Back to Journals » International Journal of General Medicine » Volume 14
Construction and Validation of Two Novel Nomograms for Predicting the Overall Survival and Cancer-Specific Survival of NSCLC Patients with Bone Metastasis
Authors Dong Q, Deng J, Mok TN , Chen J, Zha Z
Received 7 October 2021
Accepted for publication 22 November 2021
Published 2 December 2021 Volume 2021:14 Pages 9261—9272
DOI https://doi.org/10.2147/IJGM.S342596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Qiu Dong,1 Jialin Deng,2 Tsz Ngai Mok,1 Junyuan Chen,1 Zhengang Zha1
1Center for Bone, Joint and Sports Medicine, The First Hospital of Jinan University, Jinan University, Guangzhou, Guangdong, People’s Republic of China; 2School of Medicine, Jinan University, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Junyuan Chen; Zhengang Zha
Center for Bone, Joint and Sports Medicine, The First Hospital of Jinan University, Jinan University, No. 613, Huangpu Avenue West, Guangzhou, 510515, Guangdong, People’s Republic of China
Tel +86 134 8029 9854
; +86 173 6182 0624
Email [email protected]; [email protected]
Background: Bone metastasis (BM) is the most common site of metastasis in non-small cell lung carcinoma (NSCLC). We aimed to construct and validate 2 novel nomograms predicting the 3-, 6-, and 12-months overall survival (OS) and cancer-specific survival (CSS).
Methods: The clinical data of 7480 patients between 2010 and 2016 were enrolled from the Surveillance, Epidemiology, and End Results database (SEER). The patients were allocated randomly to training and validation cohorts in a 7:3 ratio. Cox proportional hazards regression models were used to identify prognostic risk factors and establish 2 nomograms. The prediction accuracy of nomograms was assessed by C-index, the area under the ROC curve (AUC), and calibration curves.
Results: A total of 244998 NSCLC patients were identified between 2010 and 2016, with 7480 found with BM, accounting for 3.1%. Overall, 7480 patients were enrolled in the OS nomogram construction and were randomized to the training set (n = 5236) and the validation set (n = 2244). Age, sex, race, marital status, histology, grade, primary site, T stage, N stage, liver metastasis, surgery, radiotherapy, and chemotherapy were found to correlate with OS. A total of 7422 samples were included in the CSS nomogram construction, randomly grouped into training set (n = 5195) and the validation set (n = 2227). Age, sex, race, histology, grade, primary site, T stage, N stage, brain metastasis, liver metastasis, surgery, radiotherapy, and chemotherapy were associated with CSS. Two nomograms were conducted to predict the 3-, 6-, and 12-months OS and CSS. The ROC curves and exhibited good performance for predicting OS and CSS.
Conclusion: We established and validated 2 high-performance nomograms to assist clinical doctors in making personalized treatment decisions.
Keywords: lung cancer, metastasis, SEER, prognosis
Introduction
Lung cancer is a malignant tumor characterized by uncontrolled cell proliferation in lung and bronchial tissues and is the most common malignancy worldwide including non-small cell lung carcinoma (NSCLC) and small cell lung carcinoma (SCLC). In recent decades, the global incidence and mortality rates of lung cancer have been increasing year by year.1–3 In the Global Cancer 2020 statistics (GLOBOCAN 2020) published by the International Agency for Research on Cancer (IARC), lung cancer ranks as the third most frequently diagnosed cancer and one of the leading causes of cancer-related deaths.4 It is estimated that more than 2.2 million people will die from lung cancer worldwide in 2020 accounting for 18% of all malignancy deaths, with more than half of all new lung cancer cases occurring in developing countries, predominantly in men (approximately 1.43 million cases/2.2 million cases, or 14.3%).4
NSCLC is the most common lung cancer type, accounting for approximately 85% lung cancer cases.5,6 NSCLC can be classified into three major subtypes: adenocarcinoma (40% of NSCLC), squamous (25–30% of NSCLC), and other subtypes.7,8 It is estimated that >50% of NSCLC patients have already developed into (at least) stage III disease, which needs multimodality therapies.9 Current therapeutic developments in NSCLC include surgery for primary and distant metastases, stereotactic radiation therapy, and chemotherapy with low-toxicity agents, especially targeted agents such as pemetrexed and gemcitabine.10 In recent years, immune-targeted therapy (ICI) and others have been shown to inhibit NSCLC.10,11 Despite significant improvements in therapeutic modalities, the survival rates for lung cancer with BM have improved, but the median survival time was still only 4 months.12 At the same time, patient outcomes have not been greatly affected, and recurrences remain very common.13 Nearly 75% of recurrences also have distant metastases, among which bone is the most common and earliest site of metastasis.14,15 BM substantially shorter survival and reduce the life quality of NSCLC patients, causing a range of skeletal-related events (SREs) including severe bone pain, pathological fractures, spinal cord compression, and hypercalcemia.16 Because current guidelines do not recommend the evaluation of BM as a routine program, a lot of NSCLC patients did not come to doctor until they got SREs.17 Therefore, it is crucial to evaluate BM in NSCLC patients. Nomograms, based on statistical regression models, can accurately estimate the prognosis of individuals and provide individualized patient survival predictions.18
Due to their utility, nomograms have been widely used in the evaluation of a variety of cancers, such as NSCLC, soft tissue sarcomas, and breast cancer.19–21 However, to our knowledge, there are few studies explicitly looking at the prognosis of NSCLC patients with BM. Therefore, we set to construct and validate two novel nomograms to predict rates of 3-, 6- and 12-months OS and CSS among NSCLC patients with BM, using the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and Methods
Patients Selection
Data of patients were extracted from the Surveillance, Epidemiology and End Results (SEER) database by SEER*Stat software version 8.3.9.1. Patients diagnosed with adenocarcinoma (ICD-O-3 histologic type: 8140–8147, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8550, 8572), squamous cell carcinoma (ICD-O-3 histologic type: 8050–8052, 8070–8078), large-cell carcinoma (ICD-O-3 histologic type: 8012–8014) and other types of NSCLC (ICD-O-3 histologic type: 8010, 8015, 8020–8022, 8030, 8036).19 To be eligible for inclusion, patients should meet the following criteria: (1) pathologically confirmed NSCLC; (2) primary site codes from C34.0 to C34.9; (3) been of adult age; (4) been diagnosed between 2010 and 2016. In contrast, patients with the following conditions were excluded from the study: (1) diagnosed by either autopsy or death certificate; (2) had the second primary tumor; (3) lacked their follow-up status; (4) lacked details of clinicopathological information; (5) without BM or BM status missing/unknown. The flowchart of patient selection is shown in Figure 1.
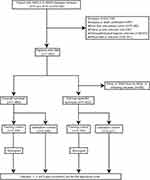 |
Figure 1 The flow chart of the study design. Abbreviation: AUC, the area under the curve. |
Data Element
The following data variables were extracted: age (years), sex (female or male), race [white, black, and other (American Indian/Alaska Native, Asian/Pacific Islander)], insurance (no or yes), marital status (married or unmarried), histology (adenocarcinoma, squamous and other), grade (I, II, III and IV), primary site (bronchus, upper lobe, middle lobe, lower lobe, and other lobes), laterality (bilateral lobe or unilateral lobe), T stage (T1–T4), N stage (N0 – N3), metastatic sites (including brain, liver, and lung), surgery (no or yes), radiotherapy (no or yes) and chemotherapy (no or yes). Detailed clinicopathological information is presented in Table 1. Overall survival (OS) was defined as the time from diagnosis to death from any cause and cancer-specific survival (CSS) was regarded as the time from diagnosis to death attributed to NSCLC. OS and CSS were used as two primary endpoints.
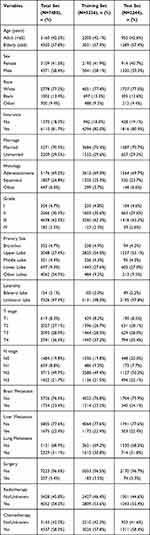 |
Table 1 Demographic Data and Baseline Characteristics of Patients |
Statistical Analysis
Eligible patients were randomly divided into the training and validation sets at a ratio of 7:3. The potential risk factors verified by univariate Cox regression analysis (P<0.05) were included in the multivariate Cox analysis. Then those variables with a P-value less than 0.05 in both univariate and multivariate Cox regression analyses were finally considered as independent prognostic factors. Based on the independent prognostic factors, the nomograms were constructed by using the “regplot” package in R software version 4.0.5. The accuracy of these nomograms was validated by c-index, receiver operating characteristic (ROC) curves, and calibration curves by the “survivalROC” package. Finally, patients were divided into low- and high-risk groups according to the average risk score, and the Kaplan–Meier technique (Log rank test) was applied to analyze the prognostic value of the nomograms.
Results
Patient Characteristics
There were 244,998 NSCLC patients diagnosed during 2010 and 2016, excluding those with unknown clinical characteristics, of whom 7480 had definite concomitant BM, with a BM rate of approximately 3.1%. A total of 7480 patients were enrolled in the OS nomogram construction and were randomized to training cohort (n = 5236) and the validation cohort (n = 2244). A total of 7422 samples were included in the CSS nomogram construction, randomly grouped into training cohort (n = 5195) and the validation cohort (n = 2227). The relevant demographic data of patients are summarized in Table 1. At baseline, 57.8% were elderly (≥65y), 41.6% (n = 3109) were female, 81.7% (n = 6110) patients had insurance, and 70.5% (n = 5271) were married. Most patients were white [77.2% (n = 5778)], followed by black [13.4% (n = 1002)], and other [9.4% (n = 700)]. In terms of tumor characteristics, adenocarcinoma comprised 69.2% (n = 5176), then squamous 24.8% (1857), and other 6.0% (n = 447). About 4.7% (n = 354) in grade I, 30.3% (n = 2266) in grade II, 62.5% (n = 4678) in grade III, 2.5% (n = 182) in grade IV. The location of primary tumor was 4.7% (n = 352) in bronchus, 27.4% (n = 2048) in upper lobe, 4.3% (n = 321) in middle lobe, 9.3% (n = 697) in lower lobe, and 54.3% (n = 4062) in other lobe. About 97.9% (n = 7326) patients showed unilateral involvement. Tumor stages T1, T2, T3, and T4 were found in 8.3% (n = 619), 27.1% (n = 2027), 28.0% (n = 2093), and 36.6% (n = 2741) patients, respectively. Stage N0, N1, N2, and N3 were found in 19.8% (n = 1484), 8.8% (n = 659), 49.7% (n = 3715), and 21.7% (n = 1622), respectively. In patients with multiple metastases, 23.4% (n = 1754) patients concomitant had brain metastases, 22.4% (n = 1675) patients had concomitant liver metastases, and 31.1% (n = 2329) patients had concomitant lung metastases. About 3.4% (n = 257) patients received surgery, 54.2% (n = 4052) underwent radiotherapy, 58.0% (n = 4337) received chemotherapy.
Prognostic Factors of OS and CSS
In the univariate Cox analysis, the following features were significantly related to OS: age, sex, race, insurance, marital status, histology, grade, primary site, laterality, T stage, N stage, liver metastasis, lung metastasis, surgery, radiotherapy, and chemotherapy. In the multivariate Cox analysis, the following variables were confirmed as independent predictors of OS: age, sex, race, marital status, histology, grade, primary site, T stage, N stage, liver metastasis, surgery, radiotherapy, and chemotherapy. In the univariate Cox analysis, the following factors were associated with CSS: age, sex, race, insurance, marital status, histology, grade, primary site, laterality, T stage, N stage, brain metastasis, liver metastasis, lung metastasis, surgery, radiotherapy, and chemotherapy. In the multivariate Cox analysis, the following factors were considered as independent predictors of CSS: age, sex, race, histology, grade, primary site, T stage, N stage, brain metastasis, liver metastasis, surgery, radiotherapy, and chemotherapy. Detailed information about the factors with prognosis (OS and CSS) is shown in Table 2.
 |
Table 2 Univariate and Multivariate Cox Regression Analyses for OS and CSS |
Nomogram Construction
According to the results of multivariable Cox regression analyses, OS and CSS nomograms were established (Figure 2A and B). Using these nomograms, the 3-, 6-, 12-months survival probability of each patient can be predicted by adding up the specific numerical value of each predictive variable. Two examples of using the nomogram to predict the survival probability of a given patient are illustrated in Figure 3.
 |
Figure 2 Nomograms for predicting 3-, 6-, and 12-months OS (A) and CSS (B). |
Nomogram Validation
The C-index of the established nomograms showed good predictive accuracy in both OS and CSS (OS, C-index 0.732; CSS, C-index 0.731). In the training set, the ROC curve analysis showed the area under the curve (AUC) for OS at 3-, 6-, and 12-months was 0.817, 0.790, and 0.785, respectively (Figure 4A), while the AUC for CSS at 3-, 6-, and 12-months 3-, 6-, and 12-months was 0.818, 0.793 and 0.779, respectively (Figure 4C). In the validation set, the ROC curve analysis showed the area under the curve (AUC) for OS at 3-, 6-, and 12-months 3-, 6-, and 12-months was 0.816, 0.779, and 0.763, respectively (Figure 4B), while the AUC for CSS at 3-, 6-, and 12-months was 0.819, 0.776 and 0.785, respectively (Figure 4D). The calibration plots for OS and CSS revealed good concordance between the predicted and observed survival probabilities in both the training set and validation set (Figure 3).
Risk Classification Systems for OS and CSS
The prognostic score of each patient was calculated by summing the individual scores for each factor. Then, all the patients were dichotomized into low- and high-risk groups according to the median cut-off value of the score. The median survival of the low-risk patients was 9 months, while high-risk patients was 2 months. The Kaplan–Meier curves showed a significant difference in the training cohort and the validation cohort between the 2 groups (Figure 5).
Discussion
In this study, we collected a large number of NSCLC patients using the SEER database to find the prognosis of overall survival and cancer-specific survival in BM patients. According to our survey, nearly 3.1% NSCLC patients were found with BM, which is much lower than the reported BM incidence (20%).22 This discrepancy could be attributed to the limitations of SEER database, with its incomplete data on clinical characteristics on the one hand and its lack of documentation of asymptomatic patients on the other hand. The BM prevalence has been underestimated, which highlights the bone screening in asymptomatic NSCLC patients. Regular skeletal examinations for these patients may aid in the early detection of BM, which may lead to the implementation of appropriate treatment regimens, reducing the occurrence of BM.23
The previous research has found multiple bone metastases, elevated lactate dehydrogenase and alkaline phosphatase were linked to a poor prognosis in individuals with NSCLC.24
Our examination included more variables and discovered that the 13 variables listed below were linked to predict their OS: age, gender, race, marital status, histology, grade, primary site, T staging, N staging, liver metastasis, surgery, radiotherapy, and chemotherapy. Clarifying the prognosis risk factors for BM, which is the most prevalent location of metastasis in NSCLC patients, would help clinicians build more specific treatment plans. Furthermore, our findings concur with recent research that has revealed that patients with adenocarcinoma with BM have poorer survival outcomes than those with other subtypes of NSCLC.
Similarly, our research found 13 characteristics associated with CSS, with brain metastases, rather than marriage, serving as an independent risk factor when compared to OS. It seems that intimacy is a good independent factor for OS, as we can see from marital status. It has been shown that the prolonged survival of cancer patients due to personal health insurance status, personal-psychological status before and after marriage, etc. can give patients a “placebo”.25 This may explain why the survival of BM in married NSCLC patients is better than that in unmarried patients. Moreover, the probability of survival at 3-, 6-, and 12-months for CSS was maintained at about 80%, which is consistent with previous studies that showed that BM of NSCLC patients with positive factors such as medical coverage and family life had higher CSS levels. In summary, it can indicate that hospice care is an important factor in good family relationships for patients with advanced NSCLC.19,25,26
We found it interesting that patients with single bone metastases in NSCLC have longer survival than patients with multiple bone metastases. Because multiple bone metastases in NSCLC usually indicate the high activity and malignancy of these tumor cells, the prognosis is poorer. Moreover, the higher the clinical stage, the shorter the survival of the patient. The clinical implication is that timely and effective treatment after the diagnosis of bone metastases is crucial to prolong survival. Meanwhile, NSCLC patients with different tumor stages have an impact on the mode of primary tumor metastasis, such as stage IV NSCLC patients with the following metastases for different primary tumor sites: patients in the upper lobe are more likely to have brain metastases and lung metastases, those in the middle lobe are more likely to have lung metastases those in the lower lobe are more likely to cause bone metastases and lung metastases.27 At the same time, we found that other metastases, especially liver metastases, usually occurred before brain metastases, which matched the specific risk of NSCLC in previous studies, that is, cancer cells from NSCLC first underwent liver metastases before brain metastases, and brain metastases were a cofactor in reducing survival time.24
However, our study has some limitations that need to be mentioned. The SEER database only provided us with patients with initially diagnosed BM, which may lead to an underestimation of those patients who progress to BM later in the disease course. By not capturing asymptomatic BM patients, the SEER database may lead to an underestimation of BM rates. Finally, the SEER database does not provide detailed diagnostic criteria for BM, as they vary from hospital to hospital and thus require further study. Moreover, with the advent of targeted therapies, mutation testing has become a standard practice in the diagnosis of NSCLC, which means that we do not know the gene expression pattern of patients. Following that, as a new type of retrospective study, there is some variation in clinical treatment protocols, uncontrollable factors such as patients’ awareness on the study results.
Conclusion
In summary, we established and validated 2 novel nomograms for NSCLC patients with BM to predict the OS and CSS, respectively. Those nomograms could serve as concise and practical tools for clinicians to anticipate the 3-, 6-, and 12-months OS and CSS for NSCLC patients with BM. Subgroup analysis showed that patients with chemotherapy, radiotherapy, surgery, liver metastasis, lower N and T stage, primary site in middle lobe, grade II, adenocarcinoma, married, other race, female and younger could get more OS benefit. While chemotherapy, radiotherapy, surgery, liver metastasis, brain metastasis, lower N and T stage, primary site in middle lobe, grade I, adenocarcinoma, other race, female and younger could get more CSS benefit. Further research and prospective studies are needed to assess its accuracy and practicability. Meanwhile, more randomized clinical trials are needed to establish the optimal treatment regimen.
Statement of Ethics
All data utilized in our study were obtained through the SEER database, which is freely accessible for research purposes. According to the ethics guidelines, informed consent and ethical approval are not required for using the public and anonymized clinical data. Therefore, the medical committee of the First Hospital of Jinan University exempted the ethical approval.
Acknowledgments
We appreciate the efforts of SEER program for the creation of SEER database, and all my colleagues who have given us advice and help on our study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant or other support funding.
Disclosure
The authors report no potential conflicts of interest.
References
1. Klebe S, Leigh J, Henderson DW, Nurminen M. Asbestos, smoking and lung cancer: an update. Int J Environ Res Public Health. 2019;17(1):258. doi:10.3390/ijerph17010258
2. Hong S, Mok Y, Jeon C, Jee SH, Samet JM. Tuberculosis, smoking and risk for lung cancer incidence and mortality. Int J Cancer. 2016;139(11):2447–2455. doi:10.1002/ijc.30384
3. Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. doi:10.1111/1759-7714.12916
4. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; 2020 [
5. Hwang JK, Page BJ, Flynn D, et al. Validation of the Eighth Edition TNM lung cancer staging system. J Thorac Oncol. 2020;15(4):649–654. doi:10.1016/j.jtho.2019.11.030
6. Shirasawa M, Fukui T, Kusuhara S, et al. Prognostic significance of the 8th edition of the TNM classification for patients with extensive disease small cell lung cancer. Cancer Manag Res. 2018;10:6039–6047. doi:10.2147/CMAR.S181789
7. Fei H, Chen S, Xu C. Interactive verification analysis of multiple sequencing data for identifying potential biomarker of lung adenocarcinoma. Biomed Res Int. 2020;2020:8931419. doi:10.1155/2020/8931419
8. Jing X, Li F, Meng X, Liu Z, Yu J, Liu B. Ovarian metastasis from lung adenocarcinoma with ALK-positive rearrangement detected by next generation sequencing: a case report and literatures review. Cancer Biol Ther. 2017;18(5):279–284. doi:10.1080/15384047.2017.1310344
9. Semrau S, Zettl H, Hildebrandt G, Klautke G, Fietkau R. Older patients with inoperable non-small cell lung cancer: long-term survival after concurrent chemoradiotherapy. Strahlenther Onkol. 2014;190(12):1125–1132. doi:10.1007/s00066-014-0710-5
10. Katsumata S, Aokage K, Ishii G, et al. Prognostic impact of the number of metastatic lymph nodes on the Eighth Edition of the TNM classification of NSCLC. J Thorac Oncol. 2019;14(8):1408–1418. doi:10.1016/j.jtho.2019.04.016
11. Liu S, Zhou F, Liu Z, et al. Predictive and prognostic significance of M descriptors of the 8th TNM classification for advanced NSCLC patients treated with immune checkpoint inhibitors. Transl Lung Cancer Res. 2020;9(4):1053–1066. doi:10.21037/tlcr-19-396
12. Zheng XQ, Huang JF, Lin JL, et al. Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study. Transl Lung Cancer Res. 2019;8(4):367–379. doi:10.21037/tlcr.2019.08.16
13. Mulshine JL, Sullivan DC. Clinical practice lung cancer screening. N Engl J Med. 2005;352:2714–2720. doi:10.1056/NEJMcp042630
14. Torok JA, Gu L, Tandberg DJ, et al. Patterns of distant metastases after surgical management of non-small-cell lung cancer. Clin Lung Cancer. 2017;18(1):e57–e70. doi:10.1016/j.cllc.2016.06.011
15. LeVasseur N, Clemons M, Hutton B, Shorr R, Jacobs C. Bone-targeted therapy use in patients with bone metastases from lung cancer: a systematic review of randomized controlled trials. Cancer Treat Rev. 2016;50:183–193. doi:10.1016/j.ctrv.2016.09.013
16. Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional Independence. Support Care Cancer. 2008;16(8):879–889. doi:10.1007/s00520-008-0418-0
17. Wood DE, Kazerooni EA, Baum SL, et al. Lung cancer screening, Version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(4):412–441. doi:10.6004/jnccn.2018.0020
18. Yap WK, Shih MC, Kuo C, et al. Development and validation of a nomogram for assessing survival in patients with metastatic lung cancer referred for radiotherapy for bone metastases. JAMA Netw Open. 2018;1(6):e183242. doi:10.1001/jamanetworkopen.2018.3242
19. Song Q, Shang J, Zhang C, Zhang L, Wu X. Impact of the homogeneous and heterogeneous risk factors on the incidence and survival outcome of bone metastasis in NSCLC patients. J Cancer Res Clin Oncol. 2019;145(3):737–746. doi:10.1007/s00432-018-02826-7
20. Liu S, Sun W, Yang S, et al. Deep learning radiomic nomogram to predict recurrence in soft tissue sarcoma: a multi-institutional study. Eur Radiol. 2021. doi:10.1007/s00330-021-08221-0
21. Li Y, Chen Y, Zhao R, et al. Development and validation of a nomogram based on pretreatment dynamic contrast-enhanced MRI for the prediction of pathologic response after neoadjuvant chemotherapy for triple-negative breast cancer. Eur Radiol. 2021. doi:10.1007/s00330-021-08291-0
22. Kuchuk M, Addison CL, Clemons M, Kuchuk I, Wheatley-Price P. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol. 2013;2(1):22–29. doi:10.1016/j.jbo.2012.12.004
23. Mallet R, Decazes P, Modzelewski R, et al. Prognostic value of low skeletal muscle mass in patient treated by exclusive curative radiochemotherapy for a NSCLC. Sci Rep. 2021;11(1):10628. doi:10.1038/s41598-021-90187-6
24. Wu XT, Zhou JW, Pan LC, Ge T. Clinical features and prognostic factors in patients with bone metastases from non-small cell lung cancer. J Int Med Res. 2020;48(5):300060520925644. doi:10.1177/0300060520925644
25. Shan Q, Li Z, Lin J, et al. Tumor primary location may affect metastasis pattern for patients with Stage IV NSCLC: a Population-Based Study. J Oncol. 2020;2020:4784701. doi:10.1155/2020/4784701
26. Jusue-Torres I, Hulbert A, Zakaria J, et al. Socioeconomic disparities in non-small cell lung cancer with brain metastases at presentation: a Population-Based Study. World Neurosurg. 2021;154:e236–e244. doi:10.1016/j.wneu.2021.07.024
27. Yang J, Zhang Y, Sun X, et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol. 2018;144(9):1835–1842. doi:10.1007/s00432-018-2702-9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



