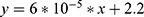Back to Journals » Clinical Ophthalmology » Volume 16
Continuous Intracorneal Ring Implantation in Keratoconus: Efficacy, Predictive Factors, and Complications
Authors Thiwa D, Linke SJ, Daxer A, Steinberg J
Received 20 May 2022
Accepted for publication 2 September 2022
Published 17 September 2022 Volume 2022:16 Pages 3055—3067
DOI https://doi.org/10.2147/OPTH.S375569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
David Thiwa,1 Stephan Johannes Linke,1,2 Albert Daxer,3,4 Johannes Steinberg1,2
1University Medical Center Hamburg (UKE), Department of Ophthalmology, Hamburg, Germany; 2Zentrumsehstärke – Augenarztpraxis am UKE, Hamburg, Germany; 3International Keratoconus Center, Wels, Austria; 4Medical University of Innsbruck, Department of Ophthalmology, Innsbruck, Austria
Correspondence: David Thiwa, University Medical Center Hamburg (UKE), Department of Ophthalmology, Martinistr. 52, Hamburg, 20246, Germany, Tel +49 157 3062 3524, Fax +49 40 429 160 64, Email [email protected]
Purpose: To examine the clinical outcomes, predictors of visual improvement and complications of continuous intracorneal ring (ICCR) implantation in patients with keratoconus and confirmed contact-lens intolerance (CLI).
Methods: This nonrandomized, multi-centric, retrospective cohort study examined visual, keratometric and clinical outcomes evaluated after a minimum follow-up of 2 months. Among the inclusion criteria for the standard treatment group (STG) were corrected distance visual acuity (CDVA) < 20/25 Snellen, no central corneal scars, minimum corneal thickness > 350μm, and central mean keratometry reading (meanK) < 55 diopters. All other eyes were classified as non-standard treatment group.
Results: A total of 118 eyes of 118 patients with aged 32 ± 11 years were included in this study. At a median follow-up of 161 days (interquartile range: 111– 372 days) ICCR implantation improved the CDVA from a mean of 0.38 to 0.15 logMAR (p< 0.0001). Our correlation analysis showed lower preoperative CDVA to be the single best predictor of CDVA improvement, with eyes of a CDVA of 20/80 or lower improving by 4.3 ± 2.0 lines on average. Eyes with a meanK > 55 diopters gained 9.04± 4.83 lines in UDVA and 2.86± 3.09 lines in CDVA. However, postoperatively these eyes had a CDVA of 0.32± 0.21 logMAR which is significantly inferior to the STG outcome (p=0.001372). Fifteen eyes (12.7%) had to undergo a ring exchange procedure because of refractive under- (9 eyes) or overcorrection (6 eyes). Two eyes (1.7%) experienced medical complications.
Conclusion: This study confirms the inclusion criteria of ICCR implantation in KC eyes with CDVA < 20/25 and CLI. Particularly in eyes with a preoperative CDVA < 20/80 and a meanK < 55 diopters, ICCR implantation should be considered due to its reversibility and low rates of serious complications. The main challenge remains in the low predictability of the magnitude of this improvement in eyes with CDVA > 20/30.
Keywords: keratoconus, MyoRing, continuous intracorneal ring, intracorneal continuous ring
Introduction
In the last 20 years, the range of treatment modalities for Keratoconus (KC) has vastly expanded. Novel contact-lens types1 can significantly improve visual acuity by correcting both lower- and higher-order aberrations. A central problem, however, is that, due to irregularities of the corneal surface, many patients begin to experience CL intolerance (CLI) and surgical measures become necessary.2
Classical corneal crosslinking (CXL) is an established surgical first step to halt KC by stabilizing the cornea and thereby prevents further ectasia and decreases in visual acuity.3–6
To improve vision in a clinically significant manner, the minimally invasive embedding of intracorneal implants have become increasingly popular. Intracorneal ring segments (ICRS) and 360° intracorneal continuous rings (ICCR) have been developed by different manufacturers and have shown their efficacy in significantly improving visual acuity in eyes with KC and in postponing or completely eliminating the need for keratoplasty.7–9 The reported advantages of ICCR compared to ICRS are the more robust improvement of spherical aberrations,7 increased biomechanical stability10,11 and their sustained effectiveness in progressive KC.8,12–15
However, a recent systematic review and meta-analysis on ICCR implantation in KC highlighted the need for high-powered studies, mainly due to heterogeneity in corrected distance visual acuity (CDVA) outcome.9 Furthermore, only few studies have reported on the surgical complications occurring after ICCR implantation and even less have reported on the need for repeated surgery to address over or under correction.16,17
To provide further insight into the efficacy of this treatment option and its complications for patients with KC, we retrospectively examined the data collected at three different centers. All treatments were planned in coordination with the manufacturer’s reading center, and all patients met the same inclusion and exclusion criteria for ICCR implantation.
Materials and Methods
This retrospective case-series was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the ethics committee of the Hamburg medical association in April 2019 with the approval number PV6017. All patients provided informed consent for anonymized data analyses.
Patient Selection
Patients were diagnosed with KC based on their medical history, slit-lamp examination results, and corneal topography and tomography findings (irregular astigmatism displayed by one or more of the following findings: a steepest K value ≥47 diopters (D); an inferior-superior K-value difference (I-S value) of >1.5 D and/or a skewed topographical axis. Patients aged 16 years or older with a thinnest pachymetry reading >350 µm, a CDVA <20/25, and without central corneal scarring were considered for ICCR implantation. Only patients with persistent contact-lens intolerance after multiple fitting attempts, one of which must have been done at specialized contact lens institute were included in the study. To prevent aniseikonia, patients with a projected postoperative anisometropia >4 D were considered ineligible for surgery. The “keratometric limit” beyond which we expected an ICCR to be insufficient for visual rehabilitation was set to a mean central simulated keratometry reading (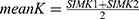 ) of 55 D.14 When patients met these criteria, the decision to perform ICCR implantation was based on the degree of subjective visual impairment. In some cases, we deviated from this treatment algorithm and included patients that did not meet all the aforementioned criteria. The eyes of these patients were defined as the non-standard treatment group (NSG). Surgery was performed for these patients when the alternative treatment (laser-assisted therapeutic keratectomy with CXL18 or keratoplasty) was not feasible for the patient.
) of 55 D.14 When patients met these criteria, the decision to perform ICCR implantation was based on the degree of subjective visual impairment. In some cases, we deviated from this treatment algorithm and included patients that did not meet all the aforementioned criteria. The eyes of these patients were defined as the non-standard treatment group (NSG). Surgery was performed for these patients when the alternative treatment (laser-assisted therapeutic keratectomy with CXL18 or keratoplasty) was not feasible for the patient.
The primary aim of the surgery was visual rehabilitation, ie an improvement of the best spectacle-corrected distance visual acuity (CDVA). Additional aims can be halting the progression of KC by stabilizing the corneal after unsuccessful CXL and to reduce anisometropia, thereby improving functional binocular CDVA. In this trial, however, eyes with previous CXL were not included.
Postoperatively, beside a consult on the first postoperative day, patients were scheduled for a follow-up 2–3 months after ICCR implantation. At this follow-up visit, a definitive evaluation of the surgical effect can be made and the need for potential ring-exchange can be assessed.19 Only patients who realized their follow-up at least 2 months after surgery were included in the study. If, at follow-up, both the physician in charge and the manufacturer’s reading center considered the visual rehabilitation to have been maximized with the ring size employed, the patient was enrolled in the study. When a second surgery was performed to exchange a ring due to refractive over- or under-correction or due to complications, we included the preoperative values of the first surgery and the postoperative values of the last surgery in the analysis. A subgroup analysis was done to compare eyes undergoing single and those undergoing two surgeries. Patients scheduled for a ring exchange at the time of data collection were excluded. We also excluded all eyes that had previously undergone ocular surgery including CXL. To avoid biasing our analysis by treating two eyes within the same patient as independent, we only included one of two eyes (selected at random) when patients had undergone surgery for both eyes.
Instruments and Procedures
Topography and tomography were performed on all patients using two different Scheimpflug-Placido camera systems (Pentacam HR, Oculus and Galilei G6, Ziemer Ophtalmic Systems AG). To implant the ICCR (MyoRing), an intra-corneal pocket with a diameter of 8.7 mm was created at a corneal depth of 300 µm. Pocket creation was carried out either using mechanical dissection with a microkeratome (Pocketmaker®, DIOPTEX GmbH, Austria) or a femtosecond laser (Femto LDV Z8®, Ziemer Ophthalmic Systems AG, Port, Switzerland) at the surgeon’s discretion. The correct MyoRing dimensions were provided by the manufacturer (DIOPTEX GmbH, Linz, Austria) based on a mathematical corneal model after uploading the relevant parameters to an online database. The treatments were performed by one of three surgeons (AD, SL, JS). All steps have been described in previous publications.11,13
Main Outcome Measures
Our primary outcome measures were the improvement of uncorrected distance visual acuity (UDVA) and CDVA as measured in logMAR chart lines. UDVA and best spectacle-CDVA were measured using a decimal scale and transformed into logMAR values for statistical analysis. As previous authors have done, the safety Index was defined as follows: the quotient between the postoperative CDVA and the preoperative CDVA.20
The rate of intraoperative and/or postoperative complications were also cataloged. Simulated central K values (SIM K1=steep, SIM K2=flat and meanK) were determined in the central 1–4-mm zone with a refractive index of n=1.3375 with the axial (sagittal) curvature map. Kmax and thinnest Pachymetry were determined on an 8-mm zone of the anterior surface.
Follow-Up
Follow-up examinations included slit-lamp examinations, assessments UDVA and CDVA, corneal topography and tomography, and corneal optical coherence tomography (OCT) examinations to assess the implantation depth of the ring. KC progression was assessed using tangential (instantaneous) anterior curvature maps and manifest refraction.
Statistical Analysis
We calculated the median, mean, standard deviation (SD) and interquartile range of all continuous variables for the included eyes. Where applicable, the data are presented as the mean ± standard deviation (SD) while non-normally distributed data are represented using the median and interquartile range (Q25:Q75).
All available variables were tested for normality using a conjunction of a graphical quantile–quantile test, and the Shapiro–Wilk test. We used the two variations of the t-test to assess for significant differences between variables sampled from a Gaussian distribution. Non-normally distributed data were analyzed using the Wilcoxon signed rank test for matched pairs (before and after) and the Mann–Whitney U-Test for independent samples.
Correlational analyses were computed with the appropriate parametric (Pearson) or non-parametric (Spearman) test using Bonferroni correction. The threshold for statistical significance was set at p <0.05. All statistical analyses were performed using the programming language Python 3.7 run on PycharmEdu2019 (JetBrains, Czech Republic) for Microsoft Windows.
For statistical analysis, all visual acuities were converted to logarithm of minimum angle of resolution (logMAR). The visual acuities of counting finger, hand motion, and light perception were converted to 0.014, 0.005 and 0.001 in decimal notation respectively as described in previous studies.21
Results
In total, 140 eyes of KC patients with full preoperative data were considered for the study. We excluded four eyes that were still scheduled for a ring exchange; one eye had previous penetrating keratoplasty; seven eyes of patients with bilateral surgery were randomly excluded to avoid overestimating the statistical power of our analysis. Finally, we excluded 10 eyes in which the final follow-up was performed before the completion of 60 days. We therefore included 118 eyes in our analysis. No patient was lost to follow-up.
Overall, the median age of patients was 32 ± 11 years with 17 eyes (14%) having to undergo additional surgeries. Visual acuity improvements postoperatively were 6.0 ± 4.8 lines in UDVA and 2.3 ± 2.1 lines in CDVA. The median follow-up period was 161 days (111:372).
Descriptive statistics on pre- and postoperative parameter values are displayed in Table 1. The statistics on the differences between pre- and postoperative values are displayed in Table 2.
 |
Table 1 Descriptive Statistics |
 |
Table 2 Difference Between Preoperative and Postoperative Values |
Figure 1 depicts the postoperative changes in distance visual acuity lines for each eye. Figure 2 shows the CDVA improvement in all eyes with respect to the day of follow-up at which this was measured.
 |
Figure 1 Simultaneously displayed changes in UDVA and CDVA. Mean: Denotes the simultaneous representation of mean changes in UDVA (6 lines) and mean changes in CDVA (2.3 lines). |
Subgroup Analyses – Multiple Surgeries and Non-Standard Treatment Group
Among the eyes that underwent multiple surgeries, 17 underwent one additional treatment. One eye had three surgeries overall. The reasons for reoperation are listed under the subheading Complications.
The outcomes comparing eyes that only underwent one surgery and the ones undergoing multiple surgeries are listed in Table 3.
 |
Table 3 Subgroup Comparison Multiple Surgeries versus Single Surgery Eyes |
We performed another subgroup analysis for the 14 eyes in the NSG. Alternative treatments were not feasible for those patients. Eight of 14 eyes were classified as non-standard for their meanK being above our threshold of 55 d with meanK at 59.18 ± 3.29 and Kmax at 73.48 ± 11.82. These eyes gained 9.04±4.83 lines in UDVA and 2.86±3.09 lines in CDVA with a postoperative vision of 0.32±0.21 CDVA significantly worse than STG eyes (p=0.001372). One patient had a min. pachy of 346µm and had a femto-laser assisted pocket creation at 290µm. Five eyes only had mild KC findings with preoperative CDVA >20/25 and Kmax values below 47D. Three of those patients were treated with the main objective of improving UDVA in myopic patients. Two of the mild KC patients underwent ICCR implantation to relieve anisometropia due to asymmetrical KC. All comparisons between STG and NSG can be found in Table 4.
 |
Table 4 Standard Treatment Group Vs Non-Standard Treatment Group |
Correlation Analysis
Table 5 shows the correlation of preoperative parameters with the UDVA lines gained and CDVA lines gained. The Pearson  correlation coefficients are found in the columns of “UDVA lines correlation coefficient” and “CDVA lines correlation coefficient”, respectively. The single best predictor of CDVA lines gained is preoperative CDVA. In Figure 3 one can observe the CDVA lines gained for each eye with respect to the preoperative CDVA in the same eye, further underlining the significant correlation between both variables.
correlation coefficients are found in the columns of “UDVA lines correlation coefficient” and “CDVA lines correlation coefficient”, respectively. The single best predictor of CDVA lines gained is preoperative CDVA. In Figure 3 one can observe the CDVA lines gained for each eye with respect to the preoperative CDVA in the same eye, further underlining the significant correlation between both variables.
 |
Table 5 Correlation of Preoperative Parameters with UDVA Lines Gained and CDVA Lines Gained |
Exemplary Case
To illustrate a typical outcome, we chose a typical patient with regards to UDVA and CDVA improvements. All his preoperative parameter values except age lie within half a standard deviation from the median. The patients’ age is within one standard deviation from the median. In the concerned eye, the preoperative unaided vision was 20/200 with a CDVA of 20/70. UDVA improved by 4.6 lines (study average: 5.9) to 20/70 while his CDVA improved by 2.4 lines (study average: 2.1) to 20/40. Figure 4 shows a top-to-bottom comparison of the curvature maps one week before and 3.5 months after surgery in our exemplary case.
Complications
In all, 15 eyes (12.7%) had to undergo a ring exchange procedure because of refractive under- (9 eyes) or overcorrection (6 eyes). One eye developed a corneal herpes simplex infection, which warranted explantation 8 months post-operatively. Re-implantation of the ICCR as performed 4 months later. Nevertheless, the patient gained 6.0 lines in UDVA and 3.9 lines in CDVA as a combined result of all procedures.
In one case, the surgical pocket created using the PocketMaker was too superficial. Initially, the patient had good postoperative vision; however, a month after surgery after minor trauma to the eye with a pillowcase, the patient started experiencing persistent epiphora. Four months after surgery, the ICCR was explanted due to the risk of extrusion. This eye gained 2.0 lines of UDVA but lost 2.0 lines of CDVA.
No eye included developed post-operative KC progression, epithelial ingrowth, or any signs of sterile inflammatory responses due to the implant or the surgical trauma.
Discussion
In this study, all analyzed visual acuity and topography parameters improved significantly (p < 0.001) with the exception of the thinnest pachymetry value (min. pachy) and topographical astigmatism (topoAsti).
The mean improvement in CDVA, was highly variable, with a standard deviation of 2.1 lines ranging from −2.0 to 10 Lines as can be seen in Figure 3. This underlines our further need for improving selection criteria by performing subgroup analyses.
Our subgroup analysis showed eyes with a higher SE were more likely to require reoperation (group 2). Eyes in group 2 had a tendency for inferior compounded CDVA gains compared to group 1 (p=0.13958).
Our correlation analysis showed preoperative vision to be the single most useful predictor of postoperative improvement in vision. In other words, high potential for improvement in CDVA was associated with a greater gain in CDVA lines. For instance, the eyes in the upper quartile of preoperative CDVA (as measured in logMAR) were eyes with a CDVA of 20/80 and lower, as measured in Snellen. These eyes gained 4.3 ± 2.0 lines in CDVA on average (Appendix A of Supplemental Data).
Our analyses further demonstrated that the degree of myopia predicts the average improvement in UDVA (Table 5), which is in line with the results in previous studies.16,22 What has not been published before, is that preoperative myopia correlates weakly but significantly with the CDVA improvement (rSE=−0.3 and p=0.0116) before applying an optional Bonferroni correction.23 This further corroborates the link between our diverging outcomes in Group 1 and 2 and higher myopia in group 1.
The inverse correlation between SE and CDVA lines gained, could in part or entirely be explained by the correlation of myopia with advanced KC and hence, high potential for improvement. To investigate whether these correlations reflected inherently positive biomechanical properties and held irrespective of potential for improvement, we did a post hoc correlation analysis between these variables and one we named the “achieved CDVA potential”.
This post hoc analysis demonstrated a weak inverse correlation that was not statistically significant (Appendix B in Supplemental Data). Further research is therefore necessary to elucidate the role of SE and a host of other parameters, as predictive factors for postoperative CDVA outcomes.
As reported by a recent review,22 reducing variability by improving inclusion criteria could yield considerably better outcomes for patients. The subgroup analysis between eyes undergoing standard and non-standard treatment showed a tendency for poorer postoperative vision (p= 0.26807). Particularly eyes with a meanK >55D had a significantly inferior CDVA outcomes postoperatively (p= 0.001372) with their CDVA being under 20/40 Snellen. We therefore argue that for KC eyes classified as stage IV with Amsler–Krumeich classification, keratoplasty may be the more efficacious visual rehabilitation albeit with a different complications profile. The emergence of novel predictive models in the field of machine learning may be a worthwhile line of future research to further improve selection criteria.
There are large gaps in reporting the distribution of CDVA gains. Some publications with large sample sizes describe no eye losing a line and some report all eyes gaining vision and not a single eye losing a CDVA line.14,16,24 With 20 eyes (17%) with CDVA lines change ≤0 at follow-up with 4 (3%) eyes losing one line or more in corrected vision, our findings suggest there may be large gaps in the reporting of CDVA gains distribution.
Very few publications have explicitly stated the percentage of eyes in which this enhancement surgery or ring centration was carried out. It has been reported that approximately 20% of eyes profit from a second procedure to adapt ring dimensions or positioning.14 At 12.7%, our study found that eyes required these additional interventions less frequently.
In an attempt to compare studies analyzing results after complete ring (ICCR)- and ring segment-implantation, we found two reviews that were published since 2015. The first one, a systematic review by Izquierdo et al,7 attributed improvements in UDVA and CDVA for all examined ICRS and ICCR after 1 year. However, they noted greater improvements in keratometry and more consistent correction of spherical aberration in patients treated with the ICCR compared with other ICRS. The second review, published by Park et al,25 concluded that complete rings (ICCR) and near-complete ring segments of arc lengths of 340° and above achieved the most robust correction of spherical equivalent in comparison to regular ICRS.
Several comparative studies on this topic have been published since 2015 that were not included in either review.
A study by Yousif et al26 comparing three intracorneal implant types found all three were effective at improving UDVA, CDVA, keratometry and corneal asphericity. They also reported a limited effect of 2-segment rings on advanced cases of KC at 6 months but a significant improvement with near-complete and complete rings.
A comparative study by Pashtaev et al27 also demonstrated that ICCR treatment lead to a greater reduction in total corneal aberration, higher order aberrations (HOAs) and spherical aberration than the Keraring in KC eyes staged at Amsler–Krumeich III. Postoperative changes in Amsler–Krumeich II KC eyes were comparable in both groups.
Bamdad et al demonstrated superior reduction of asphericity and keratometry with MyoRing treatment as with the Keraring.27
Sammour et al28 found that after 12 months the CDVA gains in eyes treated with the ICCR were significantly higher than those treated with the Ferrara Ring, mostly because of the regression of short-term (1 month) gains in the group treated with the Ferrara Ring.
Biomechanical considerations ascribing higher stabilization of the corneal by continuous rather than interrupted segments favor ICCR implantation.10,11 This is particularly relevant since some publications report of regression to baseline of CDVA improvements 12 months after ICRS implantation, particularly in progressive KC.12,26,28–31
In light of the literature and our findings, we would suggest that the comparative advantages of ICCR to ICRS are its high suitability in advanced cases of KC, in eyes with low SE and possibly in progressive KC. Remaining purported advantages of some ICRS are possibly their particular suitability in KC with a highly asymmetric cone.
The current established modality to treat severe KC remains keratoplasty, with lamellar keratoplasty being the preferred option by many surgeons, because of the reduced risk of postoperative graft rejection compared with penetrating keratoplasty.32 To our knowledge, only one study has intended to compare ICCR implantation with keratoplasty in KC.
A contralateral eye study by Yousif et al33 with 30 patients comparing femtosecond assisted DALK and MyoRing implantation in patients with advanced to severe KC and a history of recent progression demonstrated that both techniques were effective at improving visual acuity and spherical and corneal aberrations with superior improvement of corneal asphericity and HOAs after DALK. The authors deemed it an acceptable substitute for keratoplasty in advanced KC. Yet, one needs to keep in mind that loss of visual acuity through central corneal scarring cannot be remediated by ICCR.
Further investigation is necessary to explore whether this comparability is reproducible, which could render ICCR a valid alternative in regions with insufficient capacities for corneal transplants.
A potential weakness of our study is that ICCR implantation was not thoroughly standardized as to the pocket creation method. Previous studies, however, have shown no significant differences in outcomes between femtosecond and manual dissection to create the stromal pocket.34–36
Despite the success demonstrated, a significant limitation is that patients were not systematically categorized using KC grading such as the Belin–Ambrosia ABCD classification. This renders comparison with the study population more arduous.
With 91% of eyes being followed up between 2 months and 3 years, only 29% of which were followed up 12 months or more, questions could remain as to the stability of the evaluated outcomes. Nonetheless, numerous other studies with follow-up periods of 1, 2, 3 and 5 years demonstrated stable refractive and keratometry outcomes with marginal improvement of HOAs beyond the first year.9,14,15,37–39
Furthermore, this work is also limited by the lack of its consideration of HOAs and qualitative photic phenomena, which play an important role in patients’ quality of life.15,32 Only one study to date has published any patient-centered outcomes such as satisfaction scores.40 This is something that should be borne in mind in future studies.
The results of this study further confirm the efficacy of ICCR implantation as a treatment for KC. This promising technique comes with great benefits, particularly for KC patients with reduced preoperative vision (CDVA <20/70) and a low SE. The calculated safety index, low risk of complications and the reversibility of the treatment speak to its safety. The main challenge remains in the low predictability of the magnitude of this improvement in eyes with good spectacle corrected vision (CDVA >20/30).
Funding
There is no funding to report.
Disclosure
The authors Thiwa, Linke and Steinberg report no conflicts of interest in this work. The author Daxer reports an investment interest in DIOPTEX GmBH
References
1. Downie LE, Lindsay RG. Contact lens management of keratoconus. Clin Exp Optom. 2015;98(4):299–311. doi:10.1111/cxo.12300
2. Jhanji V, Sharma N, Vajpayee RB. Management of keratoconus: current scenario. Br J Ophthalmol. 2011;95(8):1044–1050. doi:10.1136/bjo.2010.185868
3. Iqbal M, Elmassry A, Saad H, et al. Standard cross-linking protocol versus accelerated and transepithelial cross-linking protocols for treatment of paediatric keratoconus: a 2-year comparative study. Acta Ophthalmol. 2019;97(4):e623–e631. doi:10.1111/aos.14275
4. Mazzotta C, Baiocchi S, Bagaglia SA, et al. Accelerated 15 mW pulsed-light crosslinking to treat progressive keratoconus: two-year clinical results. J Cataract Refract Surg. 2017;43(8):1081–1088. doi:10.1016/j.jcrs.2017.05.030
5. Mazzotta C, Ferrise M, Gabriele G, et al. Chemically-boosted corneal cross-linking for the treatment of keratoconus through a Riboflavin 0.25% optimized solution with high superoxide anion release. J Clin Med. 2021;10(6):1324. doi:10.3390/jcm10061324
6. Mazzotta C, Sgheri A, Bagaglia SA, et al. Customized corneal crosslinking for treatment of progressive keratoconus: clinical and OCT outcomes using a transepithelial approach with supplemental oxygen. J Cataract Refract Surg. 2020;46(12):1582–1587. doi:10.1097/j.jcrs.0000000000000347
7. Izquierdo L
8. Bautista-Llamas MJ, Sánchez-González MC, López-Izquierdo I, et al. Complications and explantation reasons in Intracorneal Ring Segments (ICRS) Implantation: a systematic review. J Refract Surg. 2019;35(11):740–747. doi:10.3928/1081597x-20191010-02
9. Janani L, Tanha K, Najafi F, et al. Efficacy of complete rings (MyoRing) in treatment of Keratoconus: a systematic review and meta-analysis. Int Ophthalmol. 2019;39(13):2929–2946. doi:10.1007/s10792-019-01121-9
10. Daxer A. Biomechanics of corneal ring implants. Cornea. 2015;34(11):1493–1498. doi:10.1097/ico.0000000000000591
11. Bamdad S, Sedaghat MR, Yasemi M, et al. Intracorneal stromal ring can affect the biomechanics of ectatic cornea. J Ophthalmol. 2020;2020:4274037. doi:10.1155/2020/4274037
12. Chhadva P, Yesilirmak N, Cabot F, et al. Intrastromal corneal ring segment explantation in patients with keratoconus: causes, technique, and outcomes. J Refract Surg. 2015;31(6):392–397. doi:10.3928/1081597x-20150521-05
13. Nguyen N, Gelles JD, Greenstein SA, et al. Incidence and associations of intracorneal ring segment explantation. J Cataract Refract Surg. 2019;45(2):153–158. doi:10.1016/j.jcrs.2018.09.021
14. Daxer A, Ettl A, Horantner R. Long-term results of MyoRing treatment of keratoconus. J Optom. 2017;10(2):123–129. doi:10.1016/j.optom.2016.01.002
15. Bikbova G, Kazakbaeva G, Bikbov M, et al. Complete corneal ring (MyoRing) implantation versus MyoRing implantation combined with corneal collagen crosslinking for keratoconus: 3-year follow-up. Int Ophthalmol. 2018;38(3):1285–1293. doi:10.1007/s10792-017-0593-4
16. Jadidi K, Naderi M, Mosavi SA, et al. Pre-operative factors influencing post-operative outcomes from MyoRing implantation in keratoconus. Clin Exp Optom. 2019;102(4):394–398. doi:10.1111/cxo.12859
17. Daxer A. Adjustable intracorneal ring in a lamellar pocket for keratoconus. J Refract Surg. 2010;26(3):217–221. doi:10.3928/1081597x-20100224-08
18. Rechichi M, Mazzotta C, Oliverio GW, et al. Selective transepithelial ablation with simultaneous accelerated corneal crosslinking for corneal regularization of keratoconus: STARE-X protocol. J Cataract Refract Surg. 2021;47(11):1403–1410. doi:10.1097/j.jcrs.0000000000000640
19. Daxer A, Mahmoud H, Venkateswaran RS. Intracorneal continuous ring implantation for keratoconus: one-year follow-up. J Cataract Refract Surg. 2010;36(8):1296–1302. doi:10.1016/j.jcrs.2010.03.039
20. Sedaghat MR, Momeni-Moghaddam H, Belin MW, et al. Anatomical and visual effects of the MyoRing implantation measured by the ABCD keratoconus grading system. Eye Contact Lens. 2019. doi:10.1097/icl.0000000000000595
21. Lange C, Feltgen N, Junker B, et al. Resolving the clinical acuity categories ”hand motion” and ”counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–142. doi:10.1007/s00417-008-0926-0
22. Nobari S, Villena C, Jadidi K. Predictability, stability and safety of MyoRing implantation in keratoconic eyes during one year follow-up. Iran J Opthalmol. 2014;26:136–143.
23. Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34(5):502–508. doi:10.1111/opo.12131
24. Jabbarvand M, Salamatrad A, Hashemian H, et al. Continuous intracorneal ring implantation for keratoconus using a femtosecond laser. J Cataract Refract Surg. 2013;39(7):1081–1087. doi:10.1016/j.jcrs.2013.02.054
25. Park SE, Tseng M, Lee JK. Effectiveness of intracorneal ring segments for keratoconus. Curr Opin Ophthalmol. 2019;30(4):220–228. doi:10.1097/icu.0000000000000582
26. Yousif MO, Said AMA. Comparative study of 3 intracorneal implant types to manage central keratoconus. J Cataract Refract Surg. 2018;44(3):295–305. doi:10.1016/j.jcrs.2017.12.020
27. Pashtaev NP, Pozdeeva NA, Sinitsyn MV. [Comparative analysis of corneal aberrations after intrastromal segments and MyoRing implantation using femtosecond laser in patients with keratoconus]. Vestn Oftalmol. 2017;133(3):3–8. Russian. doi:10.17116/oftalma201713334-8
28. Sammour HM, Ismail MM, Abdelghany AI, et al. Comparative study between MyoRing and Ferrara ring intracorneal implantation using femtosecond laser for treatment of keratoconus. Egypt J Hosp Med. 2017;68(1):910–922. doi:10.12816/0038191
29. Abreu AC, Malheiro L, Coelho J, et al. Implantation of intracorneal ring segments in pediatric patients: long-term follow-up. Int Med Case Rep J. 2018;11:23–27. doi:10.2147/imcrj.S151383
30. Alio JL, Vega-Estrada A, Esperanza S, et al. Intrastromal corneal ring segments: how successful is the surgical treatment of keratoconus? Middle East Afr J Ophthalmol. 2014;21(1):3–9. doi:10.4103/0974-9233.124076
31. Vega-Estrada A, Alió JL, Plaza-Puche AB. Keratoconus progression after intrastromal corneal ring segment implantation in young patients: five-year follow-up. J Cataract Refract Surg. 2015;41(6):1145–1152. doi:10.1016/j.jcrs.2014.08.045
32. Macsek M, Steinberg J, Linke S, et al. Corneal intrastromal implantation surgery by means of MyoRing corneal implant for the treatment of keratoconus: a review. Int J Keratoconus Ectatic Corneal Dis. 2018;7(1):50–60. doi:10.5005/jp-journals-10025-1159
33. Yousif MO, Said AMA. Contralateral eye study of refractive, topographic and aberrometric outcomes after femtosecond assisted MyoRing implantation and DALK for management of keratoconus. Int J Ophthalmol. 2018;11(10):1621–1630. doi:10.18240/ijo.2018.10.08
34. Alio JL, Pinero DP, Daxer A. Clinical outcomes after complete ring implantation in corneal ectasia using the femtosecond technology: a pilot study. Ophthalmology. 2011;118(7):1282–1290. doi:10.1016/j.ophtha.2010.12.012
35. Daxer B, Mahmood H, Daxer A, et al. MyoRing Treatment for Keratoconus: DIOPTEX PocketMaker vs Ziemer LDV for Corneal Pocket Creation. International Journal of Keratoconus and Ectatic Corneal Diseases 2012 doi:10.5005/jp-journals-10025-1029
36. Abdellah MM, Ammar HG. Femtosecond laser implantation of a 355-degree intrastromal corneal ring segment in keratoconus: a three-year follow-up. J Ophthalmol. 2019;2019:6783181. doi:10.1155/2019/6783181
37. Vega-Estrada A, Chorro E, Sewelam A, et al. Clinical outcomes of a new asymmetric intracorneal ring segment for the treatment of keratoconus. Cornea. 2019;38(10):1228–1232. doi:10.1097/ico.0000000000002062
38. Studený P, Křížová D, Straňák Z, et al. [Clinical results after continuous corneal ring (MyoRing) implantation in keratoconus patients]. Cesk Slov Oftalmol. 2015;71(2):87–91. Czech
39. Mohammadpour M, Khoshtinat N, Khorrami-Nejad M. Comparison of visual, tomographic, and biomechanical outcomes of 360 degrees intracorneal ring implantation with and without Corneal crosslinking for progressive keratoconus: a 5-year follow-up. Cornea. 2021;40(3):303–310. doi:10.1097/ico.0000000000002407
40. Naderi M, Karimi F, Jadidi K, et al. Long-term results of MyoRing implantation in patients with keratoconus. Clin Exp Optom. 2021;104(4):499–504. doi:10.1080/08164622.2021.1878813
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.