Khon Kaen Hospital accepted that the legal age for this type of consent was 18.
Screening for gonorrhea
Specimen collection
In each participant, sterile dragon swab was used to take sample from urethra. The swab sample was immediately inoculated on agar plate and subsequently placed in transport media (10% formalin with 0.85% sodium chloride) for nucleic acid analysis. Samples were transported for 1 hour (3 km daily) from STD clinics to the laboratory in the Department of Microbiology, Khon Kaen University. The swab samples for real-time PCR were kept at 4°C (ice box) before and during shipping. After arrival at the laboratory, the swab samples were stored at 4°C in refrigerator before analysis. Usually, the DNA was extracted twice a week and checked for GRAPH gene as the internal control. Real-time PCR was performed within 2 weeks after sample collection.
Conventional culture
The specimens were inoculated onto Modified Thayer-Martin agar plates immediately (Clinical Diagnostics LTD, Bangkok, Thailand) and incubated for 24–48 hours at 37°C in 5% CO2. Morphologically suggestive colonies of N. gonorrhea were further processed for confirmation by means of Gram staining, oxidase, and glucose utilization tests for isolation and identification of N. gonorrheae. In this study, strict quality control was maintained for every step including media preparation and use positive control in parallel with culture.
Real-time PCR
Total DNA was extracted from the cultured cells using the PUREGENE DNA purification system (Puregene®; Gentra Systems Inc., Minneapolis, MN, USA). Gonococci (GC) (PorA) gene, PorA, was amplified using a forward primer (CAGCATTCAATTTGTTCCGAGTC) and a reverse primer (GAACTGGTTTCATCTGATTACTTTCCA) with an expected product size about 89 bp. A housekeeping gene, GRAPH, was used as the internal control and was amplified using a forward primer (TCATCAGCAATGCCTCCTGCA) and a reverse primer (TGGGTGGCAGTGATGGCA) with an expected product size of about 117 bp. The real-time PCR was performed by using the Light cycler®480 II (Roche, Basel, Switzerland) in a total volume of 10 μL containing 2 μL of either DNA template, 5 μL of SYBR Green Real-time PCR Master Mix (SsoAdvancedTM Universal SYBR® Green Supermix, Bio-rad, Hercules, CA, USA), and 0.2 μM of each primer. After initial denaturation at 95°C for 1 minute, the amplification was carried out through 40 cycles, each consisting of denaturation at 95°C for 15 seconds, annealing at 58°C for 15 seconds, and polymerization at 72°C for 40 seconds, followed by a final extension at 72°C for 2 minutes.
Data management and analysis
Questionnaire, clinical, and laboratory data were entered into a secure database. Individual and composite data were obtained to conduct routine accuracy checks by the researchers, including periodical reviews by the study monitors. Sociodemographic data were collected at enrollment. When visiting, MSM were asked to report what they thought about their sexual identity. This variable was used to classify MSM into three risk groups for analysis: 1) MSM reporting they were king or top (sex act as active); 2) MSM reporting quings (sex act as bisexual); and 3) MSM reporting they were queen or bottom (sex act as passive) or other categories including transgender. Information on sexual intercourse and condom use in the preceding 3 months were categorized into three outcomes: 1) “abstinence” or no sexual activity in the study period; 2) “100% condom use” where sexual activity occurred and condoms were used for all sexual acts in this period; and 3) “unprotected” where sexually activity occurred but condoms were not used for all sexual acts. Other time-dependent sexual risk behavior variables were recorded including receiving payment for sex or paying for sex. HIV status and history of their and their partners STDs were recorded. Nonsexual behavior variables including alcohol and illegal drug use before having sex were also collected. Data analysis in this study was predominantly descriptive in nature. Counts and percentages were used to describe categorical variables, whereas the mean and standard deviation was used to describe continuous variables. Data cleaning, recoding, and analysis were performed using Stata (V11; Stata Corp, College Station, TX, USA).
Results
From September to December 2014, 150 MSM were invited to undergo routine screening, and 147 (98%) agreed to participate in the study. The three participants who declined to participate cited fear of the urethral swab and/or concerns about confidentiality as the main reasons for nonparticipation (Figure 1). Upon recruitment, the characteristics of participants using the two measurement methods were similar (Table 1).
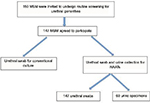  | Figure 1 Study flow and methods for specimen collection. Abbreviations: MSM, men who have sex with men; NAATs, nucleic acid amplification tests. |
Participants were predominately older (mean: 28.79 years, range: 18–55) and engaged in sex with multiple partners (63% had at least two partners and 36% had at least three partners during the previous 3 months), and all 147 participants were asymptomatic (Table 1). More than one-third (33.4%) identified themselves as transgender, and more than half of all participants (55%) stated that they used condoms 100% of the time. Nearly two-third reported (58%) that they had been previously tested for HIV antibodies in the previous 12 months; however, only 10% were HIV positive. Twenty-five MSM (17%) had a history of STDs, mainly HIV infection. In nonsexual behaviors, 41.5% reported using alcohol before sex, and 20.4% used illegal drugs before engaging in sexual activity. Of the 42 individual testing positive for gonorrhea (prevalence: 28.6%, 95% confidence interval [CI]: 24.8%–32.2%), in all cases urethral infections due to NG were detected by real-time PCR. In contrast, none of the 147 MSM tested positive for urethral NG infection based on conventional culture (prevalence: 0.0%, 95% CI: 0.0%–7.3%).
Perusal of Table 2 shows that conventional culture performed very poorly, with a very low sensitivity (0%). The very high specificity of conventional culture may be an artifact of its inability to detect NG in asymptomatic individuals (Table 2).
Examination of the crude odds ratios (ORs) in Table 3 reveals that only history of GC was significantly associated with urethral gonococcal infection. MSM with a history of GC infection had 3.75 times the odds of urethral gonococcal infection relative to MSM without a history of infection (crude OR=3.753; p<0.05). However, when we adjusted for other factors, we could not demonstrate an association between a history of GC infection and urethral gonococcal infection (adjusted OR=3.808; 95% CI: 0.903, 16.123; p=0.0686).
No other factor could be shown to be significantly associated with urethral gonococcal infection, whether in the bivariate analysis or the full multivariable model. However, some effects did have a substantial effect size. For instance, in our sample those whose partners had an unknown history of GC infection had a substantially higher risk of urethral gonococcal infection, but we could not demonstrate this association holds for the MSM population (adjusted OR=3.009; 95% CI: 0.319, 28.351; p=0.0858).
Discussion
It is widely acknowledged that many STIs are asymptomatic and can therefore be difficult to recognize and control. Consequently, the worldwide incidence of new cases of STIs is likely to be underestimated. In this cross-sectional study of asymptomatic MSM, we demonstrate a high urethral gonococcal infection rate (28.6%) in our population, considerably higher than estimated in other studies. For example, studies conducted in 20059 and 200710 reported prevalence of 10% and 7.2%, respectively.
All 42 NG cases in our study were detected using NAAT. However, the culture method failed to identify any of these cases in this asymptomatic group. This result is similar to that of Baker et al11 in 2009 who suggest that the culture method is not sensitive to urethral NG in asymptomatic patients. Regarding their history of STDs, GC (55.6%), syphilis (33.3%), and genital warts (11.1%) were the most common venereal diseases in 8 MSM. However, 8 MSM did not mention their HIV status (positive) as their history of STDs. Our results are not consistent with the generally known literature: it is generally known that HIV-infected MSM are at increased risk of GC compared with HIV-negative MSM.12
Several risk factors exist that make MSM more prone to urethral gonococcal infection than others. Whereas these risk factors are not shared by all STIs, there are many commonalities. Using alcohol before having sex, for example, is a one of the major factors facilitating the increased risk-taking behavior by MSM.13
For instance, in HIV-positive females, the identified prevalence of GC was associated with the use of alcohol before sex (OR=9.1, CI=0.59–0.15, p=0.03). However, we could not demonstrate that alcohol use was associated with urethral gonococcal infection in our study.
Our results suggest that there is an additional risk of GC found in MSM who did not know the GC infection history of their partners, although we could not show this association to be statistically significant. Regardless, this finding is congruent with study in New York which found that known history of STD of partners decreased the number of GC.14 Several risk (or protective) factors that have been identified in other studies could not be shown to be associated with urethral gonococcal infection in our population. For example, condom use has been shown to be protective factor in many other studies,15,16 and multiple partners have been shown to be a risk factor in several others.17,18 In our study, neither of these factors could be shown to be associated with UGI.There is the high prevalence of asymptomatic STDs among MSM, and thus proactive screening by health care providers will be an important part in the early diagnosis and breaking off disease transmission.19 Effective STD prevention health programs for MSM will need to be specific to the types of practices that are safe against transmission of specific STDs, and there should be routine three route screening for treatable bacterial STDs in MSM who routinely engage in unprotected sex.20 A key element of STD control is reducing the risk of HIV transmission associated with STDs. For HIV-infected MSM, diagnosis and treatment of urethral infections reduces the likelihood that they will transmit HIV.21 Most asymptomatic urethral infected MSM do not always seek screening and may still engage in sexual activity,22 and so MSM should be educated about risks of gonococcal urethral infection. They also should be educated that the greater the number of anatomic sites with sexual exposures, the greater the risk of contracting an STD.
A major limitation of our study was the modest sample size employed. Several effects were identified as potentially clinically important (eg, history of GC infection, partner history of GC infection), but we could not demonstrate them to be statistically significant, a problem that might have been avoided with a larger sample size. A further potential limitation is that the sample we tested were from STD clinics, and so may not be representative of the Thai MSM population, leading to problems with the external validity of our results. Thus, our results may be not an accurate picture of the actual epidemiology of gonorrheal infection in Northeastern part of Thailand.
Acknowledgements
The authors wish to express their gratitude to Dr Kaewjai Thepsuthammarat for help with the biostatistics and Dr Cameron P Hurst for assistance with the English-language presentation of the manuscript. The authors would also like to convey thanks to the Social Medicine Department, Khon Kaen Center Hospital, for supporting this work. Finally, the authors wish to thank the National Research University (NRU) for providing the financial support and M-REACH STD clinic for giving other facilities to perform this study.
This research article is a part of the PhD thesis entitled “Prevalence and factors associated with Neisseria gonorrhea infection and multiple resistant patterns of infection with respect to anatomical distributions among MSM”.23
Disclosure
The authors report no conflicts of interest in this work.
References
WHO/HIV_AIDS/2001-02. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overviews and Estimates; 2001. Available from: http://www.who.int/hiv/pub/sti/who_hiv_aids_2001.02.pdf. Accessed February 24, 2017. | ||
van Dyck E, Ieven M, Pattyn S, van Damme L, Laga M. Detection of Chlamydia trachomatis and Neisseria gonorrhea by enzyme immunoassay, culture, and three nucleic acid amplification tests. J Clin Microbiol. 2001;39(5):1751–1756. | ||
Geraats-Peters CWM, Brouwers M, Schneeberger PM, et al. Specific and sensitive detection of Neisseria gonorrhea in clinical specimens by real-time PCR. J Clin Microbiol. 2005;43(11):5653–5659. | ||
Ng L-K, Irene M. The laboratory diagnosis of Neisseria gonorrhea. Can J Infect Dis Med Microbiol. 2005;16(1):15–25. | ||
Centers for Disease Control and Prevention (CDC). Sexually Transmitted Disease Surveillance, 2005. Atlanta, GA: U.S. Department of Health and Human Services; 2006. | ||
Miller WC, Ford CA, Morris M, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA. 2004;291:2229–2236. | ||
Jaschek G, Gaydos C, Welsh L, Quinn TC. Direct detection of Chlamydia trachomatis in urine specimens from syptomatic and asymptomatic men by using a rapid polymerase chain reaction assay. J Clin Microbiol. 1993;31:1209–1212. | ||
Workofsky KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. Recommendations Rep. 2015;64(RR3):1–137. | ||
Charlotte KK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California. Clin Infect Dis. 2005;41:67–74. | ||
Benn PD, Rooney G, Carder C, et al. Chlamydia Trachomatis and Neisseria Gonorrhea infection and the sexual behavior of men who have sex with men. Sex Transm Infect. 2007;83:106–112. | ||
Baker J, Plankey M, Josayma Y, et al. The prevalence of rectal, urethral, and pharyngeal Neisseria Gonorrhea and Chlamydia Trachomatis among asymptomatic men who have sex with men in a prospective cohort in Washington, D.C. AIDS Patient Care STDs. 2009;23(8):585–588. | ||
Fox KK, del Rio C, Holmes KK, et al. Gonorrhea in the HIV era: a reversal in trends among men who have sex with men. Am J Public Health. 2001;91:959–964. | ||
Mansergh G, Flores S, Koblin B, Hudson S, McKirnan D, Colfax GN. Alcohol and drug use in the context of anal sex and other factors associated with sexually transmitted infections: results from a multi-city study of high-risk men who have sex with men in the USA. Sex Transm Infect. 2008;84:509–511. | ||
Du P, Coles FB, Gerber T, McNutt LA. Effects of partner notification on reducing gonorrhea incidence rate. Sex Transm Dis. 2007;34(4):189–194. | ||
Lee W, Daniel RN, Harland DA, et al. Condom effectiveness for reducing transmission of gonorrhea and chlamydia: the importance of assessing partner infection status. Am J Epidemiol. 2004;159:242–251. | ||
Zenilman JM, Weisman CS, Rompalo AM, et al. Condom use to prevent incident STDs: the validity of self-reported condom use. Sex Transm Dis. 1995;22:15–21. | ||
Rietmeijer CA, Patnaik JL, Judson FN, Douglas JM Jr. Increases in gonorrhea and sexual risk behaviors among men who have sex with men: a 12-year trend analysis at the Denver Metro Health Clinic. Sex Transm Dis. 2003;30:562–567. | ||
Jin F, Prestage GP, Mao L, et al. Incidence and risk factors for urethral and anal gonorrhea and chlamydia in a cohort of HIV negative homosexual men: the Health in Men Study. Sex Transm Infect. 2007;83(2):113–119. | ||
Makadon HJ, Mayer KH, Potter J, Goldhammer H. The Fenway Guide to Lesbian, Gay, Bisexual and Transgender Health. Philadelphia, PA: American College of Physicians Press; 2008:1–526. | ||
CDC (Centers for Disease Control and Prevention). Transmission of primary and secondary syphilis by oral sex–Chicago, Illinois (1998–2002). MMWR Morb Mortal Wkly Rep. 2004;53:966–968. | ||
Fleming DT, Wasserheit JN. Epidemiological synergy to public health practice: the contribution of sexually transmitted disease to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. | ||
Lister NA, Smith A, Tabrizi S, et al. Screening for Neisseria Gonorrhea and Chlamydia Trachomatis in men who have sex with men at male-only saunas. Sex Transm Dis. 2003;30:886–889. | ||
Budkaew J. Prevalence and factors associated with Neisseria gonorrhea infection and multiple resistant patterns of infection with respect to anatomical distributions among MSM [doctor’s thesis]. Khon Kaen: Khon Kaen University; 2016. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



