Back to Journals » Journal of Inflammation Research » Volume 17
Construction and Validation of a Nomogram Model to Predict the Severity of Mycoplasma pneumoniae Pneumonia in Children
Authors Li L, Guo R, Zou Y, Wang X, Wang Y, Zhang S, Wang H, Jin X, Zhang N
Received 20 November 2023
Accepted for publication 23 January 2024
Published 22 February 2024 Volume 2024:17 Pages 1183—1191
DOI https://doi.org/10.2147/JIR.S447569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Li Li,* Run Guo,* Yingxue Zou, Xu Wang, Yifan Wang, Shiying Zhang, Huihua Wang, Xingnan Jin, Ning Zhang
Department of Pulmonology, Tianjin Children’s Hospital (Children’s Hospital, Tianjin University) Machang Compus, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingxue Zou, Department of Pulmonology, Tianjin Children’s Hospital (Children’s Hospital, Tianjin University) Machang Compus, Tianjin, People’s Republic of China, Email [email protected]
Background: This study aimed to develop a nomogram model for early prediction of the severe Mycoplasma pneumoniae pneumonia (MPP) in children.
Methods: A retrospective analysis was conducted on children with MPP, classifying them into severe and general MPP groups. The risk factors for severe MPP were identified using Logistic Stepwise Regression Analysis, followed by Multivariate Regression Analysis to construct the nomogram model. The model’s discrimination was evaluated using a receiver operating characteristic curve, its calibration with a calibration curve, and the results were visualized using the Hosmer–Lemeshow goodness-of-fit test.
Results: Univariate analysis revealed that age, duration of fever, length of hospital-stay, decreased sounds of breathing, respiratory rate, hypokalemia, and incidence of co-infection were significantly different between severe and general MPP. Significant differences (p < 0.05) were also observed in C-reactive protein, procalcitonin, peripheral blood lymphocyte count, neutrophil-to-lymphocyte ratio, ferritin, lactate dehydrogenase, alanine aminotransferase, interleukin-6, immunoglobulin A, and CD4+ T cells between the two groups. Logistic Stepwise Regression Analysis showed that age, decreased sounds of breathing, respiratory rate, duration of fever (OR = 1.131; 95% CI: 1.060– 1.207), length of hospital-stay (OR = 1.415; 95% CI: 1.287– 1.555), incidence of co-infection (OR = 1.480; 95% CI: 1.001– 2.189), ferritin level (OR = 1.003; 95% CI: 1.001– 1.006), and LDH level (OR = 1.003; 95% CI: 1.001– 1.005) were identified as risk factors for the development of severe MPP (p < 0.05 in all). The above factors were applied in constructing a nomogram model that was subsequently tested with 0.862 of the area under the ROC curve.
Conclusion: Age, decreased sound of breathing, respiratory rate, duration of fever, length of hospital-stay, co-infection with other pathogen(s), ferritin level, and LDH level were the significant contributors for the establishment of a nomogram model to predict the severity of MPP in children.
Keywords: Mycoplasma pneumoniae pneumonia, nomogram, severity prediction
Introduction
Mycoplasma pneumoniae pneumonia (MPP) is a common community-acquired pneumonia in children, primarily caused by airway infection with Mycoplasma pneumoniae.1 Although MPP usually presents with mild symptoms and carries a favorable prognosis, once it develops into severe MPP, it can be accompanied by a variety of complications and sequelae, such as pleural effusion, lung abscess, atelectasis, necrotizing pneumonia, and bronchiolitis obliterans, all of which can significantly impair the daily life and wellbeing of infected children.2
In recent years, the incidence of severe MPP has been steadily rising. Studies have demonstrated that severe MPP can not only trigger extrapulmonary complications such as myocarditis, nephritis, encephalitis, and hemolytic anemia but also present a risk to life,3,4 thus emphasizing the importance of early detection of severe MPP as well as shortening its course to reduce comorbidity and mortality is necessary. Consequently, precise prediction and identification of severe MMP in clinical practice is of importance to reduce mortality and complications and to improve the prognosis of severe MPP. However, a unified scale or tool to evaluate and analyze the condition of children with MPP is currently unavailable in clinic, and thus, it is imperative to develop a scoring model that can promptly predict the severity of MPP so that development of severe MPP can be prevented. The current study was, therefore, designed to retrospectively analyze the risk factors related to the development of severe MPP in children, and based on the findings of the analysis, to construct and validate a nomogram model that could be applied in early identification and intervention of severe MPP.
Methods
Study Population and Data Collection
Cases of children with MPP who visited the Tianjin Children’s Hospital from January 2021 to December 2021 were retrospectively analyzed. Clinical data and results of laboratory tests for the children with MMP were collected through the electronic medical record system stored in the database of The Tianjin Children’s Hospital. Specifically, the following clinical data were collected: (1). The basic demographic information of the children including age, gender, past medical history, comorbidities, clinical symptoms, date of starting to have airway symptoms of Mycoplasma pneumoniae infection, length of hospital-stay, incidence and type of pathogen(s) of co-infection, as well as treatment and outcome. (2). Findings of physical examination including respiratory rate, heart rate, and sounds of breathing. (3). Results of routine blood test, blood biochemical panels, liver and kidney function, panels of immune function and coagulation function, and pathogenic test.
Inclusion criteria: (1). Patients under 18 years old and hospitalized with a diagnosis of MPP. (2). MPP was diagnosed by the criteria defined by The Chinese National Health Commission (Expert consensus on the diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children, version 2023),5 that is, in addition to the clinical symptoms and signs, the patients had positive results from either one of the following laboratory tests: 1. MP-DNA or MP-RNA by nucleic acid amplification test. 2. Either anti-MP antibody titer in a single test was ≥1:160, or at least fourfold increase in antibody titer during illness period by serum particle agglutination test for MP antibody. (3). Patients’ clinical data and laboratory test results were completely recorded and fully available.
Exclusion criteria: (1). Children had history of recurrent respiratory infection. (2). Because children with immunodeficiency are prone to develop into severe conditions and their laboratory test results might be different from that of children with normal immunity, children with comorbidity of severe diseases including congenital immunodeficiency were excluded.
Criteria for defining severe MPP: Severity of MPP was determined using previously published criteria.6 The MPP cases were categorized as severe MPP if the patients had any of the followings: (1). Poor general condition. (2). Disturbance of consciousness, cyanosis, and tachypnea (respiratory rate ≥ 70 times/min for an infant, or ≥ 50 times/min for children over 1 year old). (3). Assisted respiration (moaning, nasal fan, three concave sign), intermittent apnea, and oxygen saturation ≤ 92%. (4). Had extremely high fever that lasted for more than 5 days. (5). Dehydration and food refusal. (6). Chest X-ray or CT scan showed ≥2/3 unilateral lung infiltration, multi-lobar lung infiltration, pleural effusion, pneumothorax, atelectasis, lung necrosis, and lung abscess. (7). Extrapulmonary complications including complications of the neurological systems (encephalitis, acute disseminated encephalomyelitis; transverse myelitis; Guillain-Barre syndrome; or cerebral infarction); cardiovascular systems (blood clots in the heart, septic shock; myocarditis; pericarditis; Kawasaki disease; arterial or venous embolism); blood systems (immune thrombocytopenia; autoimmune hemolytic anemia; hemophagocytic syndrome; disseminated intravascular coagulation); skin and mucous membranes (urticaria; allergic purpura; erythema multiforme; Stevens-Johnson syndrome; toxic necrotic epidermolysis; mycoplasma pneumoniae-induced rash and mucositis); and others including glomerulus nephritis complicated with severe congestion or hypertensive crisis, acute kidney injury, liver function failure, acute pancreatitis, arthritis, or rhabdomyolysis syndrome.
Statistical Analysis
The data were analyzed using SPSS (version 27.0) statistical software. For continuously distributed data, normally distributed data were expressed as mean ± standard deviation (SD) and non-normally distributed data were expressed as median (interquartile range, IQR). Comparisons between groups were conducted using t-tests or rank-sum tests. Categorical data were presented as percentages (%), and discrepancies between groups were compared using the chi-square test. Factors showing significant differences were included in the logistic multivariate regression model to identify independent risk factors for severe MPP. The MPP-independent risk factors obtained from the multifactor regression analyses were used to construct nomogram models through the rms package (version 4.2.2) of the R software. The discriminatory power of the model was assessed by plotting the ROC curve and calculating the area under the ROC curve (AUC), which was equivalent to the C-statistic, using the AUC function in the R software. Meanwhile, internal validation was performed using bootstrapping with 1000 resamples. The calibration of the model was assessed using the calibration curve, and the Hosmer–Lemeshow goodness-of-fit test results were visualized. A significance level of p < 0.05 was considered statistically significant.
Results
Demographic Feature of the Enrolled Patients
A total of 1332 patients were enrolled in this study. Of them, 346 cases met the diagnostic criteria of severe MMP with a median age of 6 (4, 8) years old, and the rest 986 cases were categorized as general MPP with a median age of 4 (3,7) years old. Significance of demographic factors or clinical manifestations was examined by univariate analysis and the results are shown in Table 1. In addition to the gender ratio, factors including age, incidence of decreased sounds of breathing, incidence of hypokalemia, respiratory rate, days of fever lasted, days of hospitalstay, and incidence of co-infection with other pathogen(s) were significantly higher or longer in children with severe MMP compared to those of children with general MMP (p < 0.05, Table 1).
 |
Table 1 Comparison of the Demographic, Clinical, and Laboratory Examination Factors* |
Next, selected factors of the laboratory tests were examined by Univariate Analysis and the factors that statistically differ between the two groups are listed in Table 1. Of the examined parameters, numbers of peripheral blood lymphocyte and CD4+ T cells were significantly lower in the severe MPP than that in the general MPP (p < 0.05), while the rest of the listed parameters including C-reactive protein (CRP), procalcitonin (PCT), neutrophil-to-lymphocyte ratio (NLR), ferritin, lactate dehydrogenase (LDH), alanine aminotransferase (ALT), IL-6, and immunoglobulin A (IgA) were significantly higher in the severe MPP compared to the general MPP group (p < 0.05, Table 1).
Selection of the Predictive Factors by Stepwise Regression
Taking severe and non-severe (general) as the dependent variables, statistically significant factors in the univariate analysis were selected for Logistic Regression Collinearity Analysis. The factors for classification included in the final binary logistic were assigned values including decreased sounds of breathing (no = 0, yes = 1) and severe pneumonia (no = 0, yes = 1), as well as the continuous variables that were input with their values. It was found that age, decreased sounds of breathing, respiratory rate, duration of fever, length of hospital-stay, incidence of co-infection, blood ferritin level, and serum lactate dehydrogenase (LDH) level were the factors that could possibly predict the severity of MPP (all p < 0.05, Table 2).
 |
Table 2 Results of Multivariate Regression Analysis |
Establishment and Validation of the Nomogram Model
A nomogram model for severe MPP was constructed based on the screening results of the Logistic Regression. As shown in Figure 1, total 8 independent variables including age, decreased sounds of breathing, ferritin concentration, lactate dehydrogenase (LDH) concentration, incidence of co-infection, respiratory rate, days of having fever, and days of hospital-stay, were applied for the construction of the model. Internal validation for the nomogram model was then performed, and the results showed that the confidence interval (CI) of the area under the ROC curve of the model for predicting severe MPP was 0.862 (95% CI: 0.839, 0.886, Figure 2). Furthermore, as shown in Figure 3, this calibrated curve of the nomogram model in predicting prognosis for the children with severe MPP was close to the ideal curve. In addition, an excellent consistency of this nomogram model in predicting the risk of death in children with severe MPP was confirmed by the Hosmer–Lemeshow goodness-of-fit test (χ2 = 9.28, p = 0.3192).
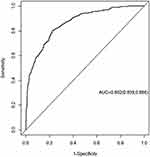 |
Figure 2 ROC curve of the nomogram model. The 8 independent risk factors presented with an area under the ROC curve (AUC) of 0.862 (95% CI: 0.839, 0.886). |
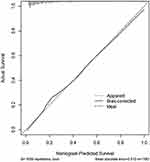 |
Figure 3 Calibration curve of the nomogram model. The observed apparent outcome (dotted line), the bias-corrected outcome (solid line), and the ideal outcome (dashed line) were presented. |
Discussion
In recent years, prevalence of MPP has been on the rise, with annual increase of the severity rate, reaching as high as 42.6% in 2016 in Suzhou, China.7 In the current study, we identified several characteristic clinical factors that might be associated with the development of severe MPP in children. These factors include age, decreased sounds of breathing, respiratory rate, fever duration, length of hospital-stay, co-infection with other pathogen(s), ferritin level, and LDH level. These independent risk factors presented in a score form to predict the severity of MPP with an area under the ROC curve of 0.862 (95% CI: 0.839, 0.886). Furthermore, internal validation showed that the calibration curve had a good fit with the standard curve, which was confirmed by Hosmer–Lemeshow test with high consistency, indicating that the nomogram model established in this study is highly accurate in predicting the severity of MPP.
The incidence of severe MPP increased with age in children due to an excessive immune response with the maturity of immunity.8–10 Decreased sounds of breathing, tachypnea, and persistent fever are common clinical features of severe airway infections, which could result in longer stays in the hospital. Consistently, the current study found that age, decreased sounds breathing, respiratory rate, days of fever lasted, and days of hospital stay were the factors that significantly contributed to the formation of a nomogram model for predicting severity of MPP.
Mycoplasma pneumoniae is a common pathogen that causes community-acquired pneumonia in children.11 Previous studies have reported that the co-infection rate of Mycoplasma pneumoniae and other pathogens ranged from 10% to 56.1%.12–14 Streptococcus pneumoniae, Epstein–Barr virus, parainfluenza virus, and respiratory syncytial virus are the most common pathogens that are co-infected with Mycoplasma pneumoniae in children. Co-infection of Mycoplasma pneumoniae with virus or bacteria could not only influence the progress of MPP but also increase the chance of extrapulmonary complications including complications of neurological systems such as encephalitis, acute disseminated encephalomyelitis; transverse myelitis; Guillain-Barre syndrome; or cerebral infarction; cardiovascular systems such as blood clots in the heart, septic shock; myocarditis; pericarditis; Kawasaki disease; arterial or venous embolism; blood systems such as immune thrombocytopenia; autoimmune hemolytic anemia; hemophagocytic syndrome; disseminated intravascular coagulation; skin and mucous membranes such as urticaria; allergic purpura; erythema multiforme; Stevens-Johnson syndrome; toxic necrotic epidermolysis; mycoplasma pneumoniae-induced rash and mucositis and others including glomerulus nephritis complicated with severe congestion or hypertensive crisis, acute kidney injury, liver function failure, acute pancreatitis, arthritis, or rhabdomyolysis syndrome. The current study found that co-infection was also a risk factor for the development of severe MPP, which is consistent with previous reports.9,14,15
The pathogenesis of severe MPP is mainly caused by the following two aspects: the virulence of the Mycoplasma pneumoniae pathogen itself, and the host immune response to defense against Mycoplasma pneumoniae infection.16 Inflammatory mediators such as reactive oxygen species and cytokines (IL-18) were produced by human host cells in response to the infection of Mycoplasma pneumoniae and released into circulation.17 These inflammatory mediators are responsible for the development of severe MPP by causing cellular death, tissue damage, and organ dysfunction. In addition, indirect interaction of immunological or allergic reaction of the host cells to Mycoplasma pneumoniae infection leads to the progress of severe MPP. In this regard, it has been reported that a positive correlation between IL-18 and LDH levels exists in severe MPP cases and that serum LDH levels were significantly higher in the cases of severe MPP compared to the control group.18–21 Consistently, we found that LDH level was one of the factors that could predict the development of severe MPP, suggesting airway cell damage caused by an excessive host-cellular response to Mycoplasma pneumoniae infection may play a critical role in the development of severe status.
Persistent fever is one of the important clinical manifestations of severe infection including severe MPP, which often indicates that the body has generated an excessively intense immuno-inflammatory response. Long-term fever may cause systemic immune dysfunction, resulting in difficulty to fight off infections and to control inflammation, which leads further to an extended fever and longer hospital-stays. Severe or refractory MPP with manifestations of pulmonary consolidation, pleural effusion, and atelectasis may be developed if systemic immune dysfunction and excessive inflammatory reaction were not resolved.3,10,22,23 As anticipated, days of fever was also one of the significant contributors to the formation of a nomogram model constructed in this study.
Procalcitonin (PCT), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are often used as indicators of systemic inflammation. However, limited studies on the application of ferritin in monitoring inflammatory reactions have been reported.24,25 In response to pro-inflammatory cytokines such as IL-1ß and TNF-α, hepatocytes could release a large amount of ferritin. High concentrations of ferritin could further promote the release of pro-inflammatory mediators and aggravate the inflammatory response by binding to T cells. Therefore, elevation of ferritin level can be an indicator of excessive inflammatory response.26,27 Here, we report that ferritin level could be one of the factors that predicts the development of severe MPP in children.
Persistent and excessive inflammatory reaction in the lung could also cause tissue damage and cell death, which results in release of LDH into the blood.16,28 As a non-specific marker of tissue damage and cellular death, LDH is often used as one of the indicators for judging the severity of the disease and prognosis. Consistent with previous reports,4,19,21 the current study found that LDH was significantly increased in severe MPP patients compared to patients with general MPP and that LDH was one of the factors significantly associated with the severity of MPP.
There are several limitations with our study. First, this was a retrospective study carried out in a single institute. Although the confidence interval of the area under the ROC curve in this retrospective study reached 0.862, a prospective cohort study is necessary to confirm that the predictive scale of the factors selected in this study can accurately distinguish between severe MPP and general MPP. Second, patients with incomplete clinical data were excluded from this study, which may cause selection bias. Third, only internal validation was used to evaluate the model in this study in that there was no external data available for us. A more effective and stronger external validation from cohort studies with other institutes remains to be conducted to confirm the findings of this study. While a larger scale and multi-center prospective study is needed to confirm the accuracy and adaptability of the current model, the clinical indicators included in this study are easy to obtain, and thus, accuracy of the nomogram model constructed in this study could be easily tested in future clinical work.
Conclusion
The current study found that age, decreased sounds of breathing, respiratory rate, fever duration, days of hospitalization, incidence of co-infection, and levels of ferritin and LDH were independent factors that significantly contributed to the construction and validation of a nomogram model for predicting the severity of MPP. This nomogram model could be a reliable tool for clinicians to make prediction on the progress of MPP so that a timely diagnosis and treatment for severe MPP could be applied in clinical work.
Data Sharing Statement
The original data included in this study are presented in this article. Further inquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was approved by the Ethic Committee of the Tianjin Children’s Hospital (No. 022-LXKY-004). All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from individual participants and their legal guardians.
Acknowledgments
We thank all participants and staff of this study and the physicians at the Tianjin Children’s Hospital affiliated to Tianjin University.
Funding
This study was financially supported by Tianjin Municipal Health Commission Key Discipline Special Fund (TJWJ2022XK038) and Tianjin Key Medical Discipline (Specialty) Construction Project (No. TJYXZDXK-040A).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kutty PK, Jain S, Taylor TH, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. 2019;68(1):5–12. doi:10.1093/cid/ciy419
2. Gao LW, Yin J, Hu YH, et al. The epidemiology of paediatric Mycoplasma pneumoniae pneumonia in North China: 2006 to 2016. Epidemiol Infect. 2019;147:e192. doi:10.1017/S0950268819000839
3. Lee KL, Lee CM, Yang TL, et al. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010–2019. J Formos Med Assoc. 2021;120(1 Pt 1):281–291. doi:10.1016/j.jfma.2020.08.018
4. Moynihan KM, Barlow A, Nourse C, Heney C, Schlebusch S, Schlapbach LJ. Severe mycoplasma pneumoniae infection in children admitted to pediatric intensive care. Pediatr Infect Dis J. 2018;37(12):e336–e338. doi:10.1097/INF.0000000000002029
5. Commission GOotNH. Guideline for diagnosis and treatment of mycoplasma pneumoniae pneumonia in children; 2023. Available from: http://www.gov.cn/zhengce/zhengceku/2023-02/16/content_5741770.htm.
6. Ni X. Diagnosis and treatment of community acquired pneumonia in children. In: General Medicine and Education. Vol. 17. Wiley Online Library; 2019:771–777.
7. Lv YT, Sun XJ, Chen Y, Ruan T, Xu GP, Huang JA. Epidemic characteristics of Mycoplasma pneumoniae infection: a retrospective analysis of a single center in Suzhou from 2014 to 2020. Ann Transl Med. 2022;10(20):1123. doi:10.21037/atm-22-4304
8. Pechous RD. With friends like these: the complex role of neutrophils in the progression of severe pneumonia. Front Cell Infect Microbiol. 2017;7:160. doi:10.3389/fcimb.2017.00160
9. Yan C, Xue G, Zhao H, et al. Molecular and clinical characteristics of severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. 2019;54(7):1012–1021. doi:10.1002/ppul.24327
10. Bi Y, Zhu Y, Ma X, et al. Development of a scale for early prediction of refractory Mycoplasma pneumoniae pneumonia in hospitalized children. Sci Rep. 2021;11(1):6595. doi:10.1038/s41598-021-86086-5
11. Gendrel D, Raymond J, Moulin F, et al. Etiology and response to antibiotic therapy of community-acquired pneumonia in French children. Eur J Clin Microbiol Infect Dis. 1997;16(5):388–391. doi:10.1007/BF01726370
12. Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19(4):293–298. doi:10.1097/00006454-200004000-00006
13. Chiu CY, Chen CJ, Wong KS, Tsai MH, Chiu CH, Huang YC. Impact of bacterial and viral coinfection on mycoplasmal pneumonia in childhood community-acquired pneumonia. J Microbiol Immunol Infect. 2015;48(1):51–56. doi:10.1016/j.jmii.2013.06.006
14. Zhou Y, Wang J, Chen W, et al. Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect Dis. 2020;20(1):633. doi:10.1186/s12879-020-05356-1
15. Liu J, Wang M, Zhao Z, et al. Viral and bacterial coinfection among hospitalized children with respiratory tract infections. Am J Infect Control. 2020;48(10):1231–1236. doi:10.1016/j.ajic.2020.01.013
16. Zou Y. Clinical significance of abnormal inflammatory markers in mycoplasma pneumoniae pneumonia. Chin J Appl Clin Pediatr. 2021;24:1209–1214.
17. Oishi T, Narita M, Matsui K, et al. Clinical implications of interleukin-18 levels in pediatric patients with Mycoplasma pneumoniae pneumonia. J Infect Chemother. 2011;17(6):803–806. doi:10.1007/s10156-011-0265-7
18. Miyashita N, Kawai Y, Inamura N, et al. Setting a standard for the initiation of steroid therapy in refractory or severe Mycoplasma pneumoniae pneumonia in adolescents and adults. J Infect Chemother. 2015;21(3):153–160. doi:10.1016/j.jiac.2014.10.008
19. Izumikawa K. Clinical features of severe or fatal mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:800. doi:10.3389/fmicb.2016.00800
20. Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60(10):1469–1475. doi:10.4187/respcare.03920
21. Liu TY, Lee WJ, Tsai CM, et al. Serum lactate dehydrogenase isoenzymes 4 plus 5 is a better biomarker than total lactate dehydrogenase for refractory Mycoplasma pneumoniae pneumonia in children. Pediatr Neonatol. 2018;59(5):501–506. doi:10.1016/j.pedneo.2017.12.008
22. Zheng HQ, Ma YC, Chen YQ, Xu YY, Pang YL, Liu L. Clinical analysis and risk factors of bronchiolitis obliterans after mycoplasma pneumoniae pneumonia. Infect Drug Resist. 2022;15:4101–4108. doi:10.2147/IDR.S372940
23. Miyashita N, Obase Y, Ouchi K, et al. Clinical features of severe Mycoplasma pneumoniae pneumonia in adults admitted to an intensive care unit. J Med Microbiol. 2007;56(Pt 12):1625–1629. doi:10.1099/jmm.0.47119-0
24. Chen Q, Shen K, X ZD. Relationship between serum ferritin level and severity of community-acquired pneumonia in children. Chin J Pract Pediatr. 2018;22(10):753–757.
25. Li G, Ye H. Correlation analysis of serum interleukin-18 and ferritin levels with disease severity in children with Mycoplasma pneumoniae pneumonia. China Maternal Child Health Care. 2022;37(12):2206–2208.
26. Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, et al. Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017;15(1):172. doi:10.1186/s12916-017-0930-5
27. Horvat CM, Fabio A, Nagin DS, et al. Mortality risk in pediatric sepsis based on c-reactive protein and ferritin levels. Pediatr Crit Care Med. 2022;23(12):968–979. doi:10.1097/PCC.0000000000003074
28. Zhou Y, Qi M, Yang M. Current status and future perspectives of lactate dehydrogenase detection and medical implications: a review. Biosensors. 2022;12:12.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

