Back to Journals » Journal of Pain Research » Volume 13
Continuous But Not Pulsed Radiofrequency Current Generated by NeuroTherm NT500 Impairs Mitochondrial Membrane Potential in Human Monocytic Cells THP-1
Authors Nishioka A, Kimura M , Sakamoto E, Nagasaka H, Azma T
Received 13 December 2019
Accepted for publication 24 June 2020
Published 13 July 2020 Volume 2020:13 Pages 1763—1768
DOI https://doi.org/10.2147/JPR.S242204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Akira Nishioka,1 Maiko Kimura,1 Eriko Sakamoto,1 Hiroshi Nagasaka,2 Toshiharu Azma1,2
1Department of Anesthesiology & Pain Medicine, Kohondai Hospital, National Center for Global Health and Medicine, Ichikawa, Chiba 272-8516, Japan; 2Department of Anesthesiology, Saitama Medical University Hospital, Iruma, Saitama 350-0495, Japan
Correspondence: Toshiharu Azma
Department of Anesthesiology & Pain Medicine, Kohondai Hospital, National Center for Global Health and Medicine, Ichikawa, Chiba 272-8516, Japan
Email [email protected]
Background: The application of pulsed radiofrequency (PRF) current to peripheral nerves with conditions related to neuropathic pain is considered to be clinically safe, while it has been reported that the destruction of mitochondria after PRF application was observed by electron microscopy. If it occurs reproducibly, PRF applied to peripheral nerves should provoke neurolysis because the impairment of mitochondria is known as the primary cause of apoptosis.
Methods: Human monocytic cells THP-1 loaded with 100 nM tetramethylrhodamine methyl ester (TMRM), a fluorescent dye that proves the mitochondrial membrane potential (MMP), were exposed to the electric field of continuous radiofrequency (CRF) or PRF current. The TMRM-related fluorescence from THP-1 cells was measured by flow cytometry.
Results: The exposure of THP-1 cells to a PRF electric field generated by NeuroTherm NT500 for 15 min with maximum power did not decrease MMP in these cells, nor did it cause the induction of apoptosis. By contrast, the application of CRF current at 70 °C for 3 min significantly decreased MMP and induced apoptosis within 10 min after CRF application.
Conclusion: We conclude from these findings that PRF application does not provoke mitochondrial injury in various types of mammalian cells because the size and the subcellular structure of the plasma membrane or mitochondria are similar among those. However, the present results cannot address the effect of PRF current on organic structure around the nervous system. Further study is required to solve the question of whether PRF current causes neurolysis or not.
Keywords: pulsed radiofrequency current, human monocytic cells, THP-1, mitochondrial membrane potential, apoptosis
Introduction
The application of pulsed radiofrequency (PRF) current to peripheral nerves is a therapeutic intervention based on a concept to maximize the intensity of the radiofrequency (RF) electric field around the subject while decreasing the power deposition that produces heat within the electric field. Because the power deposition is linearly related to time, generating two continuous RF (CRF) bursts for 20 ms in 1 s, that is one of the standard modes for PRF adopted in clinically available RF generators, reduces the power deposited in the tissue around the RF probe to 4% of CRF (for reviews, see Chua et al1).
After the first clinical application of PRF current to the dorsal root ganglion in patients with chronic radicular pain,2 accumulating clinical experience suggests that PRF current applied to peripheral nerves does not provoke neuroablation (neurolysis) that causes motor disturbance and numbness.1 A number of experimental studies from separate investigators also suggested the involvement of signal transduction pathways in the mechanisms of action of a PRF electric field to alleviate pain sensation, implicating mechanisms through neuromodulation rather than neurolysis (for reviews, see Van Boxem et al3 or the Discussion of our recent report4). Our recent observation concerning the enhanced mRNA expression for β-endorphin in human monocytic cells THP-1 applied with PRF current potently supports the concept of neuromodulation by PRF electric fields because the increase in mRNA was demonstrated even in the absence of temperature elevation while the cells were intact as confirmed by flow cytometry.4
By contrast, several observational studies using electron microscopy showed structural changes in peripheral nerves after the application of PRF, implicating mechanisms through neurolysis, including those of the enlargement of endoplasmic reticulum in DRG cells,5 the fusion of the vacuoles in these cells,5 disruption of mitochondria in A- and C-fibers,6 and the separation of myelin of axons.7
Of these histological findings, we focused on a report from Erdine et al that the destruction of mitochondria after PRF application to the afferent axon of sciatic nerves of rats was observed by electron microscopy.6 If it occurs reproducibly, PRF applied to peripheral nerves should provoke neurolysis because the impairment of mitochondria is known as the primary cause of apoptosis of mammalian cells.8 To evaluate whether the application of CRF or PRF current influences the mitochondrial membrane potential, we performed the following simple experiment.
Methods
General Experimental Protocol
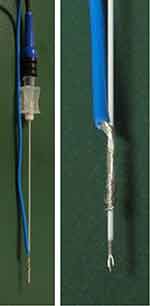
Experiments were performed according to the previously described methods with minor modification.4 THP-1 cells, purchased from Sumitomo Dainippon Pharma (Suita, Osaka, Japan), were washed three times by 200×g centrifugation for 2 min with the culture medium (RPMI-1640 containing 25 mM HEPES). Cell count was performed twice using the automated cell counter TC 20 (Bio-Rad, Hercules, CA, USA), and the average was used to re-suspend cells at 1×106 cells/mL with the culture medium supplemented with 2 mM CaCl2 and 100 nM tetramethylrhodamine methyl ester (TMRM), a reagent that proves mitochondrial membrane potential.9 A 2.5-mL portion of the suspension of THP-1 cells was transferred to a 15-mL polypropylene conical tube and was centrifuged at 1000×g for 5 min. The RF probe in a 10-cm length and 4-mm active tip guiding needle (22-gauge; Hakko, Chikuma, Nagano, Japan) was inserted into the conical tube to place the active tip in the sedimented THP-1 cells. A counter electrode was tied onto the plastic insulation of the guiding needle (Figure 1). During the application of RF current, the conical tubes were incubated in a dry thermal bath at 37 °C in the air. CRF or PRF current at a frequency of 480 kHz was applied to the sedimented cells for 3 min or 15 min, respectively, using a NeuroTherm NT500 RF generator (St. Jude Medical, Saint Paul, MN, USA). A conical tube, where the RF probe was inserted for 3 min without generating current, served as a control. The voltage generated in the CRF mode is automatically changed by the NT500 RF generator to stabilize the temperature of the RF probe at a selected point.10 The temperature of the RF probe was monitored by an LED indicator of the NT500 during the RF generation and was confirmed to be at the pre-selected level (70 °C). By contrast, the PRF mode of the NT500 system (repeated 480-kHz RF bursts of duration less than 20 ms at 2 Hz) is programmed to fix the voltage at a pre-selected level during RF generation.10 We selected the maximum voltage (>45 V) for the PRF mode and confirmed that the probe temperature reached just below 43 °C. After RF application, THP-1 cells were re-suspended by mild pipetting. The cell suspension was then split into several 500-µL portions and transferred to 5-mL polystyrene tubes, where the cell suspension was mixed with a fixed dose of CountBright (CB) absolute counting beads. The incubation in a dry thermal bath at 37 °C in the air was continued for pre-selected time periods. When evaluation for cell viability was required, propidium iodide and FITC-conjugated Annexin V (1 μg/mL and 1% (v/v) of the provided stock solution, respectively, at final concentrations) were added to the cell suspension at least 10 min before the flow cytometry.
Flow Cytometric Analysis
Particles (1×104 counts) from each tube were analyzed with a BD FACS Canto II flow cytometer, and the results were processed by the BD FACS DIVA software. FSC-A is the area of electric pulse for forward scatter (FSC) of particles detected in flow cytometry, indicative of the relative size of each particle. SSC-H is the height of the electric pulse for side scatter (SSC), which reflects the surface as well as the internal complexity of each particle. THP-1 cells, observed as the largest mass of particles with FSC values greater than those for 7-μm CB beads, were gated by a circle in the FSC-A/SSC-H plot and dotted in blue. Fluorescence related to TMRM was measured by the setting of excitation with a 488-nm blue laser beam and the detection through a 585/42-nm bandpass filter. The area of electric pulse for TMRM-related fluorescence from THP-1 cells was plotted against FSC-A of each cell. Further analyses were performed using the Kaluza flow cytometry analysis software (ver. 2.1; Beckman Coulter, Brea, CA, USA).
Statistical Analysis
Data were expressed as mean±SD. Comparison of multiple groups was performed by analysis of variance (ANOVA) followed by the Tukey HSD post-hoc test. A P value less than 0.01 was considered to be significant for statistical difference.
Results
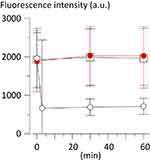
The SSC from THP-1 cells, observed at 3 min after the application of CRF current at 70 °C for 3 min, drastically increased as compared with that from these cells observed just before the CRF application. Such an increase in SSC continued during the observation period for 60 min. On the other hand, SSC from THP-1 cells observed at 60 min after the PRF application for 15 min did not change as compared with that from these cells observed just before the PRF application or that from control observed at the same timing (Figure 2). In our previous study, we observed that the increase in SSC from THP-1 cells was associated with apoptotic or necrotic cell death.4 In the present study, the number of apoptotic cells in total THP-1 cells, defined as those positively stained with Annexin-V and propidium iodide4 (85.7%, n=630), was significantly increased at 10 min after the application of CRF current as compared with that in control cells observed at the same timing (16.2%, n=983). The number of necrotic cells in total THP-1 cells (i.e., Annexin-V (–)/propidium iodide (+))4 was not influenced by the CRF application (for CRF and control, 2.6% and 1.5%, respectively). The number of apoptotic and necrotic cells in total THP-1 cells, observed at 60 min after the application of PRF current for 15 min, was not significantly changed as compared with control. Fluorescence intensity related to TMRM from THP-1 cells, observed simultaneously with SSC at 3 min after the CRF application, significantly decreased as compared with that observed before the CRF application. This finding indicates that CRF provoked an acute decrease in mitochondrial membrane potential that is known as the primary cause of apoptosis.8 The decrease in TMRM-related fluorescence intensity from CRF-applicated THP-1 cells continued during the observation period for 60 min, as expected by the change in SSC from the same cells. However, PRF application failed to decrease the fluorescence intensity related to TMRM (Figures 2 and 3).
To examine whether the function of TMRM as an indicator for mitochondrial membrane potential is influenced by heat, aliquots of stock TMRM were pre-incubated at 70 °C for 3 min or 80 °C for 5 min accurately using a thermal cycler (MiniAmp Plus; ThermoFisher Scientific, Waltham, MA, USA) before loading THP-1 cells with these aliquots. Fluorescence intensity related to TMRM from THP-1 cells, without application of RF currents, was compared with the fluorescence intensity from CB. The relative fluorescence intensity from THP-1 cells tended to decrease according to the temperature of TMRM pre-incubation, but not significantly (without pre-incubation, 13.9%±1.7; with pre-incubation at 70 °C for 3 min, 10.9%±2.1; at 80 °C for 5 min, 9.3%±2.5,;n=3 each, P=0.087), indicating approximately 78% inhibition of activity as a fluorescence probe after pre-incubation at 70 °C for 3 min. By contrast, fluorescence intensity related to TMRM from THP-1 cells applied with CRF current at 70 °C for 3 min significantly decreased to less than 40% of that from cells without RF application (Figures 2 and 3), supporting the concept that CRF current significantly suppressed the mitochondrial membrane potential in THP-1 cells.
Discussion
We have recently reported that the exposure of human monocytic cells THP-1 to a PRF electric field for 15 min increases the expression of mRNA for the precursor protein of β-endorphin proopiomelanocortin (POMC) without causing cell death, where we established the method to apply RF current to cells in a test tube reproducibly.4 In the present study using the same simple experimental system, we showed that a question, whether the exposure of human cells to a PRF electric field provokes apoptosis, can be examined using flow cytometry with TMRM, a reagent that proves mitochondrial membrane potential.
We demonstrated that the significant decrease in mitochondrial membrane potential occurred at 3 min after the application of CRF current to THP-1 cells at 70 °C for 3 min, and that the acute induction of apoptosis in these cells occurred as early as several minutes following the mitochondrial depolarization. The acute increase in SSC and decrease in TMRM-related fluorescence intensity from THP-1 cells was observed in virtually every cell applied with CRF, indicating that the exposure of THP-1 cells to the RF electric field is appropriately performed in this experimental setting. In the same setting, we have confirmed that the exposure of THP-1 cells to the PRF electric field generated by the NeuroTherm NT500 for 15 min with maximum power did not decrease the mitochondrial membrane potential in these cells, nor did it provoke cell death.
Use of the experimental system established in our previous study,4 where the cells are sedimented as a pellet in a microtube during the exposure to the PRF electric field, is a strong point of this study because a homogeneously treated mass of cells was measured using flow cytometry that minimizes the bias included in the experimental system. Even though other investigators implied the mitochondrial injury provoked by the application of PRF currents using histological methods as shown above,6 it is likely from our findings using this reproducible experimental system that PRF application does not provoke mitochondrial injury in virtually every type of mammalian cells because the size and the subcellular structure of the plasma membrane or mitochondria are similar among various types of mammalian cells.
The limitation of this study is that we used human monocytic cells THP-1, not nerve cells. Therefore, we cannot assert fully that the findings observed in THP-1 cells are applicable to nerve cells. We used THP-1 cells because monocytes are viable in a floating condition while many types of cells, including nerve cells, are not. However, monocytes possess several properties mimicking nerve cells, including those of the expression for β-endorphin and polymodal receptors such as transient receptor potential vanilloid14 through which monocytes are suggested to be involved in analgesia in inflammatory conditions.11 The precise interpretation of this experimental study is only that PRF current does not provoke mitochondrial injury in mammalian cells within the electric field of such current, but this finding is very important to know how PRF application acts in vivo. The present study cannot address the effect of PRF current on organic structure around the nervous system, while observational studies from others using electron microscopy showed the destruction of tissue components such as the myelin sheath that may influence the conduction of afferent nerve fibers.7 Further study is required to solve the question of whether PRF causes neurolysis through destructing interstitial structures.
In conclusion, we demonstrated that a continuous but not pulsed RF current generated by the NeuroTherm NT500 impairs mitochondrial membrane potential in THP-1 cells.
Abbreviations
BD, Becton Dickinson (Franklin Lakes, NJ, USA); CB, CountBright (Thermo Fisher Scientific, Waltham, MA, USA); CRF, continuous radiofrequency, FITC; fluorescein isothiocyanate isomer I; FSC, forward scatter; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; PRF, pulsed radiofrequency; RF, radiofrequency; SSC, side scatter; TMRM, tetramethylrhodamine methyl ester.
Acknowledgment
This work was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (grant nos. 22591748, 25462451, 16K10979, 16K15684, and 18K08841).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chua NHL, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir (Wien). 2011;153(4):763–771. doi:10.1007/s00701-010-0881-5
2. Sluijter M, Cosman E, Rittman W, van Kleef M. The effects of pulsed radiofrequency fields applied to the dorsal root ganglion – a preliminary report. Pain Clin. 1998;11:109–117.
3. Van Boxem K, Huntoon M, Van Zundert J, Patijn J, van Kleef M, Joosten EA. Pulsed radiofrequency: a review of the basic science as applied to the pathophysiology of radicular pain: a call for clinical translation. Reg Anesth Pain Med. 2014;39(2):149–159. doi:10.1097/AAP.0000000000000063
4. Azma T, Nishioka A, Ogawa S, Nagasaka H, Matsumoto N. Enhanced expression of gene coding for β-endorphin in human monocytic cells exposed to pulsed radio frequency electric fields through thermal and non-thermal effects. J Pain Res. 2018;11:2887–2896. doi:10.2147/JPR.S171974
5. Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9(3):251–256. doi:10.1016/j.ejpain.2004.07.002
6. Erdine S, Bilir A, Cosman ER, Cosman Jr. ER. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9(6):407–417. doi:10.1111/j.1533-2500.2009.00317.x
7. Tun K, Cemil B, Gurcay AG, et al. Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72(5):
8. Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10(6):709–717. doi:10.1038/sj.cdd.4401231
9. Scaduto RC, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76(1):469–477. doi:10.1016/S0006-3495(99)77214-0
10. Gauci C, Cosman E, Cosman EJ. The physics of radiofrequency & pulsed radiofrequency. In: Manual of RF Techniques: A Practical Manual of Radiofrequency Procedures in Chronic Pain Management.
11. Machelska HSC. Analgesic effects of immune–cell–derived opioids. In: DeLeo JA, Sorkin LSWL, editors. Immune and Glial Regulation of Pain. Seattle: IASP press; 2007:107–119.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.