Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Continuous Negative Pressure Drainage with Intermittent Irrigation Leaded to a Risk Reduction of Perineal Surgical Site Infection Following Laparoscopic Extralevator Abdominoperineal Excision for Low Rectal Cancer
Authors Han Z, Yang C, Wang Q, Wang M, Li X, Zhang C
Received 16 February 2021
Accepted for publication 8 April 2021
Published 22 April 2021 Volume 2021:17 Pages 357—364
DOI https://doi.org/10.2147/TCRM.S306896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Zhongbo Han, Chunxia Yang, Qingfeng Wang, Meng Wang, Xi Li, Chao Zhang
Department of Gastrointestinal Surgery, Zibo Central Hospital, Shandong University, Zibo, Shandong, People’s Republic of China
Correspondence: Chao Zhang
Department of Gastrointestinal Surgery, Zibo Central Hospital, Shandong University, 54 West Gongqingtuan Road, Zibo, Shandong, 255000, People’s Republic of China
Tel +86 05333570671
Fax +86 05333570672
Email [email protected]
Purpose: High rate of perineal surgical site infection (SSI) is the most common complication following abdominoperineal resection (APR), especially for extralevator abdominoperineal excision (ELAPE). The purpose of this study was to investigate the effect of continuous negative pressure drainage combined with intermittent irrigation (CNPDCII) in the presacral space on the perineal SSI following laparoscopic ELAPE for low rectal cancer.
Patients and Methods: The clinical data of 99 patients with low rectal cancer who underwent laparoscopic ELAPE surgery were retrospectively analyzed. Among the 99 patients, 46 patients received CNPDCII and 53 patients received conventional drainage in the presacral space after ELAPE. Self-made irrigation drainage tube: took a silicone drainage tube, cut 3 side holes at every 2cm intervals at the front end, and fixed a flexible tube of an intravenous needle at the front end of the silicone drainage tube. The conventional drainage tube or self-made irrigation drainage tube was placed in the presacral space and poked out from the inside of the ischial tuberosity. The incidence of SSI and other perioperative indicators between the two groups was compared within 30 days after surgery.
Results: There was no statistical difference in clinicopathological features between the two groups of patients (p> 0.05). A statistically lower rate of SSI was found in CNPDCII group (17.4%, 8/46) than the conventional drainage group (35.8%, 19/53). The drainage tube retention time (7.8± 1.2 d VS 9.4± 1.6 d) and the postoperative hospital stay (9.7± 1.4 d VS 11.9± 2.3 d) in CNPDCII group were significantly shortened than the conventional drainage group. There was no statistical difference in operating theatre time and intraoperative blood loss between the two groups. Multivariate analysis confirmed that CNPDCII was an independent protective factor for SSI after ELAPE.
Conclusion: CNPDCII can effectively reduce the incidence of SSI following laparoscopic ELAPE, which is simple, safe and effective.
Keywords: low rectal cancer, extralevator abdominoperineal excision, negative pressure drainage, surgical site infection
Introduction
Although anal-preserving surgery is increasingly used in low rectal cancer, there are still some patients who inevitably undergo abdominoperineal resection (APR). However, due to the defect and dead space caused by large-scale of tissue resection, the incidence of perineum surgical site infection (SSI) after APR has been reported to range between 10.1% and 41%.1–3 Therefore, the management of SSI following APR is still a challenge for surgeons.
Extralevator abdominoperineal excision (ELAPE) is an improved surgical procedure for low rectal cancers with wider resections which removing the totality of levator ani muscles.4 Compared with traditional APR, ELAPE is a more radical approach, which may have a lower reduction in circumferential resection margins (CRM) involvement and potentially better oncological outcome.5,6 It has gradually become the first choice for locally low advanced rectal cancer. However, compared with APR, ELAPE increases the resection involvement of the pelvic floor muscles and surrounding tissues of the rectum, which results in larger defects and dead spaces, and further increases the risk of perineal wound complications.4,7 It had been reported that the perineal wound complications were increased (range from 20% to 38%) after ELAPE.8,9 A latest study reported that the rates of SSI after ELAPE was 26.3%.10 In addition, preoperative radiotherapy and/or chemotherapy are often required for patients with low rectal cancer, which may also affect the healing of the surgical incision and increase the risk of SSI to approximately 31%.3,11
The incidence of SSI is associated with prolonged hospital stay and increased medical costs.12,13 Therefore, the reduction of SSI following ELAPE is of important clinical importance. However, how to effectively improve the healing of the perineal incision after APR or ELAPE and reduce the incidence of SSI is still a problem that plagues rectal cancer surgeons.14 Scholars has also taken various efforts to reduce the incidence of SSI, mainly by reducing the dead space and preventing fluid accumulation, but the effects were not satisfactory.15 Negative pressure drainage is considered to be an effective technique for the treatment of complex wounds such as severe fractures and soft tissue injuries. It can make wounds heal quickly, especially for those with large soft tissue defects.16,17 A study by Kaneko reported that incisional continuous negative pressure wound therapy could reduce the incidence of SSI following APR.18 Recently, the use of prophylactic negative-pressure wound therapy for prevention of wound-related complications showed encouraging results in terms of reduction of SSI after APR.19 But this continuous negative pressure device was set on the incision surface. Therefore, it played a minor role in deep or presacral space infection.
In the present study, we aimed to investigate the effect of continuous negative pressure drainage combined with intermittent irrigation in the presacral space on the SSI following laparoscopic ELAPE for low rectal cancer. We expected to find a simple and practical management that could significantly reduce the incidence of SSI following ELAPE.
Patients and Methods
Patients
The clinical data of 99 patients with low rectal cancer who underwent laparoscopic ELAPE surgery in the department of Gastrointestinal Surgery of Zibo Central Hospital, Shandong University, from January 2017 to December 2019 were retrospectively analyzed. Among the 99 patients, 46 patients received continuous negative pressure drainage combined with intermittent irrigation and 53 patients received conventional drainage in the presacral space after ELAPE. Patient inclusion criteria: preoperatively diagnosed as rectal cancer by colonoscopy and biopsy pathology; the lower edge of the tumor was within 5 cm from the anal verge; preoperative imaging examination by Magnetic Resonance Imaging (MRI) showed that the tumor was locally advanced (cT3-T4); no operative contraindications were confirmed by preoperative multidisciplinary consultation and discussion; laparoscopic ELAPE was successfully performed without conversion to laparotomy. Patient exclusion criteria: patients with distant metastasis by MRI and enhanced CT before operation; patients with multiple primary cancers; patients with recurrent rectal cancers. There was no significant difference in clinical characteristics between the two groups, as shown in Table 1. After review and approval by the Ethics Committee of Zibo Central Hospital, Shandong University, written informed consent was obtained from all patients.
 |
Table 1 Clinicopathological Features of Patients Between Conventional Drainage Group and CNPDCII Group |
Perioperative Treatment
All patients were given intestinal preparation by oral compound polyethylene glycol electrolyte powder before operation. Skin preparation was performed 2 hours before operation. Intravenous Cefuroxime was infused half an hour before the operation. If the operation time is more than 3 hours, an additional use was made. The preventive use of antibiotics after the operation did not exceed 24 hours. If incision infection, turbid or purulent drainage occurred after surgery, sensitive antibiotics would be used based on the results of drug sensitivity test. Neoadjuvant chemoradiotherapy was administered in locally advanced cancers that exhibited a clinical T category of 3 or 4 and/or positive lymph nodes according to preoperative pelvic MRI evaluation. Of the 99 patients, 35 received preoperative radiotherapy and chemotherapy. The total dose of radiotherapy was 50 Gy, 2.0 Gy per dose, 5 days per week for 5 weeks. The concurrent chemotherapy which contained capecitabine (Xeloda, 850–1000 mg/m2, d1-14) was usually administered to the patients every 3 weeks. Surgery was performed 6–8 weeks after the end of radiotherapy.
Surgical Procedures
The surgical procedures strictly followed the TME and standard oncologic practices. After dissecting the rectum to the level of the levator ani muscle, the surgical technique of ELAPE were followed.20 More specifically, the separation of the back of rectum starts from the front of the apex of the coccyx. Starting from the levator ani muscle, use an ultrasonic knife to cut the levator ani muscle on both sides, then enter the space between the ischium and the anal canal, and enter the anterior rectovaginal space (or rectal-prostatic space) in front. The same group of surgeons performed all surgical operations.
Conventional drainage tube: cut 3 side holes with a diameter of 0.5cm every 1.5cm at the front end of a silicone tube (9.0 mm/F28), and arranged them intermittently and inversely. Self-made irrigation drainage tube: sutured and fixed a flexible tube of an intravenous needle at the front end of the silicone drainage tube, as shown in Figure 1A and B. Placement of drainage tube: the conventional drainage tube or self-made irrigation drainage tube were placed in the presacral space and drawn out from the inside of the ischial tuberosity on one side of the perineum (Figure 1C). The drainage tube was connected to the drainage bag for natural drainage for 24 hours. When there was no obvious bleeding in the drainage tube, connected the drainage tube to a silicone negative pressure drainage ball and kept the negative pressure ball in a negative pressure state (10–20kPa). When the drainage fluid became turbid, this flexible infusion set tube was externally connected with normal saline to syringe the wound cavity, 3–5 times a day, 200mL each time.
Observation and Surveillance
The main outcome indicator was the incidence of SSI. SSI was diagnosed according to Surgical Site Infection (SSI) Event (Centers for Disease Control and Prevention). SSI was divided into superficial incision infection, deep incision infection and organ or space infection. The perineal incision was evaluated by surgeons once a day. In order to avoid the confirmation bias, the final determination of SSI was made by an independent blinded observer. The healing evaluation index for surgical incision: the edges of the wound fit together snugly with a complete barrier function of the skin; The color of the wound is similar to or slightly different from that of the surrounding healthy skin; The wounds can withstand certain tension without wound dehiscence under appropriate physical activity intensity.
Other indicators include operating theatre time, intraoperative blood loss, drainage tube retention time and postoperative hospital stay. Removal standards for the drainage tube: the drainage volume is less than 10mL for 2 consecutive days under the condition that the drainage tube remains unobstructed; There is no sign of SSI after evaluated by an independent blinded observer. A small number of patients were discharged with the drainage tube and returned to the hospital regularly for follow-up.
Statistical Analysis
Continuous data were tested for normal distribution by Shapiro–Wilk normality test and evaluated by unpaired Student’s t-test. Categorical data were analyzed using a chi-square test or Fisher’s exact probability test. Logistic regression analysis was used to analyze the risk factors of SSI. All statistical analyses were done by SPSS 26.0 (SPSS Inc., Chicago, IL). Differences were considered statistically significant when p < 0.05.
Results
Comparison of Intraoperative and Postoperative Data Between the Conventional Drainage Group and CNPDCII Group
There was no statistical difference between the two groups in operating theatre time (p=0.632, Table 2) and intraoperative blood loss (p=0.357, Table 2). No serious complications occurred after the operation, and all the patients were discharged as scheduled. The retention time of drainage tube in the conventional drainage group was longer than the CNPDCII group (p=0.044, Table 2). The postoperative hospital stay in CNPDCII group was significantly shorter than the conventional drainage group (p=0.041, Table 2).
 |
Table 2 Comparison of Perioperative Parameters Between Conventional Drainage Group and CNPDCII Group |
Comparison of SSI Incidence Between the Conventional Drainage Group and CNPDCII Group
In the conventional drainage group, there were 10 cases of superficial incision infection, 8 cases of deep wound infection and 1 case of pelvic cavity infection; In the CNPDCII group, there were 5 cases of superficial incision infection, 3 cases of deep incision infection and 0 case of pelvic cavity infection. All SSI cases were diagnosed during patients’ hospital stay. The rate of SSI in CNPDCII group (8/46, 17.4%) was significantly lower than in the conventional drainage group (19/53, 35.8%), as shown in Table 3.
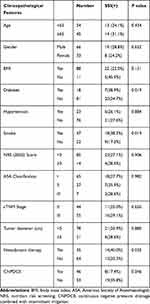 |
Table 3 Univariate Analyses of SSI in the Presacral Space and Perineal Incision After Laparoscopic ELAPE for Rectal Cancer |
Univariate and Multivariate Analysis of SSI Risk Factors After ELAPE
The rate of SSI in the whole group was 27.3% (27/99). Univariate logistic regression analysis showed that, in addition to CNPDCII, diabetes, smoking and preoperative neoadjuvant chemoradiotherapy were closely related to SSI following laparoscopic ELAPE (Table 3). In the multivariate logistic regression analysis model, the results suggested that CNPDCII was an independent protective factor for postoperative SSI, as shown in Table 4.
 |
Table 4 Multivariate Logistic Regression Analysis of SSI in the Presacral Space and Perineal Incision After Laparoscopic ELAPE for Rectal Cancer |
Discussion
Because of the enlarged resection scope of perineum and fixed bone structure of the pelvic, it is difficult to close the subcutaneous space and fat during the operation of ELAPE, which results in large dead space. Compared with the traditional abdominoperineal resection, SSI is more likely to occur because the dead spaces cannot be filled and closed by granulation tissues in a short period of time after ELAPE. This in turn may lead to more local exudation.10 Additionally, SSI will extend the length of hospital stay, increase the readmission rate, and increase the difficulty and cost of home care.21
In the present study, we demonstrated that continuous negative pressure drainage combined with intermittent irrigation could reduce the rate of SSI after laparoscopic ELAPE from 35.8% to 17.4%, and no patient had pelvic floor hernia or intestinal rupture during the negative pressure irrigation. Multivariate analysis by logistic regression confirmed that continuous negative pressure drainage combined with intermittent irrigation was an independent protective factor for SSI after ELAPE.
Reducing the accumulation of blood clots and exudate in the presacral space is an important strategy to prevent SSI after ELAPE.18 The disadvantage of conventional natural drainage is that it cannot be adequately drained, which is more likely to cause local exudate accumulation and increases the incidence of incision infection to a certain extent.22 Some scholars have proposed that continuous irrigation could reduce the infection rate of perineal incision.17,23–25 A recent study by Kaneko demonstrated that the SSI rate was significantly decreased from 32.6% to 7.8% by using incisional negative pressure wound therapy after APR.18
However, continuous irrigation requires patients to maintain a certain position in the bed, which affects the early activities of patients and limits the development of enhanced recovery after surgery (ERAS) after laparoscopic ELAPE. Therefore, we adopt a modified method by using continuous negative pressure drainage combined with intermittent irrigation. The continuous negative pressure device was also replaced by a negative pressure ball. Compared with the conventional natural drainage, this modified method adds a negative pressure suction device, which can ensure adequate drainage and reduce residual cavity effusion in presacral space. The pressure of negative suction can be controlled actively, so as to prevent the surrounding tissue or small intestine from being sucked into the drainage tube by excessive negative pressure. Furthermore, the negative pressure drainage ball is convenient for patients to carry about. On the other hand, when needing to be irrigated, the patient can be irrigated intermittently through the infusion set. Compared with continuous irrigation, our modified method does not limit patient’s early postoperative activities, which is more in line with the ERAS and shortened postoperative hospital stays.26 Therefore, our modified method is more reasonable than conventional natural drainage or continuous irrigation, which not only ensures patient’s irrigation and drainage, but also ensures the development of ERAS.
In recent years, negative pressure drainage and irrigation for the prevention and treatment of SSI have been gaining credence by most scholars. Favorable clinical effects of negative pressure drainage-assisted irrigation have been reported in patients with severe maxillofacial multiple-space infections.27,28 A few meta-analyses have confirmed that the use of negative pressure wound therapy could reduce the risk of SSI and other wound-related complications after APR.17,19,29 Negative pressure suction with irrigation has been reported to be an efficient technique for the management of deep SSI.30 Therefore, negative pressure drainage with irrigation is becoming a main trend in the prevention and treatment of complicated deep SSI.
Due to the high incidence of SSI and a great impact on the recovery of patients, many scholars have tried to find the risk factors of SSI following APR and ELAPE. However, in previous studies, there have been inconsistencies on the role of risk factors for SSI following APR and ELAPE.11,31,32 In recent years, the most reported risk factor for SSI after APR is neoadjuvant chemoradiation.31,33,34 Neoadjuvant chemoradiotherapy has become the standard treatment mode for locally advanced middle and low rectal cancer and has been recommended by many guidelines or consensus.35 However, local tissue edema and fibrosis caused by neoadjuvant chemoradiotherapy may hinder the growth of incision granulation tissue and increase bacterial growth.36 Our results revealed that the incidence of SSI in the preoperative chemoradiotherapy group was higher than that in the non-postoperative chemoradiotherapy group, and multivariate analysis confirmed the correlation between preoperative chemoradiation and SSI. BMI is considered to be closely related to the incidence of SSI in all types of surgery, including type I surgical incisions.37 Compared with normal weight patients, obese and morbidly obese patients are at least 1.3 times more likely to develop SSI.38 Different from previous research results, BMI was not correlated with SSI in univariate analysis. This might be due to the limited sample size of this study and low BMI of Asian population. Besides, the present study was based on retrospective data and a relatively small sample size. Hence, whether continuous negative pressure drainage combined with intermittent irrigation will be essential for decreasing rates of SSI following ELAPE in surgical practice still requires confirmation using multi-center prospective studies with large sample sizes.
Conclusion
In summary, our study confirmed that continuous negative pressure drainage combined with intermittent irrigation could effectively reduce the incidence of SSI after laparoscopic ELAPE, which was simple, safe and effective. It was more in accordance with the concept of ERAS and was worthy of promotion and application in clinical management.
Data Sharing Statement
The data that support the results of this study are available from the corresponding author on reasonable request.
Ethics Statement
This study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Zibo Central Hospital, Shandong University. Written informed consent was obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors approved the final version of the article and its submission.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Hawkins AT, Albutt K, Wise PE, et al. Abdominoperineal resection for rectal cancer in the twenty-first century: indications, techniques, and outcomes. J Gastrointest Surg. 2018;22(8):1477–1487. doi:10.1007/s11605-018-3750-9
2. Cahill C, Fowler A, Williams LJ. The application of incisional negative pressure wound therapy for perineal wounds: a systematic review. Int Wound J. 2018;15(5):740–748. doi:10.1111/iwj.12921
3. Nakamura T, Sato T, Hayakawa K, et al. Risk factors for perineal wound infection after abdominoperineal resection of advanced lower rectal cancer. Ann Med Surg (Lond). 2017;15:14–18. doi:10.1016/j.amsu.2017.01.024
4. Tao Y, Han J-G, Wang Z-J. Extralevator abdominoperineal excision for advanced low rectal cancer: where to go. World J Gastroenterol. 2020;26(22):3012–3023. doi:10.3748/wjg.v26.i22.3012
5. Hussain A, Mahmood F, Torrance ADW, et al. Oncological outcomes of abdominoperineal resection for the treatment of low rectal cancer: a retrospective review of a single UK tertiary centre experience. Ann Med Surg (Lond). 2018;34:28–33. doi:10.1016/j.amsu.2018.06.007
6. Shen Z, Bu Z, Li A, et al. Multicenter study of surgical and oncologic outcomes of extra-levator versus conventional abdominoperineal excision for lower rectal cancer. Eur J Surg Oncol. 2020;46(1):115–122. doi:10.1016/j.ejso.2019.08.017
7. Nyandowe M, Egedovo A, Ho Y-H. A comparative study of standard versus extralevator abdominoperineal resections. Int Surg J. 2017;4(4):1222–1224. doi:10.18203/2349-2902.isj20170921
8. Han JG, Wang ZJ, Gao ZG, et al. Perineal wound complications after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum. 2019;62(12):1477–1484. doi:10.1097/DCR.0000000000001495
9. Aggarwal N, Seshadri RA, Arvind A, et al. Perineal wound complications following extralevator abdominoperineal excision: experience of a regional cancer center. Indian J Surg Oncol. 2018;9:211–214. doi:10.1007/s13193-018-0741-y
10. Papp G, Dede K, Bursics A. Short-term advantages of ELAPE over APR. Acta Chir Belg. 2020;1–6. doi:10.1080/00015458.2020.1778265
11. Thorgersen E, Goscinski M, Spasojevic M, et al. Deep pelvic surgical site infection after radiotherapy and surgery for locally advanced rectal cancer. Ann Surg Oncol. 2017;24(3):721–728. doi:10.1245/s10434-016-5621-5
12. Badia J, Casey A, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96:1–15. doi:10.1016/j.jhin.2017;03.004
13. Abadía P, Ocaña J, Ramos D, et al. Prophylactic use of negative pressure wound therapy reduces surgical site infections in elective colorectal surgery: a prospective cohort study. Surg Infect. 2020;Online ahead of print. doi:10.1089/sur.2019.309
14. Imaizumi K, Nishizawa Y, Ikeda K, et al. Extended pelvic resection for rectal and anal canal tumors is a significant risk factor for perineal wound infection: a retrospective cohort study. Surg Today. 2018;48:978–985. doi:10.1007/s00595-018-1680-5
15. Wells CI, Ratnayake CB, Perrin J, Pandanaboyana S. Prophylactic negative pressure wound therapy in closed abdominal incisions: a meta-analysis of randomised controlled trials. World J Surg. 2019;43(11):2779–2788. doi:10.1007/s00268-019-05116-6
16. Seidel D, Diedrich S, Herrle F, et al. Negative pressure wound therapy vs conventional wound treatment in subcutaneous abdominal wound healing impairment: the SAWHI randomized clinical trial. JAMA Surg. 2020;155(6):469–478. doi:10.1001/jamasurg.2020.0414
17. Shiroky J, Lillie E, Muaddi H, et al. The impact of negative pressure wound therapy for closed surgical incisions on surgical site infection: a systematic review and meta-analysis. Surgery. 2020;167(6):1001–1009. doi:10.1016/j.surg.2020.01.018
18. Kaneko T, Funahashi K, Ushigome M, et al. Incisional negative pressure wound therapy to reduce perineal wound infection after abdominoperineal resection. Int Wound J. 2021;18(1):103–111. doi:10.1111/iwj.13499
19. Meyer J, Roos E, Abbassi Z, et al. The role of perineal application of prophylactic negative-pressure wound therapy for prevention of wound-related complications after abdomino-perineal resection: a systematic review. Int J Colorectal Dis. 2020;36(1):19–26. doi:10.1007/s00384-020-03732-6
20. Stelzner S, Holm T, Moran BJ, et al. Deep pelvic anatomy revisited for a description of crucial steps in extralevator abdominoperineal excision for rectal cancer. Dis Colon Rectum. 2011;54(8):947–957. doi:10.1097/DCR.0b013e31821c4bac
21. Mahmoud NN, Turpin RS, Yang G, et al. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect. 2009;10(6):539–544. doi:10.1089/sur.2009.006
22. Phillips J, O’Grady H, Baker E. Prevention of surgical site infections. Surgery. 2014;32:468–471. doi:10.1016/j.mpsur.2014.06.011
23. Kuper TM, Murphy PB, Kaur B, et al. Prophylactic negative pressure wound therapy for closed laparotomy incisions: a meta-analysis of randomized controlled trials. Ann Surg. 2020;271(1):67–74. doi:10.1097/SLA.0000000000003435
24. Gologorsky R, Arora S, Dua A. Negative-pressure wound therapy to reduce wound complications after abdominoperineal resection. Perm J. 2020;24:
25. Javed AA, Teinor J, Wright M, et al. Negative pressure wound therapy for surgical-site infections: a randomized trial. Ann Surg. 2019;269:1034–1040. doi:10.1097/SLA.0000000000003056
26. Gustafsson UO, Hausel J, Thorell A, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg. 2011;146(5):571–577. doi:10.1001/archsurg.2010.309
27. Zhao N, Liu Y, Yue J, et al. Negative pressure drainage‐assisted irrigation for maxillofacial space infection. Oral Dis. 2020;26(7):1586–1591. doi:10.1111/odi.13421
28. Qiu Y, Li Y, Gao B, et al. Therapeutic efficacy of vacuum sealing drainage-assisted irrigation in patients with severe multiple-space infections in the oral, maxillofacial, and cervical regions. J Craniomaxillofac Surg. 2019;47(5):837–841. doi:10.1016/j.jcms.2019.01.031
29. Sahebally SM, McKevitt K, Stephens I, et al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg. 2018;153(11):e183467. doi:10.1001/jamasurg.2018.3467
30. Zeng J, Sun X, Sun Z, et al. Negative pressure wound therapy versus closed suction irrigation system in the treatment of deep surgical site infection after lumbar surgery. World Neurosurg. 2019;127:e389–e395. doi:10.1016/j.wneu.2019.03.130
31. Sutton E, Miyagaki H, Bellini G, et al. Risk factors for superficial surgical site infection after elective rectal cancer resection: a multivariate analysis of 8880 patients from the American College of Surgeons National Surgical Quality Improvement Program database. J Surg Res. 2017;207:205–214. doi:10.1016/j.jss.2016.08.082
32. Xu Z, Qu H, Kanani G, et al. Update on risk factors of surgical site infection in colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35(12):2147–2156. doi:10.1007/s00384-020-03706-8
33. Ikeda A, Fukunaga Y, Akiyoshi T, et al. Wound infection in colorectal cancer resections through a laparoscopic approach: a single-center prospective observational study of over 3000 cases. Discov Oncol. 2021;12(1):2. doi:10.1007/s12672-021-00396-8
34. Chapman BC, Hosokawa P, Henderson W, et al. Impact of neoadjuvant chemoradiation on perioperative outcomes in patients with rectal cancer. J Surg Oncol. 2017;115(8):1033–1044. doi:10.1002/jso.24613
35. Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–448. doi:10.1097/SLA.0000000000003471
36. Reggiani Bonetti L, Manenti A, Gallo G, et al. Rectal-cancer radiotherapy damages the perineal muscle floor. Gastroenterol Rep. 2020;8(4):331–332. doi:10.1093/gastro/goaa037
37. Meijs AP, Koek MB, Vos MC, et al. The effect of body mass index on the risk of surgical site infection. Infect Control Hosp Epidemiol. 2019;40(9):991–996. doi:10.1017/ice.2019.165
38. Meijs AP, de Greeff S, Vos M, et al. The effect of body mass index on the risk of surgical site infection. Antimicrob Resist Int. 2015;4:1. doi:10.1186/2047-2994-4-S1-O29
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

