Back to Journals » Clinical Ophthalmology » Volume 18
Considerations for the Identification and Management of Geographic Atrophy: Recommendations from an Expert Panel
Authors Regillo CD, Nijm LM, Shechtman DL, Kaiser PK, Karpecki PM , Ryan EH, Ip MS, Yeu E , Kim T, Rafieetary MR, Donnenfeld ED
Received 20 October 2023
Accepted for publication 25 January 2024
Published 2 February 2024 Volume 2024:18 Pages 325—335
DOI https://doi.org/10.2147/OPTH.S445755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Carl D Regillo,1 Lisa M Nijm,2 Diana L Shechtman,3 Peter K Kaiser,4 Paul M Karpecki,5 Edwin H Ryan,6 Michael S Ip,7 Elizabeth Yeu,8 Terry Kim,9 Mohammad R Rafieetary,10 Eric D Donnenfeld11
1Mid Atlantic Retina, Wills Eye Hospital, Thomas Jefferson University, Philadelphia, PA, USA; 2Warrenville Eye Care and LASIK Center, Warrenville, IL, USA; 3Eye Centers of South Florida, North Miami Beach, FL, USA; 4Cole Eye Institute, Cleveland Clinic Lerner College of Medicine, Cleveland, OH, USA; 5Kentucky College of Optometry, University of Pikeville, Pikeville, KY, USA; 6Retina Consultants of Minnesota, Edina, MN, USA; 7Doheny Eye Center UCLA, Pasadena, CA, USA; 8Virginia Eye Consultants, Norfolk, VA, USA; 9Duke University, Durham, NC, USA; 10Charles Retina Institute, Germantown, TN, USA; 11Ophthalmic Consultants of Long Island, Garden City, NY, USA
Correspondence: Carl D Regillo, Mid Atlantic Retina, Wills Eye Hospital, Thomas Jefferson University, 840 Walnut St # 1020, Philadelphia, PA, 19107, USA, Tel +1 (215) 928-3300, Email [email protected]
Abstract: Newly approved treatments for patients with geographic atrophy are changing the treatment paradigm, highlighting the need for eye care providers (ECPs) to have a set of recommendations on how to best manage GA patients. Here, we outline how to identify various stages of age-related macular degeneration including geographic atrophy (GA) by examining optimal management scenarios implicating various ECPs and reviewing treatment considerations for patients with GA. Early identification of GA will lead to optimal patient outcomes, while a standardized management scenario will reduce clinical burden among ECPs treating patients with GA.
Keywords: age-related macular degeneration, patient care, retina, treatment
Introduction
Geographic atrophy (GA) secondary to age-related macular degeneration (AMD) is a severe form of dry AMD and represents a leading cause of irreversible central vision loss. The prevalence of GA increases with age and continues to rise along with the increasing worldwide population.1,2 There is a pressing need to develop consensus guidelines to optimize early identification, diagnosis, and management of patients with GA.
A hallmark of dry AMD is the accumulation of drusen, which are nodular deposits of cellular waste debris composed of proteins and lipids at the level of Bruch’s membrane in the macula.3 Drusen deposition may lead to dysfunction and degeneration of overlying retinal pigment epithelium (RPE) and photoreceptors and may eventually result in formation of atrophic lesions in the macula as the disease progresses. Early AMD is clinically defined as the presence of drusen with a diameter of ≥63 µm and <125 µm, intermediate AMD is defined as drusen ≥125 µm in diameter or when pigmentary changes occur with drusen of any size, and late or advanced dry AMD (hereby referred to as geographic atrophy) is characterized by areas of photoreceptor, RPE, and choriocapillaris loss.4 Since GA is a progressive disease, an improved understanding of prognostic indicators is essential for prompt identification, monitoring, and management.
Unlike neovascular AMD, which has had several highly effective therapies available for more than a decade, there were no treatment options for GA until recently. Intravitreal complement inhibitors have demonstrated the potential to slow the progression of GA, with the two agents of this class approved by the United States Food and Drug Administration (US FDA) in 2023. As GA progresses, visual function may become severely affected; on average, it takes only 2.5 years from initial identification and diagnosis of GA for central visual function to decline.5–7 Therefore, with current treatments only slowing GA growth, therapy would be most impactful when GA is identified early, before significant vision loss occurs. To further support for the benefits of identifying GA at an early stage, we outline a set of considerations directed toward the global eye care provider (ECP) community that aims to encourage screening for early signs of GA.
Previously, a diverse group of expert ECPs comprising optometrists, comprehensive ophthalmologists, and retina specialists convened using a modified Delphi panel method to achieve consensus on a variety of topics concerning identification and management of GA. This process involved use of a series of surveys that used a Likert scale to measure degree of consensus as well as a discussion during a synchronous virtual meeting. Here, we expand further on these topics and propose specific guidelines to aid ECPs in identifying and managing patients with GA through collaborative multidisciplinary care between the optometrist, comprehensive ophthalmologist, and retina specialist.
Methods
Eleven expert ECPs, including three optometrists, four comprehensive ophthalmologists, and four retina specialists, were selected to guide a roundtable discussion to create consensus guidelines on the management of GA. Topics included identification of GA via various imaging modalities, collaborative referral strategies, and optimal disease management. An online survey was first used to gather information concerning the greatest unmet needs in GA treatment and how best to overcome them. A virtual meeting was then held to generate discussion and achieve best consensus among these topics. Support for survey development and deployment, as well as meeting logistics, was provided by a third-party medical communications agency (i2Vision, San Diego, CA).
Results
Among all ECPs, regardless of practice specialty, there was unanimous agreement that there is currently no standardized process by which GA is identified and diagnosed, thus revealing a significant unmet need. One key takeaway from the panelists was that early identification and proper diagnosis of GA is paramount for optimal patient outcomes, strengthening the rationale for needing more complete recommendations on disease identification, timely referral, and treatment of patients with GA. Early identification of GA by non-retina specialists will decrease rates of missed diagnoses and may help mitigate disease complications and related comorbidities, ultimately improving visual outcomes. Additionally, there was consensus that anterior segment specialists should be able to identify GA to help improve patient expectations following cataract surgery, as it may be associated with an increased risk of AMD progression.8
Geographic Atrophy Imaging: Identification and Diagnosis
Eye care professionals employ a variety of imaging modalities tailored to specific clinical needs, including color fundus photography, fundus autofluorescence (FAF), fluorescein angiography, and optical coherence tomography (OCT).9 Many of these modalities can detect the presence of GA. Optical coherence tomography is a high-resolution, noninvasive rapid imaging technique that delivers comprehensive information about the various layers that comprise the retina. Panelists agreed that OCT was the preferred imaging method to identify GA because of both its ease of use and accessibility among optometrists and ophthalmologists. The most common output of ophthalmologic OCT, the B-scan, shows a cross-sectional image of the macula where the structural integrity of the inner and outer photoreceptors, RPE, Bruch’s membrane, and choroid can be readily assessed in a rapid, noninvasive manner (Figure 1A).
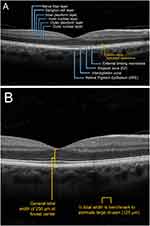 |
Figure 1 Key landmarks and features in the retina. (A) Healthy retina and retinal layers. (B) Drusen sizing estimation. |
To identify and diagnose GA at earlier stages, clinicians must first be able to recognize the specific hallmark signs of early and intermediate AMD via OCT. While most ECPs are familiar with clinical definitions of AMD and GA as outlined by current literature,4,10 the routine measurement of drusen size during imaging is uncommon. However, a simple method to estimate the size of drusen involves comparing it to the width of the central retinal vein, which measures roughly 125 µm upon examination of the macula (Figure 1B).11 On OCT, drusen generally appear as wavy, dome-shaped, triangular, or sawtooth-like disruptions under the neurosensory retina (Figure 2A).12,13 These irregularities may vary in size and reflective intensity. During evaluation of scans, clinicians should pay special attention to the RPE level, especially during earlier disease stages when abnormalities may not be particularly evident. Examples of various forms of early and intermediate AMD via OCT are shown in (Figure 2A and B).
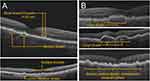 |
Figure 2 Stages of AMD as observed via OCT. (A) Early AMD. (B) Intermediate AMD. |
Of note, it is important to carefully identify and monitor lesions that may be associated with a risk of developing GA. Signs associated with high risk include hyperreflective foci and drusen volume, both of which were identified by panelists to be strong predictors of GA development. Moreover, the presence of reticular pseudodrusen, also referred to as subretinal drusenoid deposits between the RPE and inner segment/outer segment junction, is a known risk factor in the progression of AMD.14 On OCT, reticular pseudodrusen appear as hyperreflective material located above the RPE layer, unlike regular drusen, which are located below the RPE.15
The appearance of incomplete RPE and Outer Retinal Atrophy (iRORA) is an additional indicator of a high risk of developing GA and is considered to be a precursor lesion. On OCT, iRORA has been defined as hypertransmission into the choroid with a corresponding zone of attenuation or disruption of the RPE with evidence of overlying photoreceptor degeneration that does not meet the complete RPE and Outer Retinal Atrophy (cRORA) criteria (see below) (Figure 3).16 Although the term iRORA is becoming more common and increasingly recognized by ECPs, it is rarely used to support clinical decisions about treatment.
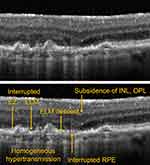 |
Figure 3 Incomplete Retinal pigment epithelium and Outer Retinal Atrophy (iRORA). |
As disease progresses, OCT remains the primary imaging modality for GA identification because of the prominent, readily identifiable hypertransmission that occurs beyond atrophic lesions. Hypertransmission is the result of increased light transmission into the choroid due to the loss of the overlying light-absorbing RPE. The location and extent of GA may vary considerably. Detailed examples of OCT images depicting variations of GA are shown in Figure 4. Similar to the presence of iRORA in the near-preceding stages of AMD-related GA, cRORA indicates the presence of GA.17 On OCT, cRORA specifically refers to a region of hypertransmission of at least 250 µm in diameter with a zone of attenuation or disruption of the RPE of at least 250 µm in diameter with evidence of overlying photoreceptor degeneration and the absence of scrolled RPE or other signs of an RPE tear (Figure 5).
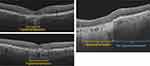 |
Figure 4 Advanced AMD/geographic atrophy via OCT. |
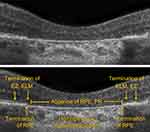 |
Figure 5 Complete Retinal pigment epithelium and Outer Retinal Atrophy (cRORA). |
In addition to the B-scan of an OCT, the technology often includes en face imaging, such as the near-infrared image, as a complementary visual guide that allows the physician to clearly observe the total lesion area of GA. En face images can provide information about both lesion location with respect to the fovea and whether the disease is unifocal or multifocal, which may have an effect on the rate of GA progression. Observing and visualizing the total lesion area leads to a more thorough comprehension of the disease and may be beneficial for patient education.
Although commonly used in clinical trial studies to measure GA lesion size, FAF has less utility in identifying early and intermediate AMD, and therefore does not have widespread clinical use among non-retina specialists. Fundus autofluorescence does not confer much additional information compared to what can be obtained from OCT (B-scan and en face). Additionally, patient tolerance to FAF imaging is lower because of the high light intensity used during acquisition.18 Fundus autofluorescence image production utilizes the naturally occurring autofluorescence of lipofuscin, a byproduct of phagocytosed photoreceptor outer segments. Although lipofuscin naturally accrues with age, excess accumulation (and therefore autofluorescence) may signal RPE cell dysfunction or other pathologic considerations indicative of GA progression. On FAF, GA lesions appear as areas of decreased autofluorescence (hypoautofluorescence) due to a lack of lipofuscin as a result of RPE cell atrophy. Similar to “en face” OCT, FAF is clinically useful to visualize the total area of GA lesions, visualize focality characteristics, and provide information concerning its location relative to the fovea. Additionally, FAF can be used to identify specific lesion characteristics through distinct patterns of hyperautofluorescence along lesion borders, which may have implications in predicting the rate of GA progression.19,20 Since the fovea appears hypoautofluorescent, determining the locality of GA lesions is often more difficult on FAF than OCT. Examples of GA via FAF are shown in Figure 6A–D.
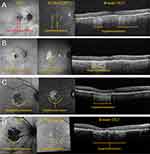 |
Figure 6 A) Early nonfoveal GA. (B) Moderate nonfoveal GA, example 1. (C) Moderate nonfoveal GA, example 2. (D) Moderate foveal GA, example 3. |
Management of Patients with GA
The optimization of patient management and collaborative multidisciplinary care is increasingly important because of the recent availability of treatments for GA. As most eye care practices currently face a high patient volume, aligning on specific guidelines of GA patient care will not only help streamline in-office visits but will potentially alleviate additional burdens. Here, we outline considerations on collaborative care for ideal referral and follow-up scenarios designed to optimize patient diagnosis and management.
For routine eye care, it is recommended that patients visit their primary ECP (optometrist or general ophthalmologist) every 12 months. The primary ECP should inquire with the patient about GA symptoms, perform a comprehensive ocular examination that includes best-corrected visual acuity assessment, and conduct a retinal examination and OCT scan to identify any signs of AMD in one or both eyes. In patients with early AMD, follow-up intervals should be every 12 months, while for intermediate AMD, follow-up may be adjusted to every 6 months, ideally with OCT at every visit. Additionally, it is suggested that the patient consider self-monitoring between visits using Amsler grid testing or with the use of preferential hyperacuity perimetry home testing. During patient visits, dietary and other lifestyle counseling, including the potential use of vitamin/mineral supplementation such as the Age-Related Eye Disease Study (AREDS) formulas, may be advised.
A nonurgent referral to a retina specialist is recommended for any suspected GA in one or both eyes, whereas a more urgent referral should be made for any patient with suspected neovascular AMD (nAMD), also referred to as “wet” AMD. A consultation with a retina specialist may be considered for patients with intermediate AMD, depending on the specific patient case or ocular circumstances, such as recent visual changes or consideration of ophthalmic surgery by the general ophthalmologist. Therefore, it is important that all ECPs be adept at identifying the various stages and forms of AMD. Taken together, these recommendations can be consolidated into a suggested care algorithm for management of patients with AMD and GA. This algorithm is represented in Figure 7 (flowchart).
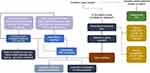 |
Figure 7 Diagnostic flow chart. |
As with many diseases, the severity and rate of progression will vary from patient to patient. Therefore, adjustments to follow-up frequency should be considered depending on the identification of signs of increased risk, such as advanced age, family history, smoking history, and whether the patient is monocular. Similarly, ECP factors influence care, including the provider’s level of knowledge and comfort with managing a patient at various stages of AMD, including GA. Timing of referral to retina specialists must be optimized to consider the burden on patients and caregivers. For example, considerations include a patient’s ability to travel and the ability of their caregivers to accompany them to clinical visits. Additionally, a specialist’s practice volume and location are important factors. For example, an optometrist in a more rural area may be hesitant to refer when the closest retina specialist may be a long distance away, placing a potential unnecessary burden on the patient and/or their family.
Treatment of Patients with GA
When it comes to recommendations of treatment for patients with GA, it is suggested that the retina specialist personalize care to maximize the patient’s quality of life. To streamline care, patients may consider monitoring (or possibly even treatment) by their comprehensive ophthalmologist under the guidance of a retina specialist, which may help maintain compliance and overall treatment fidelity. This personalization will include determining an appropriate frequency of treatment that balances the safety and efficacy of the treatment with the burden on the patient and provider. Current anti-complement therapy for GA requires treatment with office visits every 1 to 2 months. The recommended dosing for avacincaptad pegol is 2 mg (0.1 mL of 20 mg/mL solution) administered by intravitreal injection to each affected eye once monthly (approximately 28 ± 7 days) for up to 12 months, and the recommended dosing for pegcetacoplan is 15 mg (0.1 mL of 150 mg/mL solution) administered by intravitreal injection to each affected eye once every 25 to 60 days.21,22 Additionally, the treating physician may need to consider several disease treatment scenarios when choosing the ideal management for a particular patient, such as bilateral GA treatment and presence of concomitant nAMD therapy in one or both eyes.
Lesion size and location, along with other lesion characteristics such as perilesional hyperautofluoresence, are important factors that influence which eyes with GA may benefit from treatment and how often it is administered. It has been shown that extrafoveal lesions progress more rapidly than foveal lesions,20 reinforcing the importance of intervening early on in the course of GA. However, the assumed personalization of GA treatment may affect an ophthalmologist’s decision to treat. For example, if a patient presents with a far extrafoveal lesion with 20/20 visual acuity and no visual symptoms attributable to GA, the managing physician may elect to simply monitor the patient, which would spare the patient the burden of treatment. Conversely, if a patient presents with a GA lesion that is close to the center of the fovea and with visual symptoms, the physician may be more likely to initiate therapy. Studies suggest that GA develops bilaterally in as many as 65% of cases,7 in which case treatment of one or both eyes must be considered. In general, treatment decisions are influenced by patient symptoms, visual acuity, age, fellow-eye status, and ocular comorbidities, as well as the discretion of the physician and patient to conduct a comprehensive personalized risk-benefit assessment that is guided by a general evidence-based treatment algorithm. Additionally, the physician should educate the patient with regard to GA progression and treatment expectations.
As neovascular AMD and GA are late stages of the same disease, they may exist concomitantly. If an eye develops nAMD during GA treatment, nAMD treatment will need to be initiated if there are signs of exudation in the macula. Some type 1, non-exudative choroidal neovascularization lesions may not require immediate treatment but should be followed closely to detect disease progression. Ongoing treatment of GA in the eye that develops choroidal neovascularization and requires anti-vascular endothelial growth factor therapy will be at the discretion of the managing physician and patient. Combined GA and nAMD treatment is both feasible and safe based on pivotal clinical trials, but injections may need to be provided either on different clinical visits or separated by approximately 30 min if performed on the same day to minimize the risk of excessive intraocular pressure elevation.
Several circumstances may warrant discontinuation of GA treatment, such as the occurrence of severe adverse events including endophthalmitis, ischemic optic neuropathy, sustained elevation of intraocular pressure, and significant, nontransient intraocular inflammation, especially if associated with any retinal vasculitis or occlusive vasculitis. Additionally, severe central vision loss may represent a scenario where ongoing treatment risks or burdens may not significantly outweigh any potential benefits. To minimize potential adverse events, detailed clinical examination should be performed prior to treatment administration. Additionally, the treating physician should educate patients on symptoms that may signify occurrence of adverse events, such as pain, new visual changes, or new floaters. Finally, if patient compliance significantly affects the treatment regimen in a negative manner, discontinuation may be considered.
These considerations on GA patient treatment are likely to evolve as more information on best clinical practices emerge, current treatments become more established, and new therapies are developed.
Conclusion
Approved and emerging treatments for GA will cause a paradigm shift in the identification and management of GA, resulting in additional or new considerations with regard to optimal patient care. The panel agreed that because current GA treatments slow but do not stop the progression of GA, early identification of GA is important to ensure that patients with this advanced form of dry AMD have the best possible outcomes. Early intervention may lead to a reduction in the rate of vision loss and improvements in overall quality of life. Despite GA being a retinal disease, panelists believe the availability of GA treatment will affect all ECPs, including optometrists, comprehensive ophthalmologists, and retina specialists. It is imperative that all ECPs not only have disease knowledge about GA but also understand best practices for GA identification and how to collaborate with other ECPs to optimize multidisciplinary patient care.
Statement of Ethics Approval
As this study does not involve patients or study subjects, and it does not submit people to actions or impose specific behaviors on them, according to the Medical Research in Human Subjects Act (WMO), an ethical approval was not needed.
Disclosure
Dr Carl Regillo reports grants and/or personal fees from Apellis, Iveric, Annexon, NGM, Stealth, Gyroscope, and Janssen, during the conduct of the study. Dr Lisa Nijm reports personal fees from Iveric Bio, during the conduct of the study; grants and/or personal fees from Azura Ophthalmics, Bausch + Lomb, BVI Medical, Carl Zeiss Meditec, Dompe, Horizon Therapeutics Plc, Johnson & Johnson Vision, Alcon Pharmaceuticals, Ocular Therapeutix, Orasis, Oyster Point Pharma/Viatris, Rayner Intraocular Lenses Ltd, Scope Health, Sun Ophthalmics, Tarsus, Trukera Medical (TearLab Corporation), THEA, and Visus, outside the submitted work. Dr Peter Kaiser reports grants from Apellis; personal fees from Iveric Bio, Annexon, Alexion, Aviceda, Novartis, Janssen, Galimedix, Kriya Therapeutics, Stealth, and Thea, outside the submitted work. Dr Paul Karpecki reports personal fees from Iveric, Apellis, Bausch and Lomb, ScienceBased Health, and Zeiss, outside the submitted work. Dr Michael Ip reports personal fees from Alimera, Allergan, Amgen, Apellis, Clearside Biomedical, Genentech, IVERIC Bio, Novartis, Regeneron, Regenxbio, Biogen, Lineage Cell Therapeutics, ONL Therapeutics, Regenxbio, and 4DMT, outside the submitted work. Dr Elizabeth Yeu is research consultant for AcuFocus, Adaptilens, Advanced Vision Group, Aldeyra, Allergan, Bausch & Lomb, BioTissue, BVI, BlephEx, Bruder, Centricity, CorneaGen, Dompe, Elios, Expert Opinion, Eyenovia, ForSight, Glaukos, Guidepoint, Iveric Bio, J&J Vision, Kala, LayerBio, LensAR, Melt, Merck, New World Medical, Novartis, OSRX, Ocusoft, Samsara, ScienceBased Health, Sight Sciences, Surface, Thea, Visus, and Zeiss; Speakers’ Bureau Research for Alcon, consultant for and owns equity from Aurion, consultant director for Avellino; consultant director for and owns equity from STAAR, and Tarsus, outside the submitted work. Dr Mohammad Rafieetary reports personal fees from Heidelberg, personal fees from Optos, personal fees from Regeneron, personal fees from Apellis, personal fees from Iveric Bio, personal fees from Novartis, personal fees from Notal Vision, personal fees from OcuTerra, and personal fees from Zeiss, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25.
2. Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi:10.1001/archopht.122.4.564
3. Fleckenstein M, Keenan TDL, Guymer RH, et al. Age-related macular degeneration. Nat Rev Dis Primers. 2021;7(1):31. doi:10.1038/s41572-021-00265-2
4. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi:10.1016/j.ophtha.2012.10.036
5. You JI, Kim DG, Yu SY, Kim ES, Kim K. Correlation between topographic progression of geographic atrophy and visual acuity changes. Korean J Ophthalmol. 2021;35(6):448–454. doi:10.3341/kjo.2021.0037
6. Heier JS, Pieramici D, Chakravarthy U, et al. Visual function decline resulting from geographic atrophy: results from the chroma and spectri phase 3 trials. Ophthalmol Retina. 2020;4(7):673–688. doi:10.1016/j.oret.2020.01.019
7. Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168–1174. doi:10.1001/archophthalmol.2009.198
8. Yang L, Li H, Zhao X, Pan Y. Association between cataract surgery and age-related macular degeneration: a systematic review and meta-analysis. J Ophthalmol. 2022;2022:6780901. doi:10.1155/2022/6780901
9. Göbel AP, Fleckenstein M, Schmitz-Valckenberg S, Brinkmann CK, Holz FG. Imaging geographic atrophy in age-related macular degeneration. Ophthalmologica. 2011;226(4):182–190. doi:10.1159/000330420
10. Age-Related Eye Disease Study Research Group. The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the age-related eye disease study report number 6. Am J Ophthalmol. 2001;132(5):668–681. doi:10.1016/S0002-9394(01)01218-1
11. Age-Related Eye Disease Study Research Group. A simplified severity scale for age-related macular degeneration. Arch Ophthalmol. 2005;123(11):1570–1574. doi:10.1001/archopht.123.11.1570
12. Drusen characterization with multimodal imaging - PMC. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2952278/.
13. Schmidt-Erfurth U, Klimscha S, Waldstein SM, Bogunović H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye. 2017;31(1):26–44. doi:10.1038/eye.2016.227
14. Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BEK. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145(2):317–326. doi:10.1016/j.ajo.2007.09.008
15. Querques G, Canouï-Poitrine F, Coscas F, et al. Analysis of progression of reticular pseudodrusen by spectral domain–optical coherence tomography. Invest Ophthalmol Visual Sci. 2012;53(3):1264–1270. doi:10.1167/iovs.11-9063
16. Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete retinal pigment epithelial and outer retinal atrophy in age-related macular degeneration. Ophthalmology. 2020;127(3):394–409. doi:10.1016/j.ophtha.2019.09.035
17. Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125(4):537–548. doi:10.1016/j.ophtha.2017.09.028
18. Armenti ST, Greenberg JP, Smith RT. Quantitative fundus autofluorescence for the evaluation of retinal diseases. J Vis Exp. 2016;109:53577. doi:10.3791/53577
19. Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi:10.1016/j.ophtha.2013.11.023
20. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369–390. doi:10.1016/j.ophtha.2017.08.038
21. IVERIC bio, Inc. IZERVAY - avacincaptad pegol injection. Prescribing Information. Available from: https://ivericbio.com/wp-content/uploads/IZERVAY-avacincaptad-pegol-intravitreal-solution-PI_Final_8.4.23.pdf.
22. Apellis Pharmaceuticals, Inc. SYFOVRE- pegcetacoplan injection, solution. Prescribing Information. Available from: https://pi.apellis.com/files/PI_SYFOVRE.pdf.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
