Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Conventional, Complementary and Alternative Medicines: Mechanistic Insights into Therapeutic Landscape of Chronic Obstructive Pulmonary Disease
Authors Arezina R, Chen T, Wang D
Received 15 October 2022
Accepted for publication 27 March 2023
Published 3 April 2023 Volume 2023:18 Pages 447—457
DOI https://doi.org/10.2147/COPD.S393540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Radivoj Arezina,1 Tao Chen,2 Duolao Wang3,4
1Department of Medical, Stridon Clinical Research, Richmond Upon Thames, London, UK; 2Department of Public Health, Policy & Systems, Institute of Population Health, University of Liverpool, Liverpool, Merseyside, UK; 3Affiliated Hospital, Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China; 4Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, Merseyside, UK
Correspondence: Duolao Wang, Email [email protected]
Abstract: COPD (chronic obstructive pulmonary disease) is a major public health concern associated with significant morbidity and mortality worldwide. Current therapeutic guidelines for this disease recommend starting with an inhaled bronchodilator, stepping up to combination therapy as necessary, and/or adding inhaled corticosteroids as symptoms and airflow obstruction progress. However, no drug therapy exists to stop disease progression. The mechanistic definition underlying COPD pathogenesis remains poorly understood, it is generally accepted that oxidative stress and the altered immune response of low-grade airway inflammation are major factors contributing to COPD development. There are several potential therapeutic targets that are currently under investigation, including immune regulatory pathways in inflammation and lung-associated steroid resistance induced by oxidative stress signaling cascades. Patients with COPD have increased levels of inflammatory mediators, including lipid and peptide mediators, as well as a network of cytokines and chemokines that maintain inflammatory immune response and recruit circulating cells into the lungs. Many of these pro-inflammatory mediators are regulated by nuclear factor-kappaB (NF-κB) and mitogen-activated protein kinases (MAPKs), such as p38 MAPK. Increased oxidative stress is a key driving mechanism in perpetuating inflammation and lung injury. Furthermore, many proteases that degrade elastin fibres are secreted by airway resident infiltrating immune cells in COPD patients. In this perspective, we discuss novel aspects of signaling pathway activation in the context of inflammation and oxidative stress, and the broad view of potential effective pharmacotherapies that target the underlying mechanistic disease process in COPD.
Keywords: COPD, mechanistic, pharmacotherapy
Introduction
Chronic obstructive pulmonary disease (COPD) is a gradually progressive lung disease characterized by a reduced maximum expiratory flow and a slowly forced expulsion of the lungs. Inhalation of cigarette smoke (CS) is one of the primary causes of COPD, as are air pollution and noxious particles as other important factors.1 In addition, a genetic condition known as alpha-1 antitrypsin deficiency, early life events known as “childhood disadvantage factors”, and childhood respiratory infections may also contribute to COPD.1 In accordance with the Global Burden of Diseases (GBD) data, COPD is the most common chronic respiratory disease and the third leading cause of death, with 42 deaths per 100,000 individuals, constituting 4.72% of the all-cause deaths in 2017.2 COPD is thus regarded as one of the leading causes of mortality and morbidity worldwide due to its high prevalence and chronic nature of the disease.3 The hallmarks of COPD are poorly reversible airway obstructions detected by spirometry, which leads to irreversible deterioration of lung function. There are four major clinical forms of COPD, in which small airways disease (chronic obstructive bronchiolitis) and emphysema are characterized by reduced alveolar space and destruction. Additionally, coexistence of sarcopenia and abdominal obesity termed sarcopenic obese, and mixed COPD–asthma phenotype which presented characteristics of both diseases, are also frequently present among COPD patients.4,5
Cellular and Molecular Perspectives of Disease Mechanism in COPD
Chronic Inflammation in COPD
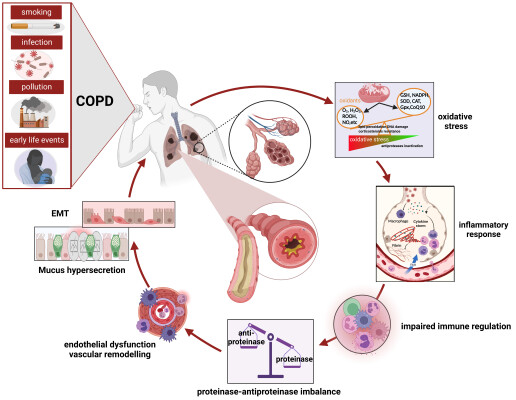
COPD is a devastating, heterogeneous disease, characterized by chronic inflammation of airways and lung parenchyma as the mechanism of its onset and progression.6 The airway inflammation in COPD is associated with activating the innate immune system, as evidenced by increased numbers of classical inflammatory cell types, such as alveolar macrophage, neutrophils, natural killer cells, and mature dendritic cells in lung tissue and the airway lumen.7 In this context, the inflammation as a result of smoking is unique since the accumulation of inflammatory cells in the lungs is not coupled with the effective innate immune system reaction, but rather results in progressive lung tissue damage. Bacterial and viral infections are considered to be a common cause of COPD exacerbations, and they facilitate secondary inflammation caused by respiratory pathogens.8 There is increasing evidence which suggests that the gut microbiota plays an important role in the development, regulation, and maintenance of healthy immune response in the lungs. Dysbiosis and subsequent dysregulation of the microbiota-related immunological processes affect the onset of COPD pathogenesis. There are several features in the newly described COPD gut metabolome that suggest altered metabolic processes. The effects of the gut metabolome on COPD are at least partially mediated by bacterial metabolites, which may influence immune responses in distant parts of the body. The best-known metabolites with demonstrated protective properties in human airway inflammation are short-chain fatty acids (SCFAs). SCFAs have also been shown to reduce inflammation in both OVA and house dust mite (HDM)-induced airway inflammation models.9,10 Moreover, oral application of SCFAs to mice during pregnancy and weaning have protect offspring from allergic lung inflammation, with butyrate potently inducing regulatory T (Treg) cells in the lungs of offspring.11 In a recent study, it was found that there was no difference in microbiome composition between smokers compared to non-smokers with COPD, supporting this as a disease-associated phenotype rather than the result of bacteria in the gut being affected by the smoke of cigarettes.12 Nevertheless, the gut is a potential source of disease inflammation in COPD based on discriminatory signals from both metagenomic and metabolomic data.13–15 Additionally, the adaptive immune system is activated in COPD, as reflected by the presence of CD8+ T cells, B cells, and both T helper type 17 and T helper type 1 forms of CD4+ T cells, as well as a reduction of regulatory T cells in the airways.16 These inflammatory cells and structure cells, including epithelial, endothelial cells, and fibroblasts secrete many inflammatory mediators that drive the inflammatory response in COPD. These mediators include lipids, free radicals, cytokines, chemokines, proteases, and growth factors, which often spill into the circulation, leading to systemic inflammation for COPD exacerbations and comorbidities potentiation. In particular, the metabolites secreted by the gut bacteria might prove useful for additional therapeutic approaches.
In COPD, repeated exposure to noxious particles, usually cigarette smoke, can activate epithelial cells and macrophages to release variety of chemotactic factors that attract inflammatory cells (eg, CD8+ T lymphocytes, neutrophils, monocytes) to the lungs, triggering a distinct inflammatory cascade in the small airways and lung parenchyma.17 In this process, activation of pro-inflammatory transcription factors, such as NF-κB), activator protein-1 (AP-1), and mitogen-activated protein kinases (MAPKs), appear to be important in potentiating the inflammatory responses that underpin COPD. There is clear evidence for the phosphorylated p38 MAPK, NF-κB activation in bronchial epithelial cells of COPD patients.18–20 In primary human lung epithelial cells, cigarette smoke exposure induced the activation of MAPK pathways, including c-Jun, extracellular regulated protein kinases (ERK), p38, as well as epidermal growth factor receptor (EGFR) ligand.21,22 Besides, increased expression of phosphoinositide-3-kinase (PI3K) and its downstream mediators (eg, protein kinase B (AKT/PKB), phosphatase and tensin homolog (PTEN), mammalian target of rapamycin (mTOR)), contributing to a vicious cycle of inflammation, innate and adaptive immunity, oxidative stress, and epithelial-mesenchymal transition (EMT), have been reported in COPD.23
Oxidant-Antioxidant Imbalance in COPD
Oxidative stress is a key driving mechanism in the pathogenesis of COPD. In COPD patients, oxidative stress is increased due to exogenous oxidants like cigarette smoke and air pollution and endogenous production of reactive oxygen species (ROS) by inflammatory and structural cells in the lungs. The ability of lung cells to maintain appropriate antioxidant responses is crucial for protecting against smoking-induced oxidative stress. A reduction in antioxidant defenses has also been recorded, with the inactivation of several antioxidant enzymes and transcription factors that regulate multiple antioxidant genes, including factor-erythroid 2-related factor (Nrf2) and forkhead box O3 (FOXO3).24,25 Numerous genetic studies have revealed alterations in the nuclear Nrf2-mediated oxidative stress pathway as well as the mitochondrial dysfunction pathway.26 Oxidative stress promotes chronic inflammation, stimulates fibrosis and emphysema, causes corticosteroid resistance, accelerates lung aging, damages DNA and activates autoantibodies.27 For instance, oxidative stress-activated pro-inflammatory pathways such as NF-κB that increased multiple inflammatory genes expression to amply the inflammatory response.28 Improved NF-κB signaling drives the expression of target genes, including mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) that is known worsening COPD pathogenesis via inducing mucus obstruction.29 The oxidative stress can also activate the enzyme PI3K to impair histone deacetylase-2 (HDAC2) expression and activity. Decreased HDAC2 abolished glucocorticoid receptor (GR) activity which could further attenuated anti-inflammation effect of GR and lead to corticosteroid resistance in COPD.30 Similarly, oxidative stress affects both the expression and activation of sirtuin family members including sirtuin1–6, altering inflammatory response, autophagy or apoptosis process via regulating NF-κB signaling, TGF-β1 signaling pathway and PI3K/mTOR signaling pathway in COPD.31 Furthermore, oxidative stress leads to telomere shortening, which activates p53, p21CIP1, and p16INK4 pathways, thereby leading to cell senescence.32 Antioxidant protection in COPD patients can be increased by aerosolization of glutathione, which has been shown to enhance airway epithelial cell host defense response to H. influenzae.33 A critical pathway that leads from the detection of H. influenzae to the increased expression of many inflammatory genes requires the induction of the transcription-activating complex NF-κB.34–36 This bacteria-induced NF-κB activation and inflammatory protein expression was decreased by glutathione augmentation, suggesting a role for oxidants in COPD treatment.33
Proteases–Antiproteases Imbalance in COPD
An imbalance between proteases and antiproteases that leads to lung parenchymal destruction in COPD is another critical factor in the genesis and development of the disease.37,38 In the healthy lung, proteases are counterbalanced by antiproteases, but the balance shifts toward proteases when cigarette smoke or other pollutants recruit neutrophils and macrophages able to produce overwhelming proteases and induce destruction of healthy lung parenchyma.39 These proteases, including elastase and matrix metalloproteinases (MMPs) released from activated macrophages, neutrophils and epithelial cells, degrade components of the extracellular matrix (ECM), elastin fibers and collagen, generating elastin fragments or collagen-derived peptides, which contributes to the development of emphysema. In addition, chemotactic peptide fragments are generated from the degraded ECM, perpetuating macrophage and neutrophil accumulation that promote the inflammatory processes of COPD.40 It is demonstrated that both MMP-9 activity and MMP-9/TIMP-1 ratio are increased in the sputum and bronchoalveolar lavage fluid from COPD patients when compared with healthy controls.41,42 Furthermore, airway epithelial and smooth muscle cell hyperplasia leading to disproportionate ECM deposits in the interstitial lung can contribute to the development of COPD and pulmonary fibrosis.43,44 The mucociliary dysfunction, specifically the excessive production of mucus coupled with a decreased ability to clear it, is a crucial feature of COPD-related lung disease.45,46 Additionally, alpha-1 antitrypsin deficiency (AATD) is genetic factor that induce COPD due to imbalances in protease-antiprotease protection in the lung.47 COPD is often associated with pulmonary vascular wall remodeling that thickens bronchial walls and narrows their lumens, which can lead to the comorbid pulmonary complication cor pulmonale.48
Other Pathogenesis Mechanisms in COPD
Isolation of bacteria, viruses and fungi implicated with either acute or chronic infections occur with increased frequency in COPD patients and showed significance for disease pathogenesis, progression and treatment. Correlatively, as the first line of defence against infection, innate immunity is impaired in COPD patients.49 Furthermore, increasing studies supported immune dysfunction and destroyed repair mechanisms, leads to exacerbations and disease severity in COPD.50,51 It is known that the incidence of COPD is high in elder persons whose age-related alterations are abnormally increased that may be induced by cell senescence, telomere shortening and defective DNA repair.52 The hallmark features of COPD pathology are displayed in Figure 1.
Current Pharmacotherapies for COPD
Current COPD treatment strategy primarily focuses on managing symptoms and reducing the frequency of exacerbations. Based on the global initiative for COPD, bronchodilation with beta2-agonists and antimuscarinic drugs, and reduction of inflammation by inhaled corticosteroids (ICS) remained a key element of pharmacological treatment for COPD patients.1 The principal bronchodilator action of beta2-agonist primarily depends on its ability to relax airway smooth muscle by binding to the active sites of beta2-adrenergic receptors, which can activate the effector molecular cyclic AMP and generate functional antagonism to bronchoconstriction.53,54 The bronchodilator activity of antimuscarinic drugs is mainly caused by blocking M3-receptors expressed in airway smooth muscles.55 There are short-acting and long-acting beta2-agonists and muscarinic antagonists. Short-acting beta2-agonist (SABA) is used as the first-line treatment for COPD exacerbation,56 and the previous evidence suggested that short-acting muscarinic antagonists (SAMA) ipratropium provided limited benefits over SABA in lung function and health status context.57 Short-acting bronchodilators should be prescribed for immediate symptom relief, but not recommended for chronic use. Long-acting beta2-agonist (LABA) and muscarinic antagonists (LAMA) together with ICS has been found to improve COPD patients’ outcomes and alleviate exacerbations.58 LABA and LAMA are considered equally efficient in COPD management and none of these has shown superior efficacy to the other. The choice within each medication category depends on the availability of medication and individual responses, preferences, and side effects. Accordingly, LABAs are suggested as the initial choice for patients with either acute urinary retention or dry mouth, while LAMAs are suggested as the first choice for COPD patients which previously experienced troublesome adverse effects using beta2-agonists, such as formoterol, salmeterol, olodaterol, and vilanterol. It is notably, the primary treatment in groups A, B, C and D COPD patients is LAMA according to the GOLD. In addition, there is evidence suggesting that LAMAs are preferred over LABAs in terms of preventing exacerbations and may be the first choice in prevention of common exacerbators.59 Furthermore, the combination of LABA and LAMA provides additional benefits over the individual components used alone without significantly increasing the risk of adverse effects. When symptoms are not controlled adequately with LAMA or LABA monotherapy, administration of LABA plus LAMA are indicated eventually via one medication device. In some patients with higher blood eosinophil counts or people with asthma-COPD overlap syndrome, a combination treatment of LABA plus ICS may be offered as initial treatment. If the symptoms persist, the triple therapy may be preferred.
Apart from the usage of major bronchodilators in combination with ICS, numerous evidences suggested phosphodiesterase-4 (PDE4) inhibitors might be effective for COPD patients. PDE-4 inhibitors act as anti-inflammatory agents that can increase cyclic AMP in inflammatory cells and inhibit the breakdown of intracellular cyclic nucleotides.60 Roflumilast, as the most used PDE-4 inhibitor, was indicated as a treatment choice to improve lung function and reduce the risk for exacerbations in patients with severe COPD (GOLD patient groups C and D).61 Roflumilast has been shown to reduce allergen-induced inflammation and stabilize lipopolysaccharide-induced systemic inflammation via decreasing inflammatory mediators and the expression of cell surface markers, particularly in chronic bronchitis, non-smoker women.62 Furthermore, in vitro and in vivo evidences have introduced macrolides exerted anti-inflammatory effects by attenuated mucus secretion and decreased accumulation of neutrophils and macrophages in the airway and have been applied in a variety of inflammatory airway diseases treatment including COPD.63 In addition, several observational studies have identified the regular use and supplementation of mucolytic agents, such as N-acetylcysteine (NAC) and carbocysteine,64,65 antioxidant agents, other anti-inflammatory drugs and vitamin D could potentially reduce the risk of exacerbation when used in susceptible patients.65,66 Since we still do not totally understand the pathophysiology of COPD development, and patients applied current drugs may acquire resistance or side effect, there is an urgent unmet need to identify other critical therapeutic targets or medicine for this disease.
Natural Medicines for COPD
It has been reported that natural medicines offer promising alternatives to COPD treatments due to their multiple therapeutic targets, low toxicity, and inherent biologic properties, making them particularly attractive. Several researchers investigating different kinds of natural medicines reported that they were able to reduce fatigue, improve quality of life and even decrease the risk of re-hospitalization or death in elderly COPD patients.67–71 Traditional Chinese medicine (TCM) therapy, though is not included in the GOLD guidelines as a recognized treatment for COPD, has been proved effective for COPD patients in increasing number of reports. Li et al first proposed a strategy based on staging and grading of TCM prevention and treatment of COPD, and which they applied in clinical practice.72 Li et al carried out clinical trials on reducing COPD symptoms using specialized TCM formulas. In a randomized, double-blind, placebo-controlled clinical trial, such specialized TCM formulas including Bu-Fei Jian-Pi, Bu-Fei Yi-Shen and Yi-Qi Zi-Shen granules significantly reduced the severity and frequency of acute exacerbations, alleviated symptoms, and improved quality of life in COPD patients.73 In addition, the combination of such specialized TCM formulas with conventional therapies shortened hospitalization times, reduced the risk of acute exacerbations, and improved overall symptoms resulting better quality of life.74 Further studies suggested these TCM formulas significantly improved the lung function of COPD, reduced the pathological damage of lung tissue, inhibited the inflammatory response, and improved overall oxidative stress responses.75–77 Their work also provided the world’s first sequential treatment regimen for AECOPD risk window (AECOPDRW), which is the period before a patient returns to a stable baseline after initial deterioration, poor lung function, and possible persistence of inflammation.78–80 This finding enriched the staging theory of COPD, and was adopted as an important part of the international clinical practice guidelines of TCM, which may provide new methods and strategies for the treatment of COPD. For the mechanistic aspects, the TCM formulas inhibited both CSE- and LPS-elevated inflammation in macrophages and epithelial cells via limiting the pathway activation of NF-κB, MAPK, STAT3 and PPAR signaling. Bu-Fei Yi-Shen formula (BYF), another specialized TCM formula, could restore unbalanced Th17/Treg ratio leading reduced inflammation.81–83Furthermore, under an effective-constituent compatibility-based screening, Li et al have purified the major effective components, including ginsenoside Rh1, icariin, nobiletin, paeonol, and astragaloside, from BYF.84 Effective-constituent compatibility of the BYF could suppress the inflammatory response in COPD rats by inhibition of NF-κB p65, c-Jun NH2-terminal kinase (JNK), and p38 mitogen-activated protein kinase signaling, and also showed synergistic effects in protection against PM2.5-induced oxidative stress via miR-155/FOXO3a signaling in COPD rats85. 86
Novel Medicines and Future Perspectives of COPD Therapies
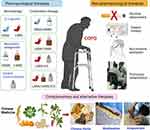
Searching for an effective treatment for COPD is still ongoing and new drugs are under development. A dual PDE3/PDE4 inhibitor ensifentrine/RPL554 was shown in a Phase 2 clinical trial to induce both bronchodilator and anti-inflammatory effects in COPD.87 Another clinical trial, which enrolled 400 individuals with severe or very severe COPD, showed that AQX-1125 ameliorates inflammatory response and that might have a therapeutic benefit for COPD by targeting the src homology 2-containing inositol-5’-phosphatase 1 (SHIP1) pathway.88 SRT1720, a SIRT1 activator, was proved to attenuated stress-induced cellular senescence and protected against emphysema by deacetylating FOXO3 transcription factor.89 Furthermore, RNA therapeutics and clinically effective RNA targeted drugs may be added to novel therapeutic options for COPD in the very near future.90 There has been a great deal of work suggesting that knocking down the mRNA coding for target proteins in COPD may have potential therapeutic effect and some siRNAs have been validated in in vivo COPD models. For instance, the levels of receptor interacting protein (RIP) 2 was found elevated in cigarette smoke-induced COPD mouse model, and intratracheal RIP2-specific siRNA was shown to reduce pro-inflammatory cytokine production and oxidative stress marker elevation, suggesting a potential role for COPD treatment.91 Additionally, in cell-based studies, CD147 siRNA and patched homolog (PCTH1) siRNA were shown to repress mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) expression and in turn inhibit mucus secretion.92,93 Specific siRNAs targeting neuron derived orphan receptor (NOR) 1, hypoxia inducible factor 1 alpha (HIF-1α) and connective tissue growth factor (CTGF) were identified as capable of reducing pulmonary vascular wall remodeling, which is the major cause of cor pulmonale, a common comorbidity in patients with advanced COPD.94–96 RNA (ncRNA) targets have also been shown to be promising in mitigating COPD. Administration of miR-181c mimics was able to attenuate CS-induced macrophage and neutrophil counts, and pro-inflammatory factors (IL6 or IL8) expression levels.97 Using anti-miR-195 lentivirus prevented neutrophil and macrophage pulmonary infiltration and phospho-Akt expression.98 Blocking long non-coding RNA (lncRNA) taurine-upregulated gene 1 (TUG1) with short hairpin RNA (shRNA) repressed CS-induced airway inflammation and remodeling in a chronic COPD mouse model.99 Conventional and complementary and alternative therapy treatments of COPD are displayed in Figure 2.
Interestingly, natural medicines as well as their purified ingredients may play an important role in anti-inflammatory and anti-oxidative stress. Ginseng and Paeonia show reduced levels of inflammatory factors in the LPS-induced alveolar epithelial cell/macrophage co-culture inflammation model.100 Ginsenoside Rg1 has extensive antioxidant and anti-inflammatory effects.101 Icariin inhibits TNF-α/IFN-γ-induced inflammatory response in human keratinocytes by regulating the P38/MAPK pathway.102 Icariin protects against LPS-induced acute lung injury and was related to the regulation of PI3K/Akt and NF-κB pathways.103 Paeonol can reduce the expression of inflammatory factors in vascular endothelial cells, reduce the adhesion of endothelial cells and monocytes, and alleviate asthma in mice by regulating TLR4/NF-κB, MAPK pathway.104
Conclusion and Future Directions
COPD is a common, preventable disease, yet an ever-growing burden to the health globally. Current therapeutic strategies are focused on reducing the symptoms and exacerbations, as well as optimizing disease management. The GOLD international guidelines provided a template for the disease diagnosis and management. Developing COPD is a complex heterogeneous processes involving a variety of inflammatory cells, inflammatory mediators, and related cell signaling pathways. The overlapping functions of molecular targeted inflammatory signals make it complexity to what extent COPD can be prevented if one pathway is inhibited alone. Genetic factors α1 that antitrypsin deficiency and telomerase reverse transcriptase mutation have been clearly shown causative in COPD. Epigenetic changes induced by gene–environment interactions, such as DNA methylation and histone modifications, could influence the development of COPD in adulthood, needs to be further explored.105 It is hoped that the study of redox epigenetic regulation in inflammation and steroid resistance will unravel the disease mechanism and identify potential epigenetic-based treatments for COPD. Hence, further research is needed to understand the functions and mechanisms of diverse target molecules and more personalized treatments for these mechanisms should be provided. The effectiveness and safety of products acting upon these target molecules will have to be evaluated in animal models and in clinical trials. In addition to small-molecules, identifying targets of natural medicines may be an increasingly important resource for drug discovery based on phenotypic screening.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pride NB, Vermeire PJERM. Definition and differential diagnosis. Eur Respir Monograph. 1998;3(7):2–5.
2. Silverman EK. Genetics of COPD. Annu Rev Physiol. 2020;82:413–431.
3. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8(6):585–596.
4. Joppa P, Tkacova R, Franssen FM, et al. Sarcopenic obesity, functional outcomes, and systemic inflammation in patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2016;17(8):712–718.
5. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guideline for COPD (GesEPOC). Arch Bronconeumol. 2014;50(Suppl 1):1–16.
6. Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci. 2017;131(13):1541–1558.
7. Pouwels SD, Heijink IH, ten Hacken NH, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7(2):215–226.
8. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365.
9. Trompette A, Gollwitzer ES, Yadava K, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166.
10. Cait A, Hughes MR, Antignano F, et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11(3):785–795. doi:10.1038/mi.2017.75
11. Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74(4):799–809.
12. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260.
13. Bowerman KL, Rehman SF, Vaughan A, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun. 2020;11(1):5886.
14. Lai HC, Lin TL, Chen TW, et al. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022;71(2):309–321.
15. Chiu YC, Lee SW, Liu CW, et al. Comprehensive profiling of the gut microbiota in patients with chronic obstructive pulmonary disease of varying severity. PLoS One. 2021;16(4):e0249944.
16. Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–3348.
17. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27.
18. Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2010;80(1):59–70.
19. Renda T, Baraldo S, Pelaia G, et al. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31(1):62–69.
20. Gaffey K, Reynolds S, Plumb J, Kaur M, Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42(1):28–41.
21. Vallese D, Ricciardolo FL, Gnemmi I, et al. Phospho-p38 MAPK expression in COPD patients and asthmatics and in challenged bronchial epithelium. Respiration. 2015;89(4):329–342.
22. Maunders H, Patwardhan S, Phillips J, Clack A, Richter A. Human bronchial epithelial cell transcriptome: gene expression changes following acute exposure to whole cigarette smoke in vitro. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1248–L1256.
23. Moradi S, Jarrahi E, Ahmadi A, et al. PI3K signalling in chronic obstructive pulmonary disease and opportunities for therapy. J Pathol. 2021;254(5):505–518.
24. Malhotra D, Thimmulappa R, Navas-Acien A, et al. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178(6):592–604.
25. Hwang JW, Rajendrasozhan S, Yao H, et al. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol. 2011;187(2):987–998.
26. Lee J, Jang J, Park SM, Yang SR. An update on the role of Nrf2 in respiratory disease: molecular mechanisms and therapeutic approaches. Int J Mol Sci. 2021;22(16):8406.
27. Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544.
28. Di Stefano A, Caramori G, Oates T, et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20(3):556–563.
29. Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–464.
30. Li J, Ye Z. The potential role and regulatory mechanisms of MUC5AC in chronic obstructive pulmonary disease. Molecules. 2020;25:19.
31. Zhang XY, Li W, Zhang JR, Li CY, Zhang J, Lv XJ. Roles of sirtuin family members in chronic obstructive pulmonary disease. Respir Res. 2022;23(1):66.
32. Birch J, Barnes PJ, Passos JF. Mitochondria, telomeres and cell senescence: implications for lung ageing and disease. Pharmacol Ther. 2018;183:34–49.
33. Manzel LJ, Shi L, O’Shaughnessy PT, Thorne PS, Look DC. Inhibition by cigarette smoke of nuclear factor-κB-dependent response to bacteria in the airway. Am J Respir Cell Mol Biol. 2011;44(2):155–165.
34. Chin CL, Manzel LJ, Lehman EE, et al. Haemophilus influenzae from patients with chronic obstructive pulmonary disease exacerbation induce more inflammation than colonizers. Am J Respir Crit Care Med. 2005;172(1):85–91.
35. Shuto T, Xu H, Wang B, et al. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci USA. 2001;98(15):8774–8779.
36. Manzel LJ, Chin CL, Behlke MA, Look DC. Regulation of bacteria-induced intercellular adhesion molecule-1 by CCAAT/enhancer binding proteins. Am J Respir Cell Mol Biol. 2009;40(2):200–210.
37. Turino GM. Emphysema in COPD: consequences and causes. Thorax. 2006;61(12):1031–1032.
38. Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51.
39. Heinz A. Elastases and elastokines: elastin degradation and its significance in health and disease. Crit Rev Biochem Mol Biol. 2020;55(3):252–273.
40. Houghton AM, Quintero PA, Perkins DL, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116(3):753–759.
41. Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1635–1639.
42. Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6(1):151.
43. Jones RL, Noble PB, Elliot JG, James AL. Airway remodelling in COPD: it’s not asthma! Respirology. 2016;21(8):1347–1356.
44. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27.
45. Ma J, Rubin BK, Voynow JA. Mucins, mucus, and goblet cells. Chest. 2018;154(1):169–176.
46. Zhou-Suckow Z, Duerr J, Hagner M, Agrawal R, Mall MA. Airway mucus, inflammation and remodeling: emerging links in the pathogenesis of chronic lung diseases. Cell Tissue Res. 2017;367(3):537–550.
47. Strange C. Alpha-1 antitrypsin deficiency associated COPD. Clin Chest Med. 2020;41(3):339–345.
48. Olivieri D, Chetta A. Therapeutic perspectives in vascular remodeling in asthma and chronic obstructive pulmonary disease. Chem Immunol Allergy. 2014;99:216–225.
49. Leung JM, Tiew PY, Mac Aogáin M, et al. The role of acute and chronic respiratory colonization and infections in the pathogenesis of COPD. Respirology. 2017;22(4):634–650.
50. Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:995–1013.
51. Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12(Suppl2):S169–175.
52. Hikichi M, Mizumura K, Maruoka S, Gon Y. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis. 2019;11(Suppl 17):S2129–s2140.
53. Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26(1):112–120.
54. Cazzola M, Page CP, Rogliani P, Matera MG. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187(7):690–696.
55. Melani AS. Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol. 2015;8(4):479–501.
56. Al-Faqawi M, Abuowda Y, Elmassry AE, Böttcher B. Management of chronic obstructive pulmonary disease exacerbations at the Nasser medical complex: a clinical audit. Lancet. 2018;391(Suppl 2):S5.
57. Appleton S, Jones T, Poole P, et al. Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;2006(3):CD006101.
58. Donner CF, Virchow JC, Lusuardi M. Pharmacoeconomics in COPD and inappropriateness of diagnostics, management and treatment. Respir Med. 2011;105(6):828–837.
59. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533.
60. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163(1):53–67.
61. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–694.
62. Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:81–90.
63. Qiu S, Zhong X. Macrolides: a promising pharmacologic therapy for chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2017;11(3):147–155.
64. Cazzola M, Calzetta L, Page C, et al. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015;24(137):451–461.
65. Rogliani P, Matera MG, Page C, Puxeddu E, Cazzola M, Calzetta L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir Res. 2019;20(1):104.
66. Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114.
67. Kuniaki H, Akihiko T, Tetsuya H, et al. Improvement in Frailty in a Patient With Severe Chronic Obstructive Pulmonary Disease After Ninjin’yoeito Therapy: a Case Report. Front Nutr. 2018;5:71.
68. Hirai K, Homma T, Matsunaga T, et al. Usefulness of Ninjin’yoeito for chronic obstructive pulmonary disease patients with frailty. J Altern Complement Med. 2020;26(8):750–757.
69. Mukaida K, Hattori N, Kondo K, et al. A pilot study of the multiherb Kampo medicine bakumondoto for cough in patients with chronic obstructive pulmonary disease. Phytomedicine. 2011;18(8–9):625–629.
70. Sasatani Y, Okauchi S, Ohara G, Kagohashi K, Satoh H. Long-term maintenance of nutritional status with ninjinyoueito in terminal patients with chronic respiratory disease: two case reports. Biomed Rep. 2020;12(3):121–124.
71. Jo T, Michihata N, Yamana H, et al. Reduction in exacerbation of COPD in patients of advanced age using the Japanese Kampo medicine Dai-kenchu-to: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:129–139.
72. Li J. International clinical practice guideline of Chinese medicine: chronic obstructive pulmonary disease. World J Tradit Chin Med. 2020;6(1):39–50.
73. Li SY, Li JS, Wang MH, et al. Effects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled study. BMC Complement Altern Med. 2012;12:197.
74. Li J, Zhang H, Ruan H, et al. Effects of Chinese herbal medicine on acute exacerbations of COPD: a randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2020;15:2901–2912.
75. Li Y, Tian YG, Li JS, et al. Bufei Yishen granules combined with acupoint sticking therapy suppress oxidative stress in chronic obstructive pulmonary disease rats: Via regulating peroxisome proliferator-activated receptor-gamma signaling. J Ethnopharmacol. 2016;(193):354–361.
76. Li JS, Zhao P, Yang, LP, et al. System biology analysis of longterm effect and mechanism of Bufei Yishen on COPD revealed by system pharmacology and 3-omics profiling. Sci Rep. 2016;6:25492.
77. Zhao P, Li JS, Li Y, et al. Systems pharmacology-based approach for dissecting the active ingredients and potential targets of the Chinese herbal Bufei Jianpi formula for the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:2633–2656.
78. Li JS, Wang HF. 基于慢性阻塞性肺疾病急性加重危险窗的袪邪扶正序贯辨证治疗策略. [Sequential syndrome differentiation by eliminating pathogen and strengthening vital Qi on the basis of acute exacerbation of chronic obstructive pulmonary disease risk window]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(9):1276–1280. Chinese.
79. Jiansheng L, Haifeng W, Suyun L, et al. Effect of sequential treatment with TCM syndrome differentiation on acute exacerbation of chronic obstructive pulmonary disease and AECOPD risk window. Complement Ther Med. 2016;29:109–115.
80. Haifeng W, Jiansheng L, Suyun L, et al. Effect of sequential treatment with syndrome differentiation on acute exacerbation of chronic obstructive pulmonary disease and “AECOPD Risk-Window”: study protocol for a randomized placebo-controlled trial. Trials. 2012;13:40.
81. Zhao P, Li J, Tian Y, et al. Restoring Th17/Treg balance via modulation of STAT3 and STAT5 activation contributes to the amelioration of chronic obstructive pulmonary disease by Bufei Yishen formula. J Ethnopharmacol. 2018;217:152–162.
82. Qin YQ, Chen YL, Zhao P et al, et al. Tiaobu Feishen therapy inhibits inflammation induced by cigarette smoke extracts in a human monocyte/macrophage cell line. J Tradit Chin Med. 2021;4(3) :360–366.
83. Chen Y et al . (2019). Three Tiaobu Feishen therapies protect human alveolar epithelial cells against cigarette smoking and tumor necrosis factor--induced inflammation by nuclear factor-kappa B pathway. J Tradit Chin Med, 39(1), 45–49.
84. Li J, Liu X, Dong H, Zheng W, Feng S, Tian Y, Zhao P, Ma J, Ren Z, Xie Y. (2020). Effective-constituent compatibility-based analysis of Bufei Yishen formula, a traditional herbal compound as an effective treatment for chronic obstructive pulmonary disease. J Integr Med, 18(4), 351–362. 10.1016/j.joim.2020.04.004
85. Li J, Wang J, Li Y, Zhao P, Tian Y, Liu X, He H, Jia R. (2021). Effective-component compatibility of Bufei Yishen formula protects COPD rats against PM2.5-induced oxidative stress via miR-155/FOXO3a pathway. Ecotoxicol Environ Saf, 228 112918 10.1016/j.ecoenv.2021.112918
86. Li J, Xie Y, Zhao P, et al. A Chinese herbal formula ameliorates COPD by inhibiting the inflammatory response via downregulation of p65, JNK, and p38. Phytomedicine. 2021;83:153475.
87. Singh D, Abbott-Banner K, Bengtsson T, Newman K. The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur Respir J. 2018;52(5):1801074.
88. Efficacy and safety of AQX-1125 in unstable COPD (FLAGSHIP); 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT01954628.
89. Chun P. Role of sirtuins in chronic obstructive pulmonary disease. Arch Pharm Res. 2015;38(1):1–10.
90. Mei D, Tan WSD, Tay Y, Mukhopadhyay A, Wong WSF. Therapeutic RNA strategies for chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2020;41(7):475–486.
91. Dong J, Liao W, Tan LH, Yong A, Peh WY, Wong WSF. Gene silencing of receptor-interacting protein 2 protects against cigarette smoke-induced acute lung injury. Pharmacol Res. 2019;139:560–568.
92. Yu Q, Yang D, Chen X, Chen Q. CD147 increases mucus secretion induced by cigarette smoke in COPD. BMC Pulm Med. 2019;19(1):29.
93. Tam A, Hughes M, McNagny KM, et al. Hedgehog signaling in the airway epithelium of patients with chronic obstructive pulmonary disease. Sci Rep. 2019;9(1):3353.
94. Wang H, Yao H, Yi B, et al. MicroRNA-638 inhibits human airway smooth muscle cell proliferation and migration through targeting cyclin D1 and NOR1. J Cell Physiol. 2018;234(1):369–381.
95. Zhou SJ, Li M, Zeng DX, et al. Expression variations of connective tissue growth factor in pulmonary arteries from smokers with and without chronic obstructive pulmonary disease. Sci Rep. 2015;5:8564.
96. Reimann S, Fink L, Wilhelm J, et al. Increased S100A4 expression in the vasculature of human COPD lungs and murine model of smoke-induced emphysema. Respir Res. 2015;16:127.
97. Du Y, Ding Y, Chen X, et al. MicroRNA-181c inhibits cigarette smoke-induced chronic obstructive pulmonary disease by regulating CCN1 expression. Respir Res. 2017;18(1):155.
98. Gu W, Yuan Y, Yang H, et al. Role of miR-195 in cigarette smoke-induced chronic obstructive pulmonary disease. Int Immunopharmacol. 2018;55:49–54.
99. Gu W, Yuan Y, Wang L, et al. Long non-coding RNA TUG1 promotes airway remodelling by suppressing the miR-145-5p/DUSP6 axis in cigarette smoke-induced COPD. J Cell Mol Med. 2019;23(11):7200–7209.
100. Shin JY, Kang ES, Park JH, Cho BO, Jang SI. Anti-inflammatory effect of red ginseng marc, Artemisia scoparia, Paeonia japonica and Angelica gigas extract mixture in LPS-stimulated RAW 264.7 cells. Biomed Rep. 2022;17(1):63.
101. Gao Y, Chu S, Zhang Z, Chen N. Hepataprotective effects of ginsenoside Rg1 - A review. J Ethnopharmacol. 2017;206:178–183.
102. Kong L, Liu J, Wang J, et al. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int Immunopharmacol. 2015;29(2):401–407.
103. Xu C-Q, Liu B-J, Wu J-F, et al. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-κB signaling pathway. Eur J Pharmacol. 2010;642(1–3):146–153. doi:10.1016/j.ejphar.2010.05.012
104. Li W, Zhao R, Wang X, et al. Nobiletin-ameliorated lipopolysaccharide-induced inflammation in acute lung injury by suppression of NF-κB pathway in vivo and vitro. Inflammation. 2018;41(3):996–1007. doi:10.1007/s10753-018-0753-3
105. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet commission. Lancet. 2022;400(10356):921–972.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.