Back to Journals » Journal of Pain Research » Volume 15
Consensus Guidelines on Interventional Therapies for Knee Pain (STEP Guidelines) from the American Society of Pain and Neuroscience
Authors Hunter CW, Deer TR , Jones MR, Chang Chien GC, D'Souza RS , Davis T, Eldon ER, Esposito MF, Goree JH , Hewan-Lowe L, Maloney JA , Mazzola AJ, Michels JS, Layno-Moses A, Patel S, Tari J , Weisbein JS , Goulding KA, Chhabra A, Hassebrock J, Wie C, Beall D, Sayed D , Strand N
Received 13 April 2022
Accepted for publication 12 August 2022
Published 8 September 2022 Volume 2022:15 Pages 2683—2745
DOI https://doi.org/10.2147/JPR.S370469
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alaa Abd-Elsayed
Corey W Hunter,1,2 Timothy R Deer,3 Mark R Jones,4 George C Chang Chien,5 Ryan S D’Souza,6 Timothy Davis,7 Erica R Eldon,2 Michael F Esposito,8 Johnathan H Goree,9 Lissa Hewan-Lowe,2 Jillian A Maloney,10 Anthony J Mazzola,2 John S Michels,11 Annie Layno-Moses,7 Shachi Patel,12 Jeanmarie Tari,1 Jacqueline S Weisbein,13 Krista A Goulding,14 Anikar Chhabra,14 Jeffrey Hassebrock,14 Chris Wie,11 Douglas Beall,15 Dawood Sayed,16 Natalie Strand11
1Ainsworth Institute of Pain Management, New York, NY, USA; 2Department of Rehabilitation & Human Performance, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 3The Spine and Nerve Center of the Virginias, Charleston, WV, USA; 4Pain Medicine of the South, Knoxville, TN, USA; 5County Medical Center, Ventura, CA, USA; 6Department of Anesthesiology, Mayo Clinic, Rochester, MN, USA; 7Source Healthcare, Santa Monica, CA, USA; 8Interventional Spine and Pain Institute, Vero Beach, FL, USA; 9Department of Anesthesiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA; 10Department of Anesthesiology, Division of Pain Medicine, Mayo Clinic, Phoenix, AZ, USA; 11Interventional Spine and Pain, Dallas, TX, USA; 12Delmarva Pain and Spine Center, Newark, DE, USA; 13Napa Valley Orthopaedic Medical Group, Napa, CA, USA; 14Department of Orthopedic Surgery, Mayo Clinic, Phoenix, AZ, USA; 15Comprehensive Specialty Care, Oklahoma City, OK, USA; 16Department of Anesthesiology, Division of Pain Medicine, University of Kansas Medical Center, Kansas City, KS, USA
Correspondence: Corey W Hunter, Email [email protected]
Abstract: Knee pain is second only to the back as the most commonly reported area of pain in the human body. With an overall prevalence of 46.2%, its impact on disability, lost productivity, and cost on healthcare cannot be overlooked. Due to the pervasiveness of knee pain in the general population, there are no shortages of treatment options available for addressing the symptoms. Ranging from physical therapy and pharmacologic agents to interventional pain procedures to surgical options, practitioners have a wide array of options to choose from – unfortunately, there is no consensus on which treatments are “better” and when they should be offered in comparison to others. While it is generally accepted that less invasive treatments should be offered before more invasive ones, there is a lack of agreement on the order in which the less invasive are to be presented. In an effort to standardize the treatment of this extremely prevalent pathology, the authors present an all-encompassing set of guidelines on the treatment of knee pain based on an extensive literature search and data grading for each of the available alternative that will allow practitioners the ability to compare and contrast each option.
Keywords: knee, knee pain, genicular nerve, ablation, regenerative medicine, platelet-rich plasma, dorsal root ganglion, peripheral nerve stimulation
Introduction
Background
Knee pain affects tens of millions of people in the United States annually – the pain can be disabling and often negatively impacts the patient’s quality of life, function and can even prevent one’s ability to simply ambulate across a room. While osteoarthritis is the most common cause of knee pain,1 there are numerous other lesser known causes that can present in a variety of ways with significant overlap between the various diagnoses. As one would expect from a condition with such a large incidence, there are a wide variety of treatments available to treat knee pain; however, there is no consensus on which treatments should be offered over others and in what order. The purpose of these guidelines is to consolidate the data into one document on the various modalities available, ranging from medication and physical therapy to interventional treatments and joint arthroplasty, thus offering practitioners a sing
These clinical guidelines are based on a systematic review of published studies examining the conservative, interventional, and surgical treatment options for the most common sources of knee pain in adults: knee osteoarthritis, post-surgical knee pain, soft tissue injury to the knee, and complex regional pain syndrome (CRPS) involving the knee joint. The intent and purpose of these guidelines is to help practitioners integrate the current evidence into clinical practice. Given the broad nature of the topic, effort was placed on reviewing recent meta-analyses and systematic reviews to summarize key findings and recommendations.
Incidence
Knee pain is a common reason for patients to present to primary care physicians and pain medicine specialists alike. An estimated 25% of Americans over the age of 55 have constant knee pain,2 and the most common underlying etiology is osteoarthritis.3 Knee osteoarthritis is more common in the geriatric population with increasing incidence with age.4 Up to one in five people over the age of 50 reports severe difficulty with physical function due to knee pain even without a formal diagnosis of osteoarthritis.4 Extrapolating this to the United States population reveals that up to 21 million Americans are suffering with reduced ability to function due to knee pain.5
As we see a rise in the aging population and obesity rates, the incidence and prevalence of knee pain is expected to rise. For the most common culprit of knee pain, knee osteoarthritis, the incidence and prevalence are difficult to accurately define, given knee osteoarthritis can exist radiographically without symptoms or clinically due to symptomatic pathology. The pooled global incidence of knee OA was 203 per 10,000 person-years in individuals aged 20 and over.6 Not all patients with knee pain suffer from symptomatic osteoarthritis, however. With the rise in access to healthcare and surgery, the incidence of persistent post-surgical knee pain after total knee arthroplasty is also a source of chronic knee pain. Despite good outcomes for most patients who undergo total knee arthroplasties, approximately 20% of the patients experience chronic pain after total knee arthroplasty (TKA).7 Among the post-arthroplasty population, the incidence of neuropathic post-surgical pain, concerning for CRPS, can be as high as 34%.8 Ultimately, the rising prevalence of chronic knee pain will present challenges within our healthcare systems as well as economic costs associated with them. These guidelines hope to provide evidenced-based decision support to physicians and other healthcare providers to improve the quality and efficiency of care.
Economic Burden of Knee Pain
Osteoarthritis is a prevalent and disabling condition currently affecting 40 to 50 million Americans, with approximately 10–30% of those afflicted having significant pain, impaired function, and decreased quality of life.1,9,10
The pain and loss of function can be debilitating; in developed countries the resultant socioeconomic burden is large, costing between 1% and 2.5% of gross domestic product.11 The socioeconomic burden of knee and hip OA alone averages more than $12,000 per patient annually in both direct and indirect costs of disease.12 In 2015, the average cost of TKA was approximately $16,000 per discharge, summing up to almost $10 billion in inpatient costs alone.13 Time lost from employment and leisure by participants and their unpaid caregivers accounted for 80% of the total cost.12 A 2017 study found that, when compared to healthy controls, knee OA patients had significantly more per-patient-per-year outpatient and pharmacy claims and costs. Knee OA patients incurred $7707 more per-patient-per-year total healthcare costs than controls.14
In a national, cross-sectional, population-based study, it was found that about one-third of the population aged 50–64 had OA and more than half were out of paid work. Only knee OA was associated with early exit from work. The estimated annual cost of early exit from work attributable to OA was €384 per capita, €1294 per OA patient and €2095 per OA patient out-of-work.15 In another study, it was estimated that mean health losses due to knee OA over people’s lifetimes are 3.44 quality-adjusted life years (QALYs) per person.16
Opioids
The United States accounts for 5% of the worldwide population yet consumes roughly 80% of opioids worldwide.17 As a nation, we have a obvious proclivity for choosing opioids to treat pain, even in cases where other treatments have been shown to be more effective and safer long term – as is the case when it comes to treating knee pain. From 2004 to 2014, 16% of the patients presenting with knee pain and osteoarthritis were prescribed opioid medications for treatment.18 Despite this, there are no data supporting the effectiveness of chronic opioids for either pain or functional improvement in patients with osteoarthritis.19 In fact, only about 35% of the patients who take opioids for osteoarthritis report pain improvement.20 This is compared to 31% of the patients who were given placebo for similar pain.20 Similar numbers are revealed when we evaluate physical functional improvements in response to opioids or placebo.20 While opioids have not demonstrated benefits, they definitely have their risks. Opioid-naïve patients who are prescribed an 8-day supply of opioids have a 13.5% probability of continuing opioid therapy at 1-year.21 If this is increased to >30 days, the probability increases to 29.9%.21 With the Centers for Disease Control (CDC) reporting 46,802 opioid overdose deaths in 2018,22 the risk of opioid therapy in this population is felt to be greater than any potential benefit, with the exception of high-risk patients with limited alternative therapies.
Despite the presence of a plethora of suitable alternatives (most of which possess high levels of evidence to support their utility), practitioners continue to recommend opioids for the treatment of knee pain – even though there is little-to-no evidence to support their use in this particular setting. One of the principal goals of these guidelines is to illustrate just how many evidence-based treatment options that are available for knee pain that go beyond a seemingly innocuous prescription for opioid pain medication.
Methods
The Consensus Guidelines on Interventional Therapies for Knee Pain (STEP) panel is composed of participants considered experts in the field and was selected by the American Society for Pain and Neuroscience (ASPN) executive board after receiving nominations. The board’s approach ensured diversity across practice locations and panelist demographics. Consideration was given to research experience, clinical experience, prior publications, work in the field, professional specialty, and speaking engagements. Invitations were sent, and upon acceptance, writing assignments were made. Database searches used replicable methods and are presented with outcomes, in each of the recommendations sections below. Multiple panelists contributed to the same topic in order to ensure consensus across experts. Panel members recused themselves from any section with actual or perceived conflict of interest. A third party was employed to edit the overall documents once panel members drafted their sections.
Literature search and summary methods were in accordance with the US Preventive Services Task Force (USPSTF) criteria for evidence level and degree of recommendation.23 In treatment areas with early or incomplete literature the expert opinion of the panel contributors was presented, expert consensus was sought to fill gaps in knowledge. USPSTF criteria for evidence levels, meaning of recommendation degrees, and strength of consensus, assuming a quorum of 80% of the participants available for vote, appear in Tables 1–3.23
 |
Table 1 USPSTF Hierarchy of Studies by the Type of Design |
 |
Table 2 USPSTF Meaning of Recommendation Degrees |
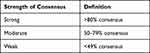 |
Table 3 USPSTF Strength of Consensus |
Evidence was quality ranked according to the following methods:
- Evidence Level 1 evidence is as a score of 39 or greater on the randomized controlled trial (RCT) and observation study score sheet (out of 48) on the Interventional Pain Management—Quality Appraisal of Reliability and Risk of Bias Assessment (IPM) score sheet24 and 10–12 on QAREL score sheet25 and 10–13 on Cochrane.26
- Level 2 evidence is a score between 29 and 38 on the RCT and observational study score sheet (out of 48) on IPM procedure scores sheet24 and 8–9 on QAREL25 and 8–9 on Cochrane.26
- Level 3 is a score between 16 and 28 on IPM procedures score sheet24 and 6–7 on QAREL25 and 6–7 on Cochrane.26
- Is a score <16 on IPM score sheet24 and <6 on QAREL25 and Cochrane score sheet.26
Additionally, a qualitative modified approach to grading of evidence is in Table 4, modified from Manchikanti et al27
 |
Table 4 Level I–V Definitions |
Lastly, a guide for strength of recommendations is also available via the National Guideline Clearinghouse Extent Adherence to Trustworthy Standards (NEATS) instrument (see Table 5).28
 |
Table 5 NEATS Recommendations |
Patient Evaluation and Imaging
Physical Exam
As in all areas of medicine, a thorough physical exam is a crucial step in the work-up and diagnosis of knee pain. While imaging can provide a clear answer as to what pathology may or may not present within the knee joint, it may not be able to definitively diagnose what the pain generator is. An examination of the knee should start with basic inspection to assess for swelling or skin changes. There are a number of pathologies in the knee that may cause swelling or edema in or around the joint (ie, osteoarthritis, Baker’s cyst, infection, soft tissue injury, etc) and would likely require further work up. Skin changes, such as erythema, shiny appearance, or loss of hair, may suggest CRPS or infection depending on the precipitating factors leading up to the symptoms.
Next, the provider should perform a cursory exam on the joint that includes
- Muscle strength: extension (quadriceps femoris, L3, femoral nerve); flexion (hamstrings, S2, sciatic nerve)
- Range of motion: 0° to 135°
- Sensation: L3 – medial aspect, L4 – anterior aspect, L5 – lateral aspect, S1 – posterior-lateral aspect, S2 – posteriod-medial aspect
- Palpation: posterior tenderness is common with Baker’s cysts; medial or lateral tenderness may indicate a soft tissue injury (ie, meniscus, collateral ligament, etc)
The final, and perhaps most important, part of the exam involves performing a series of “special” maneuvers – these are a series of standardized examination techniques that specifically stress certain parts of the knee to reveal if a particular part or aspect has been injured (Table 6).29–31
 |
Table 6 “Special” Maneuvers for Examining the Knee |
X-ray, MRI, CT, Bone Scan
- X-ray: According to the American College of Radiology (ACR) Appropriateness Criteria, radiographs should be the initial imaging modality utilized for the evaluation of knee pain. Radiographs provide adequate evaluation of the joint space, osteophyte and subchondral cyst formation, displaced or chronic stress fractures, joint effusions, and sclerosis in the subarticular region.32,33
- Magnetic resonance imaging (MRI): If radiographs are normal, or demonstrate a joint effusion, the ACR recommends an MRI without intravenous (IV) contrast as the next most appropriate study. MRI accurately demonstrates the soft tissues of the knee, without the use of ionizing radiation, and depicts tendon and ligamentous damage, tears and other abnormalities of the meniscus, the presence of synovitis, the extent of effusions, the presence and/or rupture of a popliteal cyst, the extent of cartilage loss, bone marrow lesions, subchondral insufficiency fractures, tibial stress fractures, and osteonecrosis.32,34
- MRI with and without IV contrast is not usually indicated when initial radiograph is negative or a joint effusion is detected. However, the utilization of IV contrast may be beneficial to help more accurately diagnose such causes of chronic knee pain as infrapatellar bursitis, adhesive capsulitis, patellofemoral friction syndrome (“runner’s knee”), pigmented villonodular synovitis (PVNS), and Hoffa’s fat pad syndrome.32,35
- Computed tomography (CT): While not a first-line imaging modality for the evaluation of knee pain, according to the ACR, under certain circumstances, a CT without IV contrast may be indicated for the evaluation of knee pain, especially in settings where MRI may be contraindicated (ie, in a patient with a non-MRI compatible pacemaker, spinal column stimulator, or deep brain stimulator). CT offers enhanced bony detail and may be useful to confirm a prior osseous injury such as a subtle acute non-displaced fracture or chronic stress fracture. In the setting of chronic knee pain related to patellofemoral syndrome, CT may be helpful in evaluating the patellofemoral anatomy.32,35 CT with IV contrast is not usually indicated but may be used to evaluate the menisci, articular cartilage, and the presence of loose bodies.32
- Bone scan: Bone scan is not usually indicated to evaluate patients with knee pain. Bone scan has low specificity and decreased anatomic resolution when compared to CT or MRI. However, bone scan may help to distinguish between bone and soft-tissue origins for pain.32,33,35
- Ultrasound: Ultrasound is not often useful as a diagnostic tool for the comprehensive examination of the knee. It may be appropriate to use ultrasound to confirm a suspected effusion or popliteal cyst, and can be utilized to guide in aspiration of synovial fluid which can be evaluated for crystal disease or infection.32 It also may be utilized to evaluate for synovial pathology, where the use of power Doppler ultrasound can demonstrate increased synovial blood flow that is associated with knee pain.32,36,37 Ultrasound is also useful for following patients with iliotibial (IT) band syndrome and in evaluating medial plicae.32,34 Ultrasound can also demonstrate an extrusion of the meniscus (with is suggestive of an underlying meniscal tear) and, on occasion, can detect peripheral meniscus tears, and chondrocalcinosis.32,34
Kellgren–Lawrence Scale
The Kellgren and Lawrence system is a widely used method by which osteoarthritis (OA) is graded radiographically. Originally published in 1957, and still in use today, the method classifies OA by five grades:
- Grade 0 (none): nothing on X-ray
- Grade 1 (doubtful): suspect OA
- Grade 2 (minimal): OA definitely present, yet minimal
- Grade 3 (moderate) moderate osteophytes, joint space narrowing, may be some sclerosis or bony deformations
- Grade 4 (severe) severe joint space narrowing, large osteophytes, severe sclerosis and bony deformity.38
The scale has both interobserver and intra-observer validation for reliability and with diagnostic accuracy.39 The Kellgren–Lawrence scale is limited by its ability to comment on disease progression and detect changes as it assumes linear disease progression starting with osteophytes and then to joint space narrowing, ending in joint deformation, the latter not being able to be measured without the former being present.39
Common Conditions Causing Knee Pain
Sprains
- Background: Knee sprains include injury to any of the ligamentous structures within the knee joint. Major ligaments include the anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament (MCL), and lateral collateral ligament (LCL) and will be discussed here. Additionally, there are the anterolateral ligament (ALL), transverse meniscal ligament, posterior meniscofemoral ligament, oblique popliteal ligament, arcuate ligament, popliteofibular ligament, and the ligaments that form the joint capsule.
- Etiology: Ligamentous knee sprains are due to trauma or sports injury, both with contact and non-contact forces. The ACL is commonly injured during plant and cut maneuvers due to increased knee abduction during hip adduction and knee valgus during hip internal rotation.40 Additionally, internal rotation of the tibia increases risk of ACL strain. These three movements are combined in landing from jump with hip extended and internally rotated, knee extended and valgus, tibia internally rotated, and foot planted.40 Hewett et al evaluated ACL injury mechanics in female athletes and found that abduction angle was 8° greater in ACL injury and those with ACL injury had 2.5 times greater abduction moments.41 PCL injuries are usually concomitant with injury to other ligaments particularly when varus or valgus stress or rotational force is involved. The PCL is injured with posterior translation of the tibia in relation to the femur. Force is required and a PCL tear is commonly known as a “dashboard injury” which occurs when the flexed knee hits a motor vehicle dashboard during a collision and is translated posteriorly, or during hyperflexion of the knee with the foot in plantar flexion, such as falling onto the flexed knee.42
- MCL injuries can occur in isolation or in combination with the ACL or PCL. The MCL originates at the medial epicondyle of the tibia deep to the pes anserine and inserts 5–7 cm below the joint line. It functions as the primary medial knee stabilizer and resists valgus stress, anterior tibial translation, and internal rotation. The most common mechanism of injury to the MCL is direct lateral force to the knee or maneuvers that induce severe valgus stress to the knee, and injury most often occurs at the femoral insertion.43 The LCL is the primary stabilizer of the lateral knee, originates at the lateral epicondyle of the femur and inserts on the medial fibular head. It resists varus stress, external rotation and posterior displacement of the fibula; therefore, it can be injured by contact and non-contact forces in these planes. Common mechanism of injury is direct varus force to the medial knee or hyperextension stress across the knee.44
- Diagnostic Criteria: Sprains are graded based on severity: Grade 1 – mild, painful stretching or minimal tearing of fibers, Grade 2 – moderate, painful, partial tearing of fibers, Grade 3 – severe, painful or sometimes not painful, complete rupture of ligament and may demonstrate instability. Clinical physical exam findings: ACL – knee line pain, positive Lachman’s test. PCL – dimple sign, posterior drawer test, posterior sag test, quadriceps active test, external rotation recurvatum test.42 MCL – medial joint line pain and tenderness, positive valgus stress test. LCL – lateral joint line pain and tenderness, lateral joint edema, positive varus stress test, lateral compartment laxity with figure 4 position. History and physical exam support the clinical diagnosis, and imaging should include weight—bearing knee series and stress radiographs; however, the gold standard for diagnosis of ligament injury in the knee is MRI.
- Treatment: Symptomatic pain emanating from knee sprains is typically managed conservatively with topical or oral non-steroidal anti-inflammatory drugs (NSAIDs) in those without contraindications as well as alternating ice and heat. From an orthopedic perspective, grade I and II sprains are treated with progressive weight bearing as tolerated, bracing, gentle active assisted range of motion, and strengthening of surrounding supportive musculature and are specific to each ligament involved. Some isolated Grade 3 sprains are treated with a longer period of immobilization and bracing, toe touch weight bearing, followed by aggressive range of motion (ROM); however, combined injury or severe Grade 3 sprains with complete ligament rupture can be treated surgically, particularly if the ACL is involved.43
Meniscal Injuries
- Background: The menisci function to absorb shock and transmit load forces across the femorotibial joint and protect the articular cartilage. The medial and lateral menisci are composed of layers of collagen fibers. The superficial layer is finely woven, the surface layer comprises randomly oriented fibers, and the deepest layer is a combination of circumferential and radial fibers. The menisci are located between the medial and lateral femorotibial joints. The medial meniscus is attached to the tibial plateau by the coronary ligaments and is C-shaped. The lateral meniscus is circular and more mobile with loose attachments. The outermost 3 mm is the “red zone”, is well vascularized from the peri-meniscal plexus off the genicular arteries. Most tears in the red zone will heal. Within 3–5mm from the capsular junction is the “white zone”, with reduced perfusion, some tears will heal. The remaining meniscus >5mm from the capsular junction makes up the inner two-thirds, is avascular, receives nutrients from the synovial fluid, and most tears will not heal. Meniscus tears cause knee pain by direct effect on the nociceptors in the meniscus tissue and synovium, as well as by elevated levels of intra-articular cytokines.45
- Types of traumatic tears:
- Longitudinal vertical tear: if lesion stable then conservative management. Surgical debridement vs repair for unstable lesions.
- Radial tear: debridement vs surgical repair.
- Root tear: often associated with ACL tear, debridement vs surgical repair.
- Types of degenerative tears:
- Horizontal cleavage in young athletes: rare, due to overuse, if cessation of the activity fails then proceed to surgical intervention.
- Degenerative meniscal lesions in the elderly: prevalence increases with age, 60% asymptomatic, associated with osteoarthritis, conservative management.45
- Diagnostic criteria: Clinical presentation includes joint line pain, knee locking, and positive special tests including McMurray, Ege, and Thessaly due to their high sensitivity and specificity.45 Radiologic evaluation with weightbearing AP, lateral, and Schuss views are first-line evaluation to assess for alternative sources of pain such as osteoarthritis. If pain persists despite conservative therapy, MRI is obtained to evaluate the menisci.
- Treatment: Similar to sprains, pain secondary to meniscal injuries is managed symptomatically (ie, PT and NSAIDs). If the pain fails to respond to conservative care and/or persists beyond the acute phase, intra-articular injections should be considered.
- Intra articular platelet-rich-plasma injection has not been identified to improve meniscus healing. If the joint is unstable and/or non-invasive treatment modalities fail, surgical intervention is usually indicated, depending on the type and severity of the tear.46
Tendinopathy
- Background: Tendinopathy is characterized by pain and dysfunction in a tendon, commonly due to excessive load or strain. In active patients and athletes, overuse can lead to chronicity.47 The most common tendinopathy in the knee is of the patellar tendon, is colloquially known as “jumper’s knee” since it is commonly seen in jumping athletes, and can lead to microtears at the tendon insertion at the distal pole.
- Diagnostic criteria: Diagnosis is based on history and physical exam. Patients with patellar tendinopathy present with anterior knee pain that is worse with activity, tenderness to palpation at the inferior pole of the patella. Other tendinopathies present with tenderness to palpation and pain at the tendon insertion site with activation of the muscle. Radiologic imaging will be normal. Ultrasound can assist in confirming diagnosis and will show loss of the normal fibrillar pattern, increased spacing between fibrillar lines, and generally reduced echogenicity.48 MRI will show increased signal at the tendon insertion.
- Treatment: Pain secondary to tendinopathy is treated symptomatically in the acute phase – this includes NSAIDs and physical therapy with a progressive loading program. Additional adjuncts for pain control such as cryotherapy, peritendinous injection with corticosteroids or platelet-rich plasma (PRP), and extracorporeal shockwave therapy can be considered if the pain fails to respond to NSAIDs and PT and/or the pain persists beyond the acute phase.49 Ultrasound-guided needle tenotomy, percutaneous needle scraping, and high volume injection, and stem cells are at the forefront of regenerative medicine for tendinopathy and are currently being evaluated.50 If non-invasive management fails, surgical intervention would be the next step.49
Bursitis
- Background: A bursa is a sac filled with synovial fluid that acts as a friction cushion between structures. There are 10 bursa within and around the knee, and the 4 major bursa are the prepatellar, suprapatellar, infrapatellar, and pes anserine bursa. When friction or trauma irritates a bursa, it can become inflamed and be a significant source of knee pain. Bursitis is clinically recognized by localized pain, point tenderness, and edema at the sight of the bursa. Septic bursitis may also present with erythema, warmth, and systemic symptoms such as fevers and leukocytosis.
- Etiology: Superficial to the patella is the subcutaneous prepatellar bursa, which can become inflamed with kneeling activities, and is colloquially called “housemaid’s knee or carpenter’s knee”. Inferior to the patella are two bursae, subcutaneous infrapatellar bursa which lies superficial to the patellar ligament, and the deep (subtendinous) infrapatellar bursa which lies deep to the patellar ligament. Inferomedial knee pain may be caused by anserine bursitis. The anserine bursa lies deep to the pes anserinus comprising the tendinous attachments of the semitendinosus, gracilis, and sartorius. Proximal to the anserine bursa and deep to the semimembranosus tendon is the semimembranosus bursa. There are three lateral bursae: the bursa deep to the iliotibial tract, bursa deep to the lateral collateral ligament, and the inferior subtendinous bursa deep to the biceps femoris tendon. Posteriorly, there are two subtendinous bursa, one deep to the medial and lateral heads of the gastrocnemius. A bursa can become inflamed by overuse leading to increased friction across the bursa. Septic bursitis requires bacteria to enter the bursa and is associated with trauma.
- Diagnostic criteria: Physical exam is significant for pain and point tenderness overlying the affected bursa and may be associated with swelling. Prepatellar bursitis will present with egg-shaped swelling superficial to the patella. Septic bursitis may present with erythema and warmth. Radiologic knee series are usually normal. Evaluation with ultrasound will show a hypoechoic fluid filled sac or discrete fluid collection at the site of pain indicating bursitis.51,52
- Treatment: The treatment of bursitis-related knee pain is typically focused on reducing the inflammation of the bursa, itself – this includes ice, NSAIDs, activity modifications (such as knee pads for prepatellar bursitis), injections of corticosteroid, and therapeutic aspiration. For patients with active lifestyle or occupational demands and non-septic bursitis, intrabursal injection of corticosteroids provides acute pain relief. If septic bursitis is suspected, infectious workup includes blood sampling for evaluation of leukocytosis and aspiration of bursal fluid with gram stain and cell count. Treat septic bursitis with antibiotics. Recurrent or persistent severe bursitis may benefit from surgical interventions such as bursectomy.53,54
Osteoarthritis
- Background: Osteoarthritis of the knee is a common source of knee pain, increases with advancing age, and is more prevalent in women than in men.
- Etiology: It is a disorder that develops after macro and micro-trauma leads to maladaptive repair response, activation of the pro-inflammatory immune response, and is characterized by cell stress and extracellular matrix degradation.55 Mechanical stress, varus and valgus malalignment lead to excessive loading of bone and subsequent development of bone marrow lesions, abnormal focal remodeling, and loss of articular cartilage. Osteoarthritis can involve one or both knees and can occur in any of the three compartments of the knee, most commonly in the medial compartment. Symptom onset is gradual, can be localized or generalized joint pain that is worse with weight bearing and joint motion, and relieved with rest. Morning stiffness resolves in less than 30 minutes and the joint may stiffen briefly after inactivity. Clinical exam findings include bony enlargement, small effusions that are body temperature, and crepitus with joint motion.56
- Diagnostic criteria: Diagnosis can be made based on history and physical exam. Standing radiographs may not show pathology in early OA so normal radiological findings do not exclude OA. Radiologic findings include osteophytes, subchondral cysts, subchondral sclerosis, and joint space narrowing which is graded using the Kellgren–Lawrence grading system (discussed previously).56,57 MRI is used to assess soft tissue if associated injury is suspected; however, it is rarely indicated for diagnosis of osteoarthritis. Ultrasound is an inexpensive and portable way to visualize the knee joint and assess for effusions and osteophytes; however, it is not accurate when assessing degree of joint space narrowing.56
- Treatment: With the exception of joint arthroplasty and regenerative therapies, most treatments for knee pain secondary to OA are palliative in nature. Treatments are tiered depending on the stage and level of disability of the patient. Conservative management includes activity modifications, weight loss if appropriate, exercise, physical therapy, and education. NSAIDs should be considered first line for “as-needed” symptomatic pain control.19,20,58,59 Opioids have minimal benefit in OA and are associated with undesirable side effect profile and safety risks, therefore use is discouraged.60 Intra-articular injections with corticosteroid or hyaluronic acid (HA) are well-accepted treatments for temporary symptomatic relief. Biologics such as PRP or mesenchymal stem cells have been proposed to attenuate the pro-inflammatory degradation in OA and potentially remodel the joint.61 Several adjunct medications such as cathepsin K inhibitors, Wnt inhibitors, anabolic growth factors, nerve growth factor inhibitors are being studied as a means of not only reducing the pain from knee OA but potentially stopping the progression of structural damage. For patients with advanced knee osteoarthritis who failed conservative therapy and who are candidates, total joint replacement is definitive treatment.56
CRPS
- Background: CRPS is a chronic neurologic pain condition caused by trauma and is characterized by the presence of autonomic dysfunction, persistent regional inflammatory changes, lack of dermatomal distribution. Clinical presentation often includes allodynia, hyperalgesia, and skin temperature changes. It is formally known as “causalgia” or “reflex sympathetic dystrophy”. CRPS is divided into Type I (formerly reflex sympathetic dystrophy) due to trauma with no associated major nerve injury, and Type II (formerly causalgia) due to trauma or surgical insult in the presence of major nerve injury.62 CRPS occurs in the peripheral limbs and therefore is a source of knee pain.
- Etiology: CRPS is a combination of nervous system sensitization, autonomic dysfunction, and inflammatory changes in response to injury. Shortly after injury, inflammatory factors (tumor-necrosis factor alpha and prostaglandin E2) are released which leads to peripheral nociceptive sensitization and subsequent hyperalgesia. It is thought that persistent firing of Aδ and C afferent neurons fire persistently leading to engagement of the autonomic nervous system and the distinct CRPS symptomatology. Peripheral nociceptors become sensitive to catecholamines after injury. There is probable chronologic change in the peripheral nervous system leading to degradation of large somatomotor A⍺ neurons and preservation of Aδ fibers.
- Continuous firing of peripheral nerves also leads to increased synaptic firing in the dorsal horn. This process is mediated by neuropeptides including glutamate and Substance P, leading to allodynia and hyperpathia. Over time, the central nervous system responds by central reorganization of motor and sensation pathways.
- Autonomic dysregulation is thought to be due to coupling of adrenergic and nociceptive pathways. During the acute phase, studies have shown circulating catecholamines and norepinephrine, which explains vasodilation, edema, and change in limb temperature. Over time, this leads to catecholamine sensitivity, which explains the vasoconstriction, cold temperature, and clammy skin seen in chronic CRPS.
- The innate immune system also plays a role. Mast cells release cytokines and neuropeptide levels increase (substance P and gene-related-peptide) leading to elevated inflammatory factors (TNF, IL-1b, IL-6, nerve growth factor) and subsequent peripheral sensitization to noxious stimuli.62
- Diagnostic criteria: The Budapest criteria state that patients must meet all four criteria for diagnosis of CRPS (Table 7).63
- Nerve damage can be diagnosed by electromyography (EMG).64 Sudeck atrophy is a radiologic finding consistent with CRPS and includes diffuse osteopenia with juxtacortical demineralization, and subchondral cystic changes.65 Stellate ganglion block causing sympathetic blockade with associated reduction in symptoms has been shown to be efficacious for treatment and confirmation of CRPS Type 1 diagnosis.66
- Treatment: As is the case for the treatment of CRPS in any body part, a proactive, multidisciplinary approach that includes pain management, psychiatric, physical therapy, primary care, and case worker/social work at time of diagnosis is important. Treatment is divided into acute and chronic phases. Acute treatment focuses on pain control with local nerve blocks and rehabilitative modalities to alleviate pain, manage edema, and prevent contractures. Pain management includes systemic steroids, tramadol, gabapentin, antidepressants, ketamine, calcium channel blockers, bisphosphonates, and baclofen. Therapeutic modalities include edema control, range of motion, mirror therapy, graded motor imagery, acupuncture, biofeedback, stress loading, and aerobic conditioning. Chronic pain that is refractory to acute treatment is managed by progressing to spinal cord stimulator, dorsal root ganglion stimulator, or botulinum toxin (Botox) injection. Palliative surgical treatment includes nerve decompression, resection of neuromas, joint contracture release, and amputation.62,64,65,67
 |
Table 7 Budapest Criteria for CRPS Diagnosis |
Chondromalacia
- Background: Patellar chondromalacia is softening of the patellar articular cartilage and is a common source of anterior knee pain. Patellar chondromalacia is a precursor to patellar osteoarthritis.
- Etiology: The patella is in the trochlear groove and friction during knee flexion causes breakdown of the patellar cartilage. Malalignment of patellar tracking within the trochlear groove can increase this friction and is often due to vastus medialis oblique (VMO) weakness. The exact etiology is unclear; however, it is proposed that friction trauma to superficial chondrocytes results in a proteolytic enzymic breakdown of cartilage matrix.68
- Diagnostic criteria: Anterior knee pain that may be exacerbated by squatting, jumping, rising from sitting, or ascending/descending stairs. Retro-patellar crepitus may be present during knee range of motion, joint effusion, and >2 cm quadriceps wasting support chondromalacia. Tenderness over the medial or lateral patellar facets suggests progression to osteoarthritis. Special tests include patellar grind and patellar apprehension. Radiologic imaging with Merchant or skyline view is useful for evaluating the patellofemoral compartment.69 Sagittal-patellar tilt should be taken into consideration during evaluation if MRI is obtained. Aksahin et al70 evaluated patellar chondromalacia with MRI and found that sagittal plane malpositioning and chondral lesions might be related to chondromalacia. Tuna et al71 found that patellar tilt and trochlear dysplasia were related to the presence of chondromalacia.
- Treatment: Pain control for mild cases of chondromalacia is typically managed with NSAIDs, bracing, weight loss (if appropriate), activity modification, and a physical therapy regimen that focuses on stretching the VMO and strengthening the lower extremity kinetic chain.69 In a systematic review of outcomes, faster VMO reflex time was associated with improvement after exercise intervention.72 Moderate cases of chondromalacia may require intra-articular injections of corticosteroid or hyaluronic acid. If conservative management fails after 3–6 months, surgical intervention may be warranted.69 Biologic treatments including PRP and stem cell therapy are of particular interest due to their ability to potentially slow or even reverse progression of cartilage degradation. A study of autologous chondrocyte implantation (ACI) to patellar cartilage defects resulted in significant functional improvement at minimum 2 years and lasted up to 15 years.73 Surgical modalities include tubercle osteotomy to correct lateral maltracking, realignment, patellectomy, patellofemoral replacement, and total knee replacement.69
Post-Surgical Knee Pain (PSKP)
- Background: PSKP can be a difficult condition to diagnose, especially in the acute phase, as reports of pain after surgery are to be expected – it is not until several weeks, or even months, into the postoperative period that a practitioner will begin to suspect something is “wrong.” Even then, once any complications with the surgery (ie, infection, prosthesis malfunction, fracture, etc) have been ruled out, the usual assumptions are either malingering or drug seeking. For these reasons, PSKP tends to be underdiagnosed and its true incidence is difficult to measure as many patients reporting symptoms of PSKP are simply taken back to the operating room for subsequent surgeries. For example, many patients who undergo arthroscopic knee surgeries for knee OA eventually undergo total knee arthroplasty (TKA). A systematic review reports the overall incidence of TKA after knee arthroscopy is 2.62% and increases to 3.98% when selecting for older patients. The mean and median time from arthroscopy to TKA was 3.4 and 2.0 years, respectively.74 Annual revision rates of TKA are 0.49%, compared to revision rates of medial unicompartmental arthroscopy (1.07%), lateral unicompartmental arthroscopy (1.13%), and patellofemoral arthroscopy (1.75%).75 Nearly 20% of the patients experience suboptimal results following TKA, and residual post-TKA pain is a common complaint.76 Predictors of increased residual post-TKA pain include other pain sites, catastrophizing, and depression.77
- Etiology: Post-TKA pain can be caused by intrinsic or extrinsic etiologies. Table 8 outlines the possible causes.
- Diagnostic criteria: Assessment of residual post-TKA pain includes detailed history, review of surgical reports, and physical exam. Acuity, onset, nature, exacerbating factors can help distinguish between intrinsic and extrinsic causes. Physical exam includes visual inspection including joint alignment, palpation, range of motion, patellar tracking and stability in coronal/sagittal planes during range of motion, gait, and exam of the hip and spine. Preoperative and postoperative radiologic images should be reviewed. Obtain weight bearing anterior-posterior, lateral, and merchant views of the knee. Additionally, weightbearing films of the entire leg, hip, and ankle will assess varus or valgus abnormalities. Diagnostic intra-articular local anesthetic injection will support diagnosis of intra-articular source if pain is relieved within a few minutes of injection.76
- Treatment: Treating knee pain secondary to surgery can be extremely complicate given the fact that it may not be readily apparent what the pain is consequential to. Is it merely postsurgical discomfort that is taking longer than expected to heal, is it the result of a nerve injury (ie, CRPS Type II), a new pathology created by the surgery itself, or something else entirely? An unfortunate confounding factor that negatively impacts these situations is the presence of opioids as these patients will all inevitably be taking due to their having just had a surgical procedure. As such, multimodal analgesia is the optimal perioperative pain control regimen and reduces long-term opioid use with improved patient outcomes. This includes a combination of preemptive analgesia, neuraxial anesthesia, peripheral nerve blockade, patient controlled analgesia, local infiltration analgesia, and oral opioid/non-opioid medications.78 Yu et al report discontinuing patient-controlled analgesia and femoral nerve blocks from the multimodal regimen and replacing them with liposomal bupivacaine resulted in less overall opioid consumption, with no difference in functional recovery or reported pain control.79 In patients with centrally sensitized knee pain prior to TKA, duloxetine should be considered to minimize post-TKA pain.80 For patients with persistent post-TKA pain, physical therapy, range of motion, and transcutaneous electrical nerve stimulation (TENS) are conservative measures that are effective.81 Interventional therapies to consider include nerve blockade, peripheral nerve neuromodulation, and dorsal root ganglion neuromodulation.
 |
Table 8 Intrinsic or Extrinsic Etiologies of Post-TKA Pain |
Recommendations Regarding Conservative Care
Medication
NSAIDs
Twenty-one randomized control trials were included in this systematic review of oral NSAIDs for knee pain. Overall, three separate knee pain diagnostic groups had sufficient evidence to warrant inclusion. Twelve studies were included on knee osteoarthritis;82–93 eight studies were included on pain following total knee arthroplasty;82–85,94–101 and one study was included on patellofemoral pain syndrome.102 Of the studies regarding knee OA, five studies reported level 1 evidence in support of NSAID use, four studies reported level 2 evidence in support of NSAID use, and two presented level 3 evidence in support of NSAID use. Each of the supporting studies reported at least 30% reduction in knee pain with NSAID use. Of note, one study reported non-superiority of naproxen over placebo.85 Of considerable interest, cyclooxygenase-2 (COX-2) inhibitors were as effective as non-selective NSAIDs across studies, with several reporting enhanced analgesia and all describing a decreased incidence of adverse side effects, primarily GI upset, with COX-2 inhibitors. One RCT even described superiority of celecoxib 20 mg qd over tramadol 300 mg extended release, with each superior to placebo.89
Regarding pain following total knee arthroplasty, each of the eight included studies supported the use of NSAIDs, with seven reporting level 1 evidence and one study reporting level 2 evidence. Consistent with the bulk of evidence for COX-2 inhibitors in knee osteoarthritis, COX-2 inhibitors were also supported across all studies, with sustained analgesic benefit noted after from COX-2 inhibitor use in the first weeks following arthroplasty.
Lastly, one study described a significant analgesic benefit of acute NSAID use in patellofemoral syndrome versus placebo, with a 5-point reduction in visual analog scale (VAS) score in comparison to 2 point with placebo102 (see Table 9).
 |
Table 9 Evidence Table Regarding NSAIDs |
In summary, oral NSAIDs are moderately effective in controlling pain in patients with moderate-to-severe pain due to knee osteoarthritis and pain s/p total knee arthroplasty. COX-2 selective NSAIDs are similarly effective to non-selective NSAIDs in controlling pain with the advantage of a considerably improved safety profile, especially regarding gastrointestinal upset. While all NSAIDs are associated with an increased risk of acute kidney injury, patients with a history of hypertension, heart failure, or diabetes have higher chance of developing these complications.
Consensus Points for NSAIDs
- NSAIDs are an effective treatment for mild-to-moderate pain secondary to osteoarthritis knee pain; Level 1, Grade A, Consensus Strong
- Topical NSAIDs are recommended before oral treatments because of their lower systemic exposure/toxicity; Level 1, Grade A, Consensus Strong
- Topical diclofenac 70–81 mg/day should be considered as first-line pharmacological treatment for knee OA - can be effective and generally safer than oral NSAIDs due to reduced systemic exposure and lower dose; Level 1, Grade A, Consensus Strong
- NSAIDs should not be used for patients with comorbidities due to risk of adverse events; Level 1, Grade A, Consensus Strong
- NSAIDs should not be used on a long-term basis (>3 months) due to side effect profile (cardiovascular and gastrointestinal) and lack safety data; Level 1, Grade A, Consensus Strong
- Celecoxib and non-selective NSAIDs are as effective as opioid for knee OA; Level 1, Grade A, Consensus Strong
- Due to low effect on pain and physical function, regardless of dose, the potential clinical benefit of opioids does not outweigh the potential harm in patients with knee OA; Level 1, Grade A, Consensus Strong
Topicals
Out of the 17 studies included in this study, 6 reported level 1 evidence in support of the use of topical NSAIDs for knee osteoarthritis.103–108 9 studies reported level 2 evidence in support of topical NSAIDs109–117 one reported level 3 evidence in support of topical NSAIDs;118 and one study reported level 2 evidence not in favor of topical NSAID therapy.119 Adverse events were rare in all included studies, primarily limited to minor skin irritation at the site of application. The clinical effect of topical NSAIDs was modest at best in comparison to placebo, but all studies did report statistically significant improvements in pain reduction. There was insufficient evidence to support recommendations for other topical medications (eg, capsaicin, copper). Osteoarthritis of the knee was the only diagnosis with sufficient literature to support recommendations regarding topical NSAID therapy.
Importantly, five separate studies demonstrated non-inferiority to oral NSAID therapy. Of note, one study reported inferiority of topical NSAID therapy in comparison to placebo. Indeed, the placebo effect was readily apparent in each study, which is a well-known phenomenon found in trials of topical medications (see Table 10).
 |
Table 10 Evidence Table Regarding Topicals |
To sum, topical NSAIDs have established efficacy comparable to oral NSAID therapy in the treatment of knee osteoarthritis, with a vastly improved adverse side effect profile and decreased cost. Topical NSAIDs may be preferable for patients with knee osteoarthritis older than 75, those with comorbidities or at an increased risk of renal, cardiovascular, or gastrointestinal adverse events.
Consensus Points for Topicals
- Topical NSAIDs are an effective treatment for symptomatic knee osteoarthritis and can be utilized as part of as an adjuvant analgesic treatment plan; Level 1, Grade A, Consensus Strong
- Topical NSAIDs should be utilized before oral NSAID therapy for the treatment of knee osteoarthritis; Level 1, Grade A, Consensus Strong
Opioids
Due to the US opioid crisis, the use of chronic opioids for osteoarthritic, neuropathic, and non-surgical pain syndromes is currently under scrutiny.120 Prior to 2013, 15.9% of the patients with knee osteoarthritis were prescribed an opioid.18 While opioid prescribing for this condition has not been directly studied since, opioid prescribing has declined or all comers since 2013. Despite this, opioid prescribing for chronic knee pain continues to be routinely practiced.
Welch and colleagues performed a systematic review of 22 double blinded trials (8942 participants) which compared opioids for chronic osteoarthritic knee pain versus placebo.120 These authors found based on low-quality evidence that opioids provided no clinical relevant improvement in disability and no clinically relevant pain relief of 50% or greater.120 While there was no difference in serious adverse events compared to placebo, there was a relevant dropout rate for the opioid group due to side effects.120
Krebs et al conducted a randomized controlled trial comparing acetaminophen and non-steroidal anti-inflammatory medications in patients with osteoarthritis of the back, knee, and hip.121 While this study did not directly isolate knee patients, they found that there was no difference between the two groups in pain-related function and brief pain inventory interference.121 There was significant improvement in pain intensity in the non-opioid group when compared to opioids. Lastly, the opioid group had greater medication-related side effects and adverse events.121
There is limited peer-reviewed literature evaluating the benefits of opioids for chronic post-surgical knee pain specifically, but we do know that chronic opioid use has risks of opioid use disorder and overdose. Others have also detailed that chronic opioid use prior to total knee arthroscopy is an independent risk factor for persistent opioid use after surgery.122,123
There is also little peer-reviewed literature evaluating the benefits of opioids for neuropathic knee pain. Busse et al in a systematic review of opioids for non-cancer pain found that opioids, compared to placebo, were associated with small but significant improvements in pain and physical functioning in patients with neuropathic pain but also associated with side effects.124 Vergne-Salle specifically discussed opioids for neuropathic pain of the knee in an expert review and stated that opioids should only be used when other available treatments have failed.125 This is because high doses are often needed to provide the desired effect and these are associated with high morbidity and mortality.125
Consensus Points for Opioids
- Due to low effect on pain and physical function, regardless of dose, the potential clinical benefit of opioids does not outweigh the potential harm in patients with knee OA; Level 1, Grade A, Consensus Strong
- There is no evidence to support the use of opioids over NSAIDs for the treatment of knee osteoarthritis; Level 1, Grade A, Consensus Strong
- For the treatment of knee pain, opioids should be limited to the acute postoperative post-injury/trauma period; Level 2, Grade B, Consensus Strong
Tricyclic Antidepressants and Neuroleptics
While opioids and non-steroidals are the most prescribed oral medications for chronic knee pain, utility can be limited due to risk factors, side effects, and other causes of morbidity. In these situations, some have turned to adjuvant medications including both tricyclic antidepressants (TCA) and neuroleptic medications. Unfortunately, there is little evidence of their efficacy. A group in New Zealand studied the effectiveness of nortriptyline in lowering WOMAC scores in 205 patients with painful knee osteoarthritis.126 In this double-blind, prospective study, patients were randomized to a maximum dose of nortriptyline 100 mg daily or placebo. Both placebo and nortriptyline decreased WOMAC, but the difference between the decrease in WOMAC between the groups (6 points) was insignificant. Also, the nortriptyline group had significantly more side effects including dry mouth, constipation, and sweating. In an older study (1993) published in Pain, 50 mg amitriptyline was shown to have no benefit for patients after on post-operative days 1–3 when used as an adjuvant to opioids.127 Actually, patients randomized to TCA had a higher mean VAS score.
While ramosetron has demonstrated efficacy in decreasing post-operative nausea and vomiting, there is little evidence about its pain efficacy.128 It has been used in some large postoperative pain protocols, which have shown efficacy, but it has not been isolated as the beneficial component.129 While there is another large-scale trial to study the efficacy of amitriptyline for chronic knee OA, based on the current evidence, the authors do not believe that neuroleptics or tricyclic antidepressants currently have a place in the algorithm for the treatment of knee pain.130 However, TCAs may have a place in the treatment of neuropathic knee pain (ie, PSKP, CRPS, etc) as a adjuvant treatment, or potentially even as a standalone, whereas neuroleptics have not been found to be effective – more evidence is needed.
Consensus Points for Tricyclic Antidepressants and Neuroleptics
- TCA may be effective in the treatment of neuropathic knee pain, PSKP and/or CRPS when used as adjuvants and should be utilized as a first-line therapy; Level II-3, Grade C, Consensus Strong.
Antihypertensives
Hypertension and OA have a number of shared risks (ie, aging, obesity, chronic inflammation, etc) and often coexist with one and other as comorbidities. Given the significant overlap between these patient populations, antihypertensive medications have been routinely administered to patients with OA (particularly knee OA); however, it remains unclear as to whether or not they have an impact aside from their intended value on the cardiovascular system. The five medication classes of interest for knee OA include beta‐blockers, ACE inhibitors, angiotensin receptor blockers, CCBs, and thiazide diuretics. Beta-blockers have been shown to be associated with lower WOMAC scores and statistically significantly lower risk of joint pain, whereas other authors detected no evidence of analgesic effect.131,132 Calcium channel blockers, on the other hand, have been associated with higher pain scores and a higher prevalence of joint replacement.132–134 What is more concerning is the idea that calcium channel blockers may accelerate the process of OA by impairing the proliferation of chondrocytes.135 The evidence is extremely limited on the use of these classes of medication for knee pain, and recommendations cannot be made for or against their use.
Consensus Points for Antihypertensives
There is insufficient evidence to make any recommendations on the use of these medications for knee pain – more data is required.
Physical Therapy
Osteoarthritis (OA)
Conservative measures for the treatment of OA include physical therapy (PT) with the focus placed on improving aerobic capacity, quadriceps muscle strength and/or lower extremity performance. These treatment modalities have proven to be effective when performed under supervision at least three times per week for 4 weeks. These types of programs yield similar outcomes regardless of patient attributes to include the degree of severity of the OA.136
The 2014 meta-regression analysis of RCTs titled “Impact of exercise type and dose on pain and disability in knee osteoarthritis” reviewed 48 RCTs. Over the more than 4000 patients studied, it was determined that therapy programs focusing on one single modality were more efficacious in pain reduction for patient-reported disabilities than those mixing several types of exercise with different goals within the same session.136
There is ample evidence to support focusing on one type of exercise when instituting a PT program for OA of the knee. The amount of exercise should be at least three times per week over a 4-week period to relieve pain and reduce disability.
Post-Surgical Knee Pain (PSKP)
Each surgery requires the specific surgeon who performed it to carefully balance the patient’s individual risks and benefits throughout the rehabilitation process to secure the best outcome.
This topic is so broad and the post-operative treatment modalities for individual therapy are so unique that this decision should be deferred to the surgeon who performed the procedure to generate a consensus point.
CRPS
Two authors evaluated 18 RCTs with 739 participants to test the efficacy of physiotherapy-based interventions between 1992 and 2015. There are only two studies specifically discussing CRPS Type 1 of the lower extremities (inclusive of the knee). Unfortunately, only one study is available as the other was removed from the site.137
While physiotherapy and rehabilitation remain to be first-line treatments for people with CRPS, the review could find no evidence to support or dismiss its efficacy.137
There is low-quality evidence due to the fact that most of the included trials were unclear or at high risk for bias. The trials were too broad with regard to interventions and did not allow for ample opportunity to pool data. This led to imprecision and inconsistency in the trials.
Soft Tissue Injuries
A systematic review and search was conducted from January 1, 1990 to April 8, 2015. A total of 9494 citations were screened and 11 RCTs were found of which 8 were discarded due to critical appraisal. Of the remaining 3 included, only 2 pertained to knee pain.138
The first RCT used found statistically significant improvements in pain and function illustrating the benefits of progressive combined exercises over watchful waiting for patellofemoral pain syndrome (PFPS). The second suggested supervised closed kinetic chain exercise can lead to greater symptom improvements than open chain exercises for PFPS.138
While the study found limited high-quality evidence supporting the use of PT to manage soft tissue injuries of the knee, there was anecdotal evidence that facility-based PT programs can potentially benefit patients with PFPS. Further high-quality research on this topic is needed.
Consensus Points for Physical Therapy
- PT is an effective treatment for OA of the knee; Level I, Grade A, Consensus Strong
- PT can be utilized for the treatment of CRPS of the knee; Level III, Grade C, Consensus Weak
- PT is an effective treatment for soft tissue injuries of the knee (excluding PFPS); Level II-2, Grade B, Consensus Strong
- PT can be utilized for the treatment of PFPS; Level III, Grade C, Consensus Weak
Durable Medical Equipment (DME)
As a part of the treatment of common musculoskeletal disorders, durable medical equipment (DME) refers to the medical equipment that assists in the treatment of musculoskeletal disorders, injury, illness. DME is further defined as reusable and nondisposable. Overall, the term DME refers to a wide range of equipment including devices for mobility such as bracing, orthotics, wheelchairs and canes as well as devices for activities of daily living (ADLS) such as shower chairs and even hospital bed.139 For the purpose of this paper, the following discussion will focus on DME indicated for the treatment of knee pain. DME is generally indicated for the knee used prophylactic, functional, postoperatively and for rehabilitative applications. Selecting the appropriate DME for knee pain starts with a proper diagnosis. The following discussion will focus on various types and indications for bracing and assistive devices (AD).139
For a clinician to effectively prescribe the proper DME for a patient, they must know the correct diagnosis, patient goals and the patient’s ability to comply with the DME prescription.139 In order to prescribe a brace, a clinician only needs to know generalities and it is recommended that the clinician has access to an orthotist who specializes in custom-made and off-the-shelf products.139
Bracing
The goal of functional braces is to provide stability and enhance function, while prophylactic braces prevent injury or decrease the severity of a possible injury and rehabilitative or postoperative braces allow controlled range of motion and help limit swelling.139
It is common practice for knee braces to be prescribed by physicians for OA pain, post surgically in total knee replacements (TKRs) and after ACL repairs as well as in the case of soft tissue or ligamentous injury. Types of knee braces include soft, hinge, medial and lateral offloading, hinged, compression, wrap around, band straps, open or closed patella and open or closed popliteal.140
Practitioners often prescribe bracing to relieve pain from osteoarthritis, a degenerative disease that occurs often later in life. Most commonly found in adults, OA is increasing to epidemic proportions in the US with 50 million Americans diagnosed and counting.139,141 Knee pain generated from OA is treated with medications, physical therapy, exercise, weight loss bracing and surgery. DME can assist in the management of OA pain oral medications fall out of favor, and non-medication options are gaining popularity.
The medial knee joint is more susceptible to mechanical stress which leads to overloading of the articular cartilage and can cause early degeneration.141 Unloader or offloading braces work to remove some of the medial or lateral compartment stress on the knee joint as well as improve bone alignment. These braces may in fact provide significant improvement in pain and function. In medial compartment OA, the varus deformity that develops can be offset by the valgus force against the joint from a brace; unloading the medial joint which has become compressed. Valgus offloading or unloader braces may be used. One study failed to show a difference both radiographically and clinically between the N=50 Bledsoe Thruster brace and the N=50 SofTec OA brace at 2 and 12 weeks follow-up, showing that both braces are effective in treating of varus medial knee OA (Level 1, Grade C).142 One paper concludes with a low quality of evidence that wearing a knee brace past 12 months as compared to not wearing one does not provide a difference in pain reduction or increased joint function and there is evidence that wearers may discontinue use due to this lack of effect.143 A Cochrane review showed bracing to be effective in treating unicompartmental OA, especially medial compartment allowing for improved function, which improved activity levels, thus allowing more opportunity for strengthening and weight loss.139 The European League Against Rheumatism (EULAR), the OsteoArthritis Research Society International (OARSI), and the American College of Rheumatology (ACR) recently put forth that knee OA management suggests the use of medications, exercise, strength training. There is however a lack of consensus regarding knee offloader braces.141 The OARSI did not recommend offloading braces citing “inconclusive evidence” of their symptomatic benefit, yet were “strongly recommended” in the new ACR guidelines.141
In the treatment of OA, the VER‐brace, which is a medial compartment unloader brace that works by applying valgus force combined with and external rotation, was found by one randomized crossover trial to be more comfortable and decrease pain as compared to a valgus three‐point bending system brace V3P‐brace or a standard stabilizing brace using post-injury (ACL). These findings were suggestive of increasing compliance in bracing treatment (Level 1, Grade B).144
One observational study showed a decrease in pain and improved function with a multidisciplinary non-operative approach in the setting of patellofemoral OA or tibiofemoral OA, yet donning a patellofemoral or a tibiofemoral knee brace did not seem to give additional benefits (observational study Level II-3).145 When comparing a standard knee offloading brace to new knee OA ankle brace (ankle foot orthotic; AFO), one study found newer AFO clinically as effective in treating medial knee OA (multicenter randomized control) (Level II-1, Grade C).146
- Soft knee braces: Knee braces made from soft flexible material work by reducing dynamic instability found in OA.147 Thus, wearing a soft knee brace has been shown to provide increased leeway in activity in patients with OA at the knee (Level II-3 Grade B).148 Whether the brace fit tightly made no difference in outcome.147 Soft braces are thought to work by improving proprioception at the knee (Level II-3 Grade B.148 One meta-analysis of both randomized and nonrandomized controlled trials showed moderate effects of soft braces on pain mild-to-moderate changes to subjective reports of physical function in knee OA. However, the authors cited low-quality level of evidence due to some lack of blinding (Level I, Grade C).149
- PFPS: One study showed that the use of bracing for PFPS provided immediate reduction of pain and quadriceps activation. Variability in pain symptoms was seen in individuals, so they were grouped into two groups, one group more and one with less pain. Some study participants felt uncomfortable pressure on the patella from the design of the knee brace, no hole present, thought by the researchers to be from a silicone ring within the front of the brace. They suggest prescribers of this brace for PFPS should instruct patient to only wear during pain-provoking activities. No adverse reactions. They cite that they do not fully understand why these braces reduce pain in PFPS remains unclear and cite that it could be due to the increase in contact area and change in the abnormal joint movement which may be reducing stress on the joint.150
- Hole cut-out: One study reported knee bracing without a patella hole cut-out as superior citing the thought that the cut-out gives the wearer more dynamic somatosensory stimulation and control (Level 1 Grade B or C).151 For anterior knee pain in the case of PFPS and tendinitis, the knee sleeve, either elastic or neoprene, compresses the knee, and patellar strap can be used to control pain. The patella cut-out in the knee sleeve provides comfort and not function.
The patella strap attaches underneath the patella and gives it a slight push up in efforts to lower traction across the patellar tendon. Limited evidence, citing a Cochrane review; unable to make concrete recommendations on their use. They may have some use as a second-line treatment in pain reduction as they are inexpensive and without contraindication. Patients can simply stop using them if no benefit is found.
Knee braces may improve post-surgical kinesiophobia, or fear of movement, in short-term follow-up (2 and 6 weeks) with PFP compared with minimal intervention (single-blind randomized controlled trial (1:1)).152 Practitioners may consider prescribing knee bracing when clinically relevant for rehabilitation of PFP (single-blind randomized controlled trial (1:1)).152 One literary review found strong evidence that the higher the level of kinesiophobia, the higher the pain intensity and disability levels (Level 1 Grade B).153
- Prophylactic braces: These aim to prevent injury to medial collateral ligament (MCL) due to excessive valgus force as literature claims these braces may provide more than 10% to 30% resistance to this force as compared to a brace-less knee. They are most commonly used in American football leagues; however, both the American Academy of Orthopedic Surgeons and the American Academy of Pediatrics claim there is insufficient evidence to support their use. Some studies do show benefits in high-risk conditions; however, these braces are not shown to prevent MCL injury.139
- Functional braces: These help stabilize the joint after a meniscus or ligamentous injury with the use of sturdy material, with a goal to prevent more injury. There is little difference between custom versus off-the-shelf and functional braces.139
In the case of a PCL injury, strength evidence has shown favorable outcomes in the use of newly developed dynamic bracing when integrated into a conservative management plan.154 These braces work by applying an anterior counterforce to the posterior tibial translation proximally.154
In bracing after ACL reconstruction, meta-analysis of seven studies with a total number of 440 participants, there was no significant difference between the bracing and not bracing group. Thus, researchers concluded that knee bracing post repair likely does not provide clinical improvements and they recommend against routinely prescribing bracing for these patients. However, the same meta-analysis found adverse effects of bracing, noting this was subjectively scored. Adverse effects may include thigh atrophy, soft tissue compression and a loss of flexion (Level I, meta-analysis. Grade D).149 One study cited that up to 87% of the orthopedic surgeons prescribed functional knee bracing post ACL repair and that these braces, which may lead to thigh muscle atrophy and decrease strength, do not significantly impact the laxity in the knee joint nor have a significant impact on pain reduction or an effect on joint laxity, pain, or satisfaction. Can add support but not replace rehabilitative therapies.139
- Orthotics and shoe inserts: Shoe orthotic inserts may be prescribed by practitioners for OA knee pain.139 One paper showed, with low quality of evidence, little to no difference in pain reduction when using a lateral wedge shoe insert on knee OA pain as compared to a no insole and probably little to no pain reduction or improvement of function or quality of life compared to the use of a neutral insole more than 12 months.143 Additionally, lateral wedges compared to valgus knee braces showed the possibility of little to no difference in pain reduction and increased function after 6 months of wear.143 One paper showed, with a low quality of evidence, that people with OA who use knee braces experience little to no pain relief or increase in function.143 There is moderate quality of evidence to suggest lateral wedged and neutral insoles give little to no pain relief or increase in function.143 One meta-analysis did not find clinical significance between the use of lateral wedge insoles and neutral insoles in pain reduction in the treatment of medial compartment knee OA pain (meta-analysis Level 1, Grade C).155
- Taping: Kinesiology tape or “kinesio taping” has been shown in meta-analysis to be effective in relieving pain and increasing function in patients suffering from knee OA; however, the results should be interpreted cautiously due to the low quality of evidence as there was a small sample size in most of the RCTs and lack of good comparison to drug standards (Level I, Grade C).156
- Bracing complications and noncompliance: Multiple studies cite noncompliance as an ongoing issue with bracing to relieve knee pain and in particular varus bracing (Level I, Grade C).139,142 Adverse effects of knee bracing have been reported to include pain in the posterior knee, low back, leg and plantar aspect of the foot, skin irritation and bruising; poor fit may worsen these symptoms.139,143 While comparing an AFO brace for OA to a standard knee offloading brace one study noted significantly lower side effects in the AFO group. However, this study was limited in that those in the AFO group, less knee contacting brace, used more bandaging and more therapy, which begs the question of whether these factors contributed to the perception of less side effects or actually prevented some of the side effects on their own (multicenter randomized control) (Level II-1 Grade C).146 Need for scoring side effects, which impact compliance. Braces are cumbersome and uncomfortable, shoe inserts require bigger bulkier and less stylish shoes. Long-term study are needed to look at braces and orthoses against conservative care.143
Assistive Devices
Practitioners often prescribe assistive walking devices (eg, cane, crutch, walker), to relieve pain from OA.139 Our search did not find any studies showing a superior device when comparing cane to walker to crutches.
- Canes: The cane has been shown to reduce the intra-articular loading forces by more than 10% and are most effective for medial and lateral compartment disease with less efficacy in patellofemoral disease.139 When a person starts using a cane, the energy expenditure is increased for about the first month and then diminishes as the user habituates to its daily use.157 There is about 1 month of decreased efficacy where the user gets used to using the cane; in month 2, energy expenditure decrease as well as pain.157 In knee OA prescribers should ensure proper cane height and instruct patients to utilize the cane on the contralateral side, having the cane advance with the affected leg while the patient is walking.139 Canes have been shown to reduce pain and improve function as well as some quality of life (QOL) aspects. One single blinded study showed the use of a cane to decrease pain with ambulation and decrease the use of NSAIDs (Level I Grade),157 with diminished returns over 3 months.158
- Walkers and crutches: Walkers provide stability and are safer for patients with danger of falls. Long-term use of a walker has a high association with older age and poorer prognosis. Crutches are generally better suited for patients with post of knee pain or knee injury that demonstrate good safety awareness and upper body strength (“crutch muscles”).
Thus, in summary, the optimal choice for an orthosis remains unclear, and long‐term implications are still to be determined.143 One study cites the quality of evidence, and studies are poor and need to be improved focusing on randomization and blinding, and prior to increasing lengths of studies, short-term efficacy should be shown to justify long-term efficacy. A period of 5 years, in the case of knee pain from OA, is likely necessary because of the chronicity of the disease. Additionally, this study suggests a standardized knee score when pooling data (such as WOMAC).143
Consensus Points for Durable Medical Equipment (DME)
- DME is an effective treatment modality for knee pain; Level II-2, Grade B, Consensus Strong
- The choice DME to be utilized for knee pain should be based on the individual diagnosis and takes into account comfort and compliance; Level I, Grade C, Consensus Weak
- Unicompartmental unloading bracing: medial compartment unloading braces are an effective treatment for knee OA and superior to other bracing options; Level II-1, Grade B, Consensus Strong
- Lateral shoe wedge is an effective treatment for OA knee pain as compared to a neutral insole. The patients may benefit from the feeling of protection which could decrease their likelihood of kinesiophobia and improve recovery as motion is liberalized; Level II-3, Grade C, Consensus Weak
- Patella strap, neoprene sleeve and taping should be utilized for knee pain; Level II-3, Grade C, Consensus Weak
- A cane is an appropriate treatment for OA knee pain, with the proper training size selection and guidance by the prescriber; Level II-1, Grade B, Consensus Strong
- A walker can be used for chronic pain secondary to knee OA and in the elderly to improve gait stability in those patients with higher risk of falls, as well as for ambulation in short distances; Level II-1, Grade B, Consensus Strong
- Crutches should be utilized post knee injury in younger patients with good balance and upper body strength; Level II-2, Grade C, Consensus Strong
Recommendations Regarding Injection-Based Therapies
Corticosteroid Injections
Intra-articular corticosteroid (IAC or IACS) injections were pioneered by Dr Jollander in 1953.159 The first trial for IAC injections was performed by White and Norton in 1958.160 IAC injections have become standard therapy for patients with knee osteoarthritis (OA). However, there is still debate on the efficacy of IAC injections for knee OA. A Cochrane review in 2015 evaluated 27 trials with a total of 1767 participants comparing IAC injections with a sham injection or no treatment for patients with knee OA.161 The quality of evidence was “low” due to a high or unclear risk of bias in many studies. There was also a significant amount of inconsistencies regarding the dosage of corticosteroid and the type of corticosteroid used. The review did find IAC injections reduced pain and improved function more effectively than control interventions. The authors reported an improvement of pain after IAC injection was moderate at 1–2 weeks post-injection, mild to moderate at 4–6 weeks, and minor at 13 weeks. There was no statistically significant evidence of treatment effect at 26 weeks. The Cochrane review concluded, given the poor quality and variability in the studies, it is uncertain if there is a significant advantage of IAC injections for knee OA after 6 weeks post-injection.
Conversely, a more recent systematic review and meta-analysis evaluating the magnitude and duration of the effect of IAC for knee OA found moderate evidence for IAC injections to reduce pain related to knee OA.162 The treatment effect was up to 3 months after the injection in the meta-analysis. The number needed to treat was 10. Unfortunately, as was such the case in the Cochrane review, there is significant heterogeneity between studies evaluating IAC for knee OA. The inconsistency between the Cochrane review and the meta-analysis underscores the need for RCTs with specific corticosteroids used and a fixed-dose to consider further the efficacy of pain relief and duration of effect (see Table 11).
 |
Table 11 Evidence Table Regarding Intra-Articular Corticosteroid Injections |
In summary, IAC may provide short-term mild-to-moderate pain relief for patients with knee osteoarthritis.
Consensus Points for Intra-Articular Corticosteroid Injections
- Intra-articular corticosteroids (IACS) may provide short-term pain relief for patients with symptomatic OA of the knee refractory to conservative medical management; Level 1, Grade B, Consensus Moderate
- IACS is superior to intra-articular hyaluronic acid only in the short-term (2–4 weeks), and is more effective in patients with severe knee pain secondary to OA; Level 1, Grade B, Consensus Moderate
- IACS is associated with an increase in cartilage volume loss. Caution should be exercised with repeat injection to prevent progression of disease; Level II, Grade B, Consensus Moderate
Hyaluronic Acid
HA, also known as hyaluronan, is a large glycosaminoglycan that is one of the natural components of cartilage. This is not to be confused with hylan, which is the modified form of hyaluronic acid. Hylgan has a higher viscosity to theoretically increase the time in the joint and therefore increase the efficacy of treatment.163 Studies have shown that HA can stimulate the synthesis of cartilage and reduce inflammation.164 Broadly, these injections are referred to as viscosupplementation. There has been debate over both the cost-effectiveness and efficacy of HA injections to treat knee osteoarthritis. There also is a lack of consensus about the number of injections needed and the type of viscosupplementation utilized.
A meta-analysis published in JAMA in 2003 reviewed a total of 22 trials with a review of a total of 2927 patients. Overall, there was a small noticeable effect of HA injections when compared to placebo. Furthermore, the authors found a publication bias which confounds the reported benefit of HA injections.165 A systematic review and meta-analysis of randomized trials, which included a total of 831 patients, showed that HA injections had statistically significant improvement in knee pain but without clinically meaningful outcomes when compared to oral NSAID use. The long-term side effect profile of HA is better than with oral NSAID use which could support its use over NSAIDs, particularly in the elderly population.166 A meta-analysis of randomized controlled trials published in 2018 compared HA injections to methylprednisolone injections for knee osteoarthritis. Five studies with a total of 1004 patients showed that both HA and methylprednisolone injection therapies were safe interventions that were effective in pain and physical function and stiffness at multiple time points (4 weeks, 12 weeks, and 26 weeks).164 In addition, Dai et al compared the efficacy and safety of HA versus hylan. Their meta-analysis showed that there was no clinically significant difference between either treatment. However, due to the higher cost of hylan, it was recommended that its use be discouraged from treating knee osteoarthritis pain.163
Another area of debate is the number of injections that are needed for HA therapy. A meta-analysis of single-injection products utilizing a post hoc placebo comparison showed that a single injection could produce results similar to multi-injections. One of the limitations is that the study was funded by LCA Pharmaceuticals, and the author is an employee and shareholder of the company that makes a single injection HA product.167 Another systematic review compared the effectiveness of different dosing regimens of HA. This study identified 11 studies and found no difference in outcomes between a series of three and five injections. The authors’ conclusion based on their results suggested that there appears to be a similar efficacy with single injections with greater cost-effectiveness.168 A review of the effectiveness and safety of Supartz (sodium hyaluronate) from pooled clinical trials showed that single 5-week injections could provide reductions in pain and improve function without any significant side effects. A total of 1155 patients were included in the analysis, and the intervention was compared to placebo169 (see Table 12).
 |
Table 12 Evidence Table Regarding Hyaluronic Acid |
Unfortunately, there are many conflicting conclusions from systematic reviews and meta-analyses on viscosupplementation for the treatment of knee osteoarthritis. Overall, viscosupplementation is a safe, well-tolerated procedure that has been shown to have an improvement in pain and function. However, there is no evidence to suggest that one type of viscosupplementation product is superior to another, and also no evidence to suggest that a series of injections are better than a single injection.
Consensus Points for Hyaluronic Acid
- Intra-articular hyaluronic acid (IAHA) is a safe and effective therapeutic option for patients with symptomatic OA of the knee refractory to conservative medical management; Level 1, Grade A, Consensus Strong
- IAHA demonstrates superior, longer-lasting efficacy post-injection in comparison to intra-articular corticosteroids in patients with knee pain secondary to OA; Level 1, Grade A, Consensus Strong
- Current evidence suggests there is no difference between the reduction in knee pain secondary to OA following a 3-week course of IAHA versus a 5-week course; Level II, Grade B, Consensus Moderate
- HA formulation differences including molecular weight and cross-linkage have been proposed as more or less effective; Level III, Grade C, Consensus Weak
Recommendations Regarding Genicular Nerve Ablations
Genicular nerve ablation (GNA) is a percutaneous, needle-based therapy option designed to palliatively treat knee pain. Analogous to an intra-articular injection with corticosteroid, GNA is not intended to remedy the root cause of pain or structurally alter the joint in any way; rather, the goal is to block/interrupt the transmission of pain signals from the knee, itself, thus eliminating the perception of pain by the brain. Like facet rhizotomy, GNA utilizes radiofrequency energy (aka radiofrequency ablation or RFA) focused on the active tip of a needle or cannula to create a focal energy field that will coagulate targeted sensory nerves caught in its path, thus preventing their ability to communicate with the central nervous system – in the case of the knee, the targeted nerves, for the most part, are the genicular nerves.
The genicular nerves provide innervation to the majority knee via a network of different branches that collectively communicate pain from different locations around the joint (Figure 1):
- Superior medial (SM)
- Superior lateral (SL)
- Inferior medial (IM)
- Inferior lateral (IL)
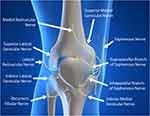 |
Figure 1 Illustration of the innervation of the knee. |
Additional innervation to the knee is provided by branches from the saphenous and the fibular nerves (Figure 1):
- Recurrent fibular nerve (RFN)
- Infrapatellar branch of the saphenous nerve (IPB)
- Suprapatellar branch of the saphenous nerve (SPB)
- Medial retinacular nerve (MR)
- Lateral retinacular nerve (LR)
Not all of these nerves need be ablated to provide significant relief as the majority of the evidence for GNA involves the ablation of only the SM, SL and IM genicular nerves. The initial publications on GNA were a number of case series demonstrating the efficacy of the therapy on pain after knee arthroplasty surgery.170–172 Over time, the procedure evolved in its application and began to be used prior to surgery. At the time of this manuscript, eight RCTs on GNA have been completed, all of which demonstrated efficacy in reducing pain and safety by ablating the SM, SL and IM genicular nerves.173–175 The first of these was published by Alcidi et al in 2007 on 40 patients treated with GNA and then followed for 1 month. The authors demonstrated significant improvements in pain and function as compared to control (TENS).176
In 2011, Choi et al published the results of a double-blind RCT on 38 patients with knee OA receiving either GNA or sham.177 The authors showed statistically significant improvements in VAS and function at 4 and 12 weeks, with no adverse events noted. The authors showed significant reductions in pain and improvements in quality-of-life scores. El-Hakeim et al published similar results in 2018 on 60 patients out to 6 months.178 In 2017, Qudsi-Sinclair et al published the results of an RCT on 30 patients with knee pain refractory to total knee arthroplasty (TKA) by targeting the SM, SL and IM nerves. The authors showed significant improvements in pain and function out to 12 months in the treatment group.179
In 2018, Davis et al published the results of the largest prospective RCT to date – 151 patients with knee osteoarthritis treated with cooled GNA or intra-articular steroid (IAS) injections.174 The authors showed statistically significant improvements in pain and function out to 12 months in patients treated with GNA. In a follow-up study, Hunter et al published the 18- and 24-month data on this subject group showing sustained improvements in pain and function with no safety concerns identified.180
While the bulk of the evidence on GNA demonstrates efficacy by ablating the “three main genicular nerves” (ie, SM, SL and IM), there remains some debate as to whether they are the most optimal targets, or if others should be done in conjunction with the main three to provide for a more complete denervation of the knee. In 2011, Ikeuchi et al published the results of a non-randomized, prospected trial on 35 patients treated with ablation of the MR and IPB.181 The authors demonstrated significant improvements in knee pain and quality of life scores out to 6 months. In stark comparison to the studies published on GNA using the 3 main nerves, Ikeuchi’s study reported minor bleeding and prolong hypoesthesia in 67% and 78% of the subjects, respectively, suggesting that while the improvements are comparable, targeting these nerves instead of SM, IM and SL may come with a higher incidence of adverse events. The prevalence of hypoesthesia is not surprising given that the MR is responsible for cutaneous innervation of the knee as a branch of the medial femoral cutaneous nerve. This complication could be potentially averted by using a pulsed ablation instead. The results publihed by Ikeuchi suggest that the MR and IPB should be considered as supplemental targets in those patients with refractory pain to ablation of SM, SL and IM nerves.
Of the 10 RCTs, 5 used thermal RFA (TRFA) (70° to 90°C; 90–270 seconds) 4 used cooled RFA (CRFA) (60°C for 150 seconds, and 1 used pulsed RFA (PRFA) (42°C for 10 minutes). At the time of this manuscript, there are no comparative studies suggesting which RFA modality provides better results for knee pain. As it pertains to pulsed RFA, aside from the one RCT by Gulec et al,182 which did not have a control arm and only compared bipolar to monopolar pulsed GNA, the remaining evidence is limited to prospective and retrospective case series and case reports173 – therefore, there is no high-level evidence to support its use for knee pain (see Table 13).
 |
Table 13 Evidence Table Regarding Genicular Nerve Ablations |
Consensus Points for Genicular Nerve Ablation
- RFA of the SM, SL and IM genicular nerves is a safe and effective therapeutic option for treating knee pain secondary to OA as well as pain refractory to TKA; Level 1, Grade A, Consensus Strong
- RFA of the SM, SL and IM genicular nerves can significantly reduce knee pain and improve function in patients with knee OA and pain refractory to TKA; Level 1, Grade A, Consensus Strong
- Thermal or cooled RFA should be utilized when performing GNA; Level 1, Grade A, Consensus Strong
- In patients with persistent knee pain after GNA targeting the SM, SL and LM genicular nerves, one may consider targeting IL, MR and/or IPB for supplemental treatment; Level III, Grade B, Consensus Moderate
Recommendations Regarding Regenerative Therapies
Platelet-Rich Plasma
PRP was first introduced in the 1970s for wound and bone healing in the field of oral and maxillofacial surgery.183 Recently, PRP shows promise for treating various orthopedic, musculoskeletal, and pain syndromes. PRP has varied components including platelets and other cell types, growth factors, and cytokines. The basic premise to foster an environment that promotes healing by directing cell proliferation, chemotaxis, and angiogenesis.184 These changes are seen in intra-articular PRP injection for symptomatic knee osteoarthritis where studies demonstrate a significant decrease in protein concentration of immunoglobulins associated with inflammation, including apolipoprotein A-I, haptoglobin, immunoglobulin kappa chain, transferrin, and matrix metalloproteinase. Additionally, post PRP injection proteins associated with chelation and anti-aging physiological functions increase significantly, including matrilin, transthyretin, and complement 5. Moreover, these laboratory findings are complemented with clinical success, including improvements in knee symptoms of the index of osteoarthritis severity185 and a decrease in synovial fluid volumes. This enhanced environment for healing can be an inherent advantage of PRP in comparison to other injectates. For instance, repeated intra-articular corticosteroids may have detrimental systemic and local effects, including greater cartilage volume loss. Although the clinical outcomes of such have not been fully demonstrated.186,187
It is important to discern that all PRP is not equivalent. Factors at minimum that can affect the final PRP product include volume of blood aspirated, baseline platelet count, patient health status and comorbidities, patient medications, anticoagulant of choice, centrifugation parameters, and inclusion/exclusion of leukocytes. Not distinguishing for this variability, we have summarized below the gross evidence evaluating the use of PRP for knee-related pathology.
The combined data from the reviews discussed support PRP’s excellent safety profile. A total of 26 studies (n=1051) that reported adverse events demonstrated non-significant differences between other conservative treatments and PRP injection.188 The other reviews show agreement that PRP-treated patients did not display significant increased adverse events or additional side effects.189–191 PRP intra-articular knee injection is safe with comparable risk factors to other conservative treatment for knee osteoarthritis.
- Intra-articular PRP: Several systematic reviews have analyzed the available literature regarding PRP specifically focusing on symptomatic knee osteoarthritis. In 2017, Dai et al evaluated the efficacy of PRP for the treatment of knee osteoarthritis compared to saline control and hyaluronic acid. Meta-analysis of 10 RCTs (n=1069) revealed that at 6 months post injection, PRP and hyaluronic acid had similar effects with respect to pain relief and functional improvement. However, at 12 months, PRP was associated with significantly better pain relief and functional improvement as measured by the WOMAC that exceeded the minimal clinically important difference. When comparing PRP to saline, PRP intervention was more effective for pain relief and functional improvement at 6 and 12 months with scores that again exceeded the minimal clinically important difference.189
Similarly, in 2020, Hohmann et al compared intra-articular knee injections of PRP primarily to hyaluronic acid. A pooled estimate of 12 RCTs (n=1248) supported superiority of PRP (n=636) compared to hyaluronic acid (n=612) for symptomatic knee pain at 6 and 12 months. There was significant difference in reported knee pain favoring PRP at both 6 months and 12 months. Although the data did not demonstrate significant difference in clinical outcomes utilizing the WOMAC and International Knee Documentation Committee (IKDC) scores. The authors present evidence in favor of PRP for treatment of symptomatic knee osteoarthritis pain.192
A separate systematic review in 2020 investigated PRP versus hyaluronic acid in the treatment of knee osteoarthritis by including 14 RCTs (n=1350). Compared with hyaluronic acid, PRP had higher scores in long-term (>24 weeks) VAS, IKDC, WOMAC-Pain, WOMAC-stiffness, WOMAC-Physical Function, and WOMAC-Total. The authors conclude that PRP demonstrates more advantages over hyaluronic acid in the conservative treatment of knee osteoarthritis, including reduced long-term pain and improved knee function.190
Moreover, in 2019 another systematic review found analogous evidence when comparing PRP to hyaluronic acid. Meta-analysis of RCTs (N=1314) revealed that PRP injections reduced pain and improved function more effectively than hyaluronic acid injections in patients with knee osteoarthritis. The VAS pain score showed a significant difference at 12 months. Furthermore, better functional improvement was observed in the PRP group, as measured by the WOMAC function score at 3, 6, and 12 months.191
A recent comprehensive systematic review and meta-analysis in 2020 largely supported the above findings and divided the meta-analysis by both reported pain score (VAS) and functional improvement (WOMAC). Trams et al included 22 studies (n=888) for meta-analysis, investigating reported pain via the VAS comparing PRP versus placebo (6 studies, n=190), corticosteroids (2 studies, n=53), or hyaluronic acid (15 studies, n=645). PRP showed significant improvements in VAS compared to both placebo and hyaluronic acid subgroups. The same review also analyzed functional outcomes measured by the WOMAC scale: 25 studies compared PRP versus control groups: 9 studies compared against placebo (n=264), 1 study compared against corticosteroids (n=19), and 15 studies compared against HA (n=730). The pooled estimates, as well as each subgroup, showed significant differences in favor of PRP.188
While most studies have compared PRP to hyaluronic acid injections or placebo, we reviewed two RCTs that investigated PRP versus corticosteroid. In 2017, Jubert et al randomized patients to treatment either with single leukocyte-reduced PRP (n=34) or corticosteroid intra-articular injection (n=30). Quality of life differences at 3 and 6 months were significantly improved in the PRP group and so did general health perception differences at 6 months. The authors conclude that a single PRP intra-articular injection is effective for relieving pain and improving activities of daily living and quality of life in late-stage knee OA (Kellgren–Lawrence grade III to IV). Furthermore, for patients older than 67 years, a single intra-articular injection of PRP has similar results to a single injection of corticosteroid.193
A second RCT by Guvendi et al included 50 patients diagnosed with grade III knee osteoarthritis. Patients were randomized to three groups: single corticosteroid injection group (n=17), single PRP injection group (n=19), and three PRP injection group with 1 week interval (n=14). WOMAC and Lequesne function scores at the 6-month follow-up were significantly improved in the PRP groups compared to the corticosteroid group. No significant differences were demonstrated in the single PRP treatment group compared to the three-injection group, which differs from previous studies that suggest superiority to multiple injections. The authors suggest this lack of improvement after multiple injections may be due to later-stage cartilage damage in the patients, or due to the short one-week interval between injections.194
A systematic review pooled these two aforementioned studies (n=53) to conclude there were non-significant differences in pain score via VAS in favor of PRP versus corticosteroid (p = 0.23).188 The same systematic review reported that PRP was significantly superior compared to corticosteroid in terms of functional outcomes. Compared to corticosteroid injection for symptomatic knee osteoarthritis, PRP intra-articular injection shows superior outcomes, including improved knee joint function and quality of life.
In 2021, Bennell et al published the results of an RCT comparing PRP to saline for the treatment of knee pain secondary to OA. While the results showed no statistically significant difference between PRP and placebo, the authors were not technically using PRP in the treatment arm.195 PRP is defined as a concentration of platelets that is ≥2× that of whole blood – the concentration utilized by the authors was only 1.6×, thus it cannot be considered PRP. This fact alone largely explains why the results are contrary to those published in over three dozen other studies. As such, the results of this study are not reflective of PRP as a treatment for knee pain secondary to OA. In 2022, Chu et al published the results of the largest RCT on PRP to date (n=610) comparing PRP to sham, utilizing a concentration of roughly 4.3× that of whole blood. The PRP arm showed statistically significant improvements over sham in IKDC, WOMAC, and VAS out to 60 months.196
Although there has been much variation in treatment protocols and specifically the total number of PRP injections, the optimal number of PRP injections for positive outcomes has been under investigation. In 2019, one systematic review studied the clinical effectiveness of single versus multiple (double or triple) PRP injections for knee osteoarthritis. Meta-analysis of five clinical trials (n=301) showed that, at 6 months after the intervention, there were no significant differences in pain improvement between the differing number of injection groups. However, at 6 months, there was a significant and clinically important difference in improvement in knee functionality in favor of multiple injections, with sub-analysis only evident for the results of single versus triple injections. However, the authors conclude that there is difficulty in generalizing data due to the lack of standardization between the multiple injection intervals.197
A second meta-analysis of six studies (n=255) comparing single versus multiple [two (n=85) or three times (n=170)] injections of PRP assessed significant differences in reported VAS pain scores in favor of multiple injections. However, only three injections of PRP (n=170) showed significant differences compared to a single injection.188 The same review reported functional outcomes were also analyzed in five studies (n=211) comparing single versus multiple injections and showed significant differences again in favor of multiple injections.188 Further research is needed to create frequency protocols, although data suggest multiple PRP injections may provide superior relief relative to a single injection.
- Intraosseous PRP: The benefit of intraosseous PRP injections compared to intra-articular injection alone has also been investigated. Su et al randomized 86 patients with grade II to III knee osteoarthritis to one of three groups: intra-articular PRP combined with intraosseous injection of PRP, intra-articular PRP alone, or intra-articular hyaluronic acid. Patients that received both intraosseous and intra-articular PRP received both 2 weeks apart. The group that received both intraosseous and intra-articular PRP demonstrated significantly superior VAS and WOMAC scores than the other groups up to 18-month follow-up.198
These findings support the pilot study by Sanchez et al where patients (n=13) diagnosed with severe knee osteoarthritis grade III and IV received one PRP intra-articular injection combined with two PRP intraosseous injections targeting the subchondral bone. There was a significant improvement in pain, symptoms, function, and quality of life measures from baseline at week 8 through the 24-week study period. Eight of the 13 patients who completed the study showed minimal clinically important improvement.199
Further building on these positive findings, Sanchez et al studied 60 patients suffering from severe knee osteoarthritis (grade III and IV) who either received intra-articular PRP (weekly for 3 weeks) or a combination of intra-osseous and intra-articular PRP (intraosseous plus intra-articular injection week 1, followed by 2 weekly intra-articular injections). In this observational study, the combination of intraosseous with intra-articular injection group showed significant improvement in KOOS and WOMAC. Sixteen of the 30 patients in the intraosseous group reached minimal clinically important difference at 2 and 6 months, compared to 8 out of 30 in the intra-articular PRP alone group. When comparing the response of both groups, there was a statistically significant improvement in pain reduction and functional improvement at 6 and 12 months. Of note, there were no clinically superior differences at 2 months which the authors suggest relates to the delayed time course of PRP, and interestingly, intra-articular PRP alone in this study did not show statistical benefits in either pain or functional scores.200 Both of the above studies report no significant adverse effects post intraosseous PRP injection.
- Use in soft tissue
- Chronicpatellar tendinopathy: PRP has been studied in chronic patellar tendinopathy. In 2014, Dragoo et al randomized 23 patients with patellar tendinopathy on examination and MRI who had failed nonoperative treatment to receive ultrasound-guided dry needling alone (n=13) or with injection of leukocyte-rich PRP (n=10). Both groups received standardized eccentric exercises. The PRP group had improved significantly more than the dry needling group at 12 weeks, although by greater than 26 weeks there was no significant difference in the Victorian Institute of Sports Assessment score for patellar tendinopathy. The authors conclude that PRP treatment accelerates the recovery from patellar tendinopathy relative to exercise and ultrasound guided dry needling alone, although the benefits decrease over time.201
- In 2015, Zayni et al further evaluated PRP to treat chronic patellar tendinopathy. Patients received either one or two PRP injections 2 weeks apart (n=40) under ultrasonography guidance within and around the hypoechogenic patellar tendon area; nine patients failed PRP treatment and needed surgery, the remaining patients significantly improved. Those receiving two PRP injections had better outcome measures in the Victorian Institute of Sport Assessment-Patella, VAS, and Tegner scale.202 These studies suggest, PRP may improve outcomes in functionality in treatment of chronic patellar tendinopathy within the first 12 weeks, and two injections may provide additional benefit over one.
- Pes anserinus: One study evaluated PRP in the treatment of pes anserinus pain syndrome. In 2014, Rowicki et al investigated 33 patients with chronic pain in the pes anserinus who were treated with PRP into that region. And 84.8% of these patients demonstrated total or near-total pain relief within 6 months of treatment. However, this study lacks a control group and provides low-level evidence in favor of PRP for pes anserinus pain syndrome203
- Medial collateral ligament sprains: Regarding medial collateral ligament sprains, 46 healthy athletes with high grade II or III medial collateral ligament sprains were randomly allocated to two equal groups: one group received a single PRP injection and both groups went on to participate in a 12-week functional rehabilitation program. In the PRP treatment group, only at the 4-week mark, pain was significantly reduced, while stability and Lysholm scores were noted to have no significant difference.204
- Meniscal tear: One case report describes a bucket handle meniscal tear treated with three separate PRP injections in and around the meniscus within 7 months of the diagnosis. Patient-reported resolution of pain 8 months post injury and MRI 10 months post injury and arthroscopy 47 months post injury showed complete resolution of the meniscal tear. Although promising reports for the treatment of ligaments, cartilage, and tendons further studies are required before appropriate recommendations may be made.205
In conclusion, PRP is an anabolic, safe, effective approach for knee osteoarthritis, as well as possible targeted treatments specifically for meniscus and ligament structures (see Table 14). Best practice approach is pending, but Level 1 evidence supports treatment of symptomatic knee osteoarthritis with PRP. Further research is needed for development of standardized treatment protocols demonstrating ultimately the ideal composition of injectate along with optimal dosing, timing interval, and frequency, as well as patient selection.
 |  |  |
Table 14 Evidence Table Regarding Platelet-Rich Plasma |
Consensus Points for Platelet-Rich Plasma
- Intra-articular PRP is an effective and safe treatment for knee pain secondary to osteoarthritis with Kellgren–Lawrence Scale II–III; Level 1, Grade A, Consensus Strong
- Intra-articular PRP is at least as effective as an entire series of viscosupplementation with hyaluronic acid; Level 1, Grade A, Consensus Strong
- Intra-articular PRP can improve function in patients with knee pain secondary to osteoarthritis; Level 1, Grade A, Consensus Strong
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) have also been used in hopes of promoting an anabolic healing environment. MSCs have the ability to provide signals for tissue regeneration as well as the additional potential to differentiate into a variety of connective tissue-type cells. Animal studies suggest intra-articular and intraosseous injection of MSCs appear to result in regeneration of articular cartilage in osteoarthritic models.206 However, it is important to be cognizant given their inherent nature, not all MSCs are equivalent. MSCs are found in most tissues of the human body but primarily sourced for reimplantation from the bone marrow and adipose due to ease of access. Volume of aspirate, patient health status and comorbidities, patient medications, harvesting protocol parameters and technique can all affect the final MSC product. Not distinguishing this heterogeneity, we have summarized below the gross clinical evidence evaluating using MSCs for knee-related pathology.
In 2020, Prodromos et al investigated the use of autologous MSCs (stromal vascular fraction, culture-expanded adipose-derived stem cells, bone marrow aspirate, culture-expanded bone marrow, and minimally manipulated fat graft) for the treatment of knee osteoarthritis. This extensive systematic review utilized historical controls of placebo treated patients adapted from the placebo arms of other prior knee injection studies for treatment of osteoarthritis. In the 29 studies of 1063 treated knees included, VAS pain scores were statistically significant with improvements greater than the minimally important clinical difference at a post-treatment mean of 6 months. Those post MSC injection showed continuing functional improvements greater than 6 months and continued to improve up to 1 year, whereas the placebo scores were down trending by 6 months. There was no dose–response relationship shown regarding the number of cell dose and outcomes. Also, in comparing cell type among those with large enough cohorts, culture-expanded adipose-derived stem cells, bone marrow aspirate, and culture-expanded bone marrow all show similar outcomes. This review concludes that autologous MSCs are effective to improve both pain and function for those suffering from knee osteoarthritis.207
A second review in 2020 by Migliorini et al enrolled a total of 1069 osteoarthritic knees from 18 studies (of which 3 had controls), with mean age 57.39. Seventy-two percent of all studies harvested the stem cells from the iliac crest (bone marrow-derived MSCs), whereas 28% harvested from the adipose tissue (adipose-derived MSCs). The mean VAS improved from a baseline of 55.20 to 30.98 and 36.91 at 6- and 12-month follow-up, respectively. The mean WOMAC score improved from a baseline of 25.66 to 25.23 and 15.60 at 6- and 12-month follow-up, respectively. Mean walking distance also improved from a baseline of 71.90 m to 152.22 and 316.72 m at 6- and 12-month follow-up, respectively. No significant differences were seen in VAS, WOMAC, and walking distance scores concerning the donor source. Those treated with earlier stage Grade II and III osteoarthritis did show statistically significant better outcomes.208 The study reports there were 136 (12.7%) local complications detected. Mostly pain and swelling, and rare cases of skin reaction (n=1), allergic reaction (n=2), and hematoma (n=2). The authors agree that autosomal MSCs are safe and show favorable results in improving both pain and function in treating knee osteoarthritis.
To control for the increased bias that may result from additional therapies at the time of MSC delivery, Tan et al formulated their systemic review in 2021 with the goal to pool the outcomes of treatment using intra-articular injections of MSCs alone without any adjuvant therapies for osteoarthritis. Nineteen Level I or Level II studies (n=440) were included of which 9 used bone marrow MSCs and 10 used adipose MSCs. The meta-analysis concluded intra-articular injections of MSCs without any adjuvant therapies significantly improves pain and function for knee osteoarthritis. Interestingly, differing from prior reviews, this review suggests significant better outcomes obtained with the use of bone marrow MSCs as compared with adipose MSCs. The authors also found favorability of cultured MSCs compared to uncultured MSCs. The authors suggest that due to their stringent inclusion criteria, this review provides greater homogeneity among the studies and thus fairer comparisons among the data.209
On the contrary, one systemic review by Dai et al in 2021 investigating intra-articular MSC injection for knee osteoarthritis formed an unfavorable conclusion compared to the previously discussed reviews that predominately support the use of MSCs. The authors suggest there was no significant difference found in the MSC treated osteoarthritic knee compared to a placebo control. This review included both autologous and allogeneic MSCs totaling 13 studies of which 6 were adipose-derived, 5 were bone-marrow-derived, 1 was placenta-derived, and 1 was umbilical cord-derived (MSC=250; total controls=111). In the subgroup analysis compared to placebo (n=66), MSC intra-articular injections (n=67) did not show significant differences in VAS for pain, WOMAC pain score, WOMAC function score, nor WOMAC stiffness score. However, compared to hyaluronic acid (n=77), MSC intra-articular injection (n=77) did show significantly better improvement in VAS for pain, WOMAC pain score, WOMAC total score, and WOMAC stiffness score. Although the minimum clinically important difference was not exceeded. Furthermore, in the pooled groups of controls including both placebo and hyaluronic acid (n=143), the authors did again find a statistically significant difference in favor of MSCs (n=144). In terms of function, including a total of 8 studies and pooling hyaluronic acid and all placebo controls (n=121) compared to MSCs (n=118), there was also a statistical improvement in WOMAC which did exceed minimum clinically important difference only when compared to the hyaluronic group, but not the placebo group alone. Although this study shows MSCs did not statistically outperform placebo injections, the total number of subjects evaluated to make this comparison was low (n=66) and the majority of follow-up was relatively short-term at 6 months compared to 12 month outcomes for the majority of the hyaluronic acid controls.210 In addition, pooling placebo and hyaluronic acid controls the MSCs did reach statistically significant levels of pain and function improvement.
Regarding potential cartilage repair several studies included radiological evaluations. In 2019, Kim et al published a meta-analysis of level II RCTs to investigate clinical outcomes and cartilage repair in osteoarthritis of the knee post treatment with intra-articular injections of MSCs at 12 or 24 months. Four of the studies utilized bone marrow-derived mesenchymal stem cells, while one study utilized adipose-derived stromal vascular fraction. Of note, two of these studies performed concomitant surgery (high tibial osteotomy), and three of the studies used additional injections including PRP or hyaluronic acid. Of the studies that reported VAS (4 studies: MSCs=56; controls=58), there was a significant decrease in VAS in MSC treated knees compared to controls. Additionally, in combined VAS and WOMAC pain scales (MSCs=91; controls=93) a significant decrease was also seen. Looking at functional outcomes reported in three studies (MSCs=35; controls=35), there was an improvement in WOMAC scores although this did not reach a level of statistical significance. However, two studies that reported Lysholm knee function scores (MSCs=49, controls=51) did demonstrate statistical improvement in those treated with MSCs. Moreover, the combined functional scores of both WOMAC and Lysholm (MSCs=69; controls=71) did reach statistical significance in favor of MSCs improving functional outcomes. Regarding improvements in imaging, three studies (n=96) reported MRI evaluation post treatment with a trend towards improvement; however, this did not reach a level of statistical significance. The authors conclude that MSCs did show improved pain and function in a period of 12–24 months. However, there was not clinical evidence to suggest improving cartilage repair in knee osteoarthritis.211
An additional systematic review in 2019 by Ha et al includes 17 studies (6 RCTs) focusing on intra-articular MSCs for osteoarthritis of the knee clinical outcomes and cartilage repair. Eight studies used bone marrow-derived MSCs, six used adipose tissue-derived stromal vasculature fraction, two used adipose tissue-derived MSCs, and one used umbilical cord blood-derived MSCs. Fifteen of the 17 studies reported improved clinical outcomes at final follow-up in the MSC group. Nine of 11 studies reported improvement of the cartilage state on MRI, and 6 of 7 studies reported repaired tissue on second-look arthroscopy, although some studies displayed mixed results. The authors conclude that intra-articular MSCs showed improvements in pain and function in many cases at follow-up less than 28 months.212
Similarly, Jaibaji et al in 2021 published a systematic narrative review to analyze autologous MSCs for the treatment of cartilage defects of the knee. Seventeen studies (including 5 RCTs, of which 8 using bone marrow sourced, 3 using adipose-derived, 3 using synovial-derived, 2 using peripheral blood, and 1 using bone marrow and peripheral blood) were found with a mean age of 35.1 years (n=367). The authors conclude that all studies demonstrated significant improvements in function outcomes in their patients with osteochondral lesions, suggesting MSCs, whether derived from synovium, bone marrow, adipose tissue, or peripheral blood, may have clinical efficacy in cartilage regeneration. Of note the authors do acknowledge a high risk of bias in at least one category in each of the five RCTs. Four out of five had high risk for bias due to lack of appropriate blinding.213
In 2020, Ma et al included 10 RCTs (n=335) to analyze the efficacy and safety of intra-articular injection of MSCs in the treatment of knee osteoarthritis. Five used autologous mesenchymal stem cells (adipose-derived MSC=3; bone marrow-MSC=2) and five used allogeneic mesenchymal stem cells (umbilical cord-derived=1; placenta-derived=1; bone marrow-derived MSC=2, adipose-derived MSC=1). The strength of this review includes the increased stringent exclusion criteria which included the exclusion of studies with concomitant treatment such as low/high tibial osteotomy, microfracture, and knee replacement. This meta-analysis showed significant improvement in MSC groups pain scores compared to control groups. In terms of function, all WOMAC scores also improved significantly. However, 2 of the 10 studies were not blinded. Regarding cartilage repair, there was no significant difference in the Whole-Organ Magnetic Resonance Imaging Score (WORMS), but the MSC group did show significant increase in cartilage volume (3 studies, MSC=46: control=42). There were also a significantly higher proportion of patients with adverse events reported in the MSC treatment group. All 10 studies in their review evaluated adverse events, reporting mostly mild and moderate clinical symptoms including joint pain, swelling, pain at the injection site, and joint effusion. One study reported three severe adverse effects including dyslipidemia, anemia, and muscle hemorrhage although all had complete recovery. Another study reported one patient with development of severe prepatellar bursitis that ultimately resolved. Of the six studies that reported both numbers of patients in MSCs and control arms with adverse events, there was a significant higher rate of adverse events in the MSCs group. However, as discussed, the overwhelming majority of adverse events were rare and mild. The authors conclude that intra-articular injection of MSCs is an effective and safe modality to relieve pain and improve the function of patients with knee osteoarthritis. This review states the improved findings in both pain and function may be due to the addition of newly incorporated studies of adipose tissue and umbilical cord stem cell sources.214 Furthermore, The overall good safety profile of MSCs was consistent among each of the systematic reviews we have previously discussed, suggesting MSCs are a safe treatment for knee osteoarthritis.
The overwhelming majority of systematic reviews conclude that MSCs are favorable and safe in the treatment of knee osteoarthritis, particularly demonstrating improvements of pain and function. One out of the eight reviews in their subgroup analysis concludes that MSCs did not show improvement in pain or function compared to purely placebo controls; however, in the same review, the pooled placebo and hyaluronic acid controls groups did show statistical differences favoring MSCs. This contradiction may be due to a small sample size and a relatively short follow-up. Furthermore, several reviews were also able to demonstrate increases in cartilage volumes and at the minimum suggest MSCs may play a role to delay further progression of osteoarthritic changes. However, given the inherent heterogeneity of MSCs as well as the lack of RCTs, there is insufficient evidence for ideal treatment paradigms and generalizability at this time.
Consensus Points for Mesenchymal Stem Cells
- Intra-articular MSCs are a safe treatment for knee OA; Level II-1, Grade B, Consensus Moderate
- Intra-articular MSCs are effective for treating pain and improving function in patients OA; Level II-1, Grade B, Consensus Moderate
Amniotic Tissue
Human amniotic membrane (HAM) and human amniotic fluid-derived cells (HAFCs) contain properties that present potential for alleviation of OA disease progression. Amniotic tissues consist of anti-inflammatory factors that upregulate anti-inflammatory pathways, high hyaluronic acid and proteoglycan content, as well as regenerative chondrocyte differentiation properties.
A study by Vines et al examined single intra-articular injection of cryopreserved particulated human amnion and amniotic fluid cells in six patients (n=6) with Kellgren–Lawrence (KL) grades 3 or 4 knee OA. This prospective open-label pilot study was conducted to assess feasibility and safety for future placebo-controlled trials of intra-articular amniotic suspension allograft (ASA) injections. The ASA injection did not significantly affect blood cell counts, lymphocytes, or inflammatory markers; there were, however, small but statistically significant increases in IgG and IgE levels.
Two patients developed a temporary increase in pain which resolved within 2 weeks. None of the patients developed an infection or experienced an inflammatory reaction; there was no difficulty with injection and no immediate complication.
The authors conclude that a single intra-articular injection of ASA is feasible in patients suffering from knee OA. Observed improvements in KOOS, IDKC, and single assessment numeric evaluation (SANE) scores within the small study population suggest substantial improvement if reproducible in a placebo-controlled trial.215
Another study conducted by Ramon Castellanos assessed the short-term safety and effectiveness of amniotic membrane/umbilical cord particulate (AMUC) as a treatment option for knee OA. The single-center, prospective, investigator-initiated pilot study enrolled a total of 20 subjects (n=20). Ultrasound-guided injection of 50 mg AMUC was injected, and patients were monitored at 6 weeks, 12 weeks, and 24 weeks. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was used to evaluate pain scale, with WOMAC-A evaluating pain with five questions, WOMAC-B evaluating stiffness with two questions, and WOMAC-C evaluating physical function with seven questions. Patients who did not report a greater than 30% reduction in pain using the WOMAC-A questionnaire received a second injection at 6 weeks.
The average pain score was reduced significantly from 74.3 ± 17.2mm at baseline to 45.0 ± 25.4 mm at 6 weeks (pain reduction of 37.6%), 35.4 ± 26.6 mm at 12 weeks (pain reduction of 55.1%), and 37.4 ± 26.7 mm at 24 weeks (pain reduction of 51.7%). Those patients reporting more than 50% relief included 25% (5/20) at 6 weeks, 45% (9/20) at 12 weeks, and 50% (10/20) at 24 weeks.
Patients’ physical function scores were significantly reduced from 74.2 ± 18.0 mm at baseline to 51.4 ±24.9 mm at 6 weeks (physical function improvement of 25.3%), 40.3 ± 26.8 mm at 12 weeks (physical function improvement of 42.3%), and 41.7 ± 27.3 mm at 24 weeks (physical function improvement of 44.5%).
A patient global assessment (PGA) was also performed. Seventeen of the 20 patients reported positive improvement at 6 and 12 weeks, and 14 patients reported improvement at 24 weeks. However, it is important to note that hypertension was a significant covariate.
A total of 11 patients received a second injection at the 6-week mark. Seven of the 8 (87.5%) patients with a body mass index (BMI) >30 kg/m2 received a second injection; only 4 of the 12 (33.3%) patients with BMI <30 kg/m2 qualified for a second injection. One subject received greater than 30% improvement from the first injection (38% improvement) but was still allowed the second injection per investigator discretion. Results showed significant improvement in non-obese patients at all time points, but only significant improvement for obese patients at 12 and 24 weeks. MRI evaluation of the total study population showed significant improvement in bone marrow lesions (BMLs) in seven patients.
This study suggests preliminary safety and effectiveness in pain relief and improvement in function in those with knee OA BML grades 0–3. The authors conclude that randomized placebo-controlled studies would provide more insight on AMUC as a potential treatment option for symptomatic knee OA.216
Mead et al conducted a single-center retrospective study investigating intra-articular knee injection of 100 mg lyophilized and micronized AMUC in those suffering from KL Grade 3 or 4 moderate-to-severe knee OA. Data points included patient global impression of change (PGIC), which used a 7-point scale, and global perceived improvement (GPI), which used a percentage improvement compared to baseline. The investigators used the Outcome Measures in Rheumatology (OMERACT)-OARSI responder criteria to evaluate changes in pain, function, and patient’s global assessment.
Forty-two patients were enrolled into the study, reporting an average pain score of 6.6 ± 0.5 out of 10, prior to injection. Twelve months post injection, 31 of the 42 (74%) patients reported clinically significant improvement of knee pain and function via PGIC. Pain function improved by 62 ± 24% at 1 month, 69 ± 27% at 3 months, 69 ± 27% at 6 months, and 64 ± 31% at 12 months on the GPI. This improvement lasted a mean duration of 12.1 ± 4.5 months. Treatment response was 81% (34/42) ascertained by the simplified OMERACT-OARSI criteria at the 12-month mark.
Of the KL grade 3 knee OA subjects, improvement in knee pain and function by PGIC was seen in 87% of the patients (13/15) at 12 months and lasted 13.4 ± 3.6 months; the OMERACT-OARSI treatment response rate was 93% (14/15) in these patients at 12 months. In the KL grade 4 group, 67% of the patients (18/27) reported clinically significant improvement of knee pain and function via PGIC for a duration of 11.5 ± 4.9 months. At 12 months, the OMERACT-OARSI treatment response rate was 74% (20/27).
One patient developed knee swelling within 36 hours of injection. However, s/he reported progressive improvement and, by week 6, showed significant pain relief and increased range of motion. No other adverse events were found.
The authors suggest that the intra-articular injection of AMUC particulate may be a valuable treatment option for those with moderate-to-severe knee OA and could possibly delay knee replacement; however, prospective randomized controlled trials are needed for further validation.217
In a multicenter randomized single-blinded controlled trial conducted by Farr et al, ASA was injected in subjects with knee OA and compared to saline and HA. The study included 200 subjects with a 1:1:1 randomization to ASA (68 subjects), HA (64 subjects), or saline (68 subjects). Patient-reported outcomes (PROs) including ED-5D-5L, KOOS, VAS, Tegner, and SANE were obtained at baseline, 3 months, and 6 months post injection. Those who reported unacceptable pain relief at 3 months were withdrawn which included nine patients from the ASA group (13.2%), 44 patients from the HA group (68.8%), and 51 patients from the saline group (75%). Significant differences were found between ASA and HA at the 3-month mark in the EQ-5D-5L pain and anxiety subgroup, KOOS pain, symptoms and ADLs subgroups, and VAS for pain during strenuous work, pain during normal daily living, and overall pain. At 6 months post injection, scores for EQ-5D-5L mobility, activities, pain, health subgroups, KOOS pain, symptoms, ADL, SANE scores, and VAS overall pain demonstrated superior improvement in the ASA group compared to both HA and saline groups. Moreover, there was a larger responder rate for ASA (69.1%) in comparison to HA (39.1%) and saline (42.6%) (see Table 15).
 |
Table 15 Evidence Table Regarding Amniotic Tissue |
In conclusion, this Level 1 randomized controlled trial investigating ASA in symptomatic knee OA depicts greater statistically and clinically significant improvements when compared to the saline and HA control groups. ASA may be a useful option as a non-operative treatment for symptomatic knee OA.218
Consensus Points for Amniotic Tissue
- Intra-articular ASA is an effective treatment option for knee pain secondary to OA; Level I, Grade B, Consensus Moderate
Recommendations Regarding Neurostimulation
Peripheral Nerve Stimulation (PNS)
PNS uses electrical currents to treat selected nerves. This can also be done with peripheral nerve field stimulation (PNfS), where instead of stimulating a specific nerve, the small nerve endings in the tissue are stimulated.219 When selecting patients for PNS, the main complaint of the patient should be a burning paresthesia in the peripheral nerve distribution that supplies the knee. A diagnostic block of the infrapatellar branches of the saphenous and the articular branch of the peroneal nerve should be done prior to consideration of PNS. If the patient responds with transient relief, they may be a good candidate for PNS/PNfS.220 While there are a number of case reports or series supporting the use of PNS for knee pain, there is only one RCT with a small sample size addressing PNS for knee pain221; therefore, there are limited data to support its use currently.222 Prior to pursuing permanent implant, a trial should be performed demonstrating at least 50% reduction of the patient’s pain. The placement of the leads for PNS/PNfS can be placed under fluoroscopic or ultrasound guidance.220 When placed under ultrasound guidance, the nerves can be visualized allowing for the safe placement of the PNS leads while avoiding neurovascular damage.223 The sensory innervation of the knee comes mainly from the femoral and sciatic nerves with a small portion of the posterior aspect of the knee being supplied by the posterior branch of the obturator nerve.224,225 Therefore, placement of the leads should be done under image guidance with the goal to place the leads in the area of the femoral and sciatic nerves.222
Consensus Points for Peripheral Nerve Stimulation (PNS)
- PNS is an effective treatment option for chronic post-surgical and neuropathic knee pain; Level IV, Grade I, Consensus Moderate
Dorsal Root Ganglion Stimulation (DRG)
In contrast to PNS, in the short time since the introduction of DRG, there have been a number of studies supporting its use for chronic knee pain, specifically, postsurgical joint pain in the knee.226 In a single-center retrospective study of 14 patients implanted with DRG systems, 8 patients underwent a single L3 lead implanted, 1 patient had a single L4 lead implanted, and 3 patients had 2 leads implanted (L3 and L4). Twelve of the 14 had greater than 50% reduction of their pain.227 Similarly, in the FOCUS study, 12 patients with total knee replacements had successful treatment of their pain after DRG stimulation.228 The efficacy of DRG treatment for post-operative pain associated with TKA has additionally been supported by Morgella et al’s outcomes, with 27 patients implanted with DRG systems demonstrating a reduction in VAS of approximately 69%.229 Localization of where to place the DRG stimulation leads was based on a proposed method of predicting target levels using sensory stimulation of the DRG with a radiofrequency cannula.230 In a sample size of 23 patients, the most impactful DRG stimulation occurred at L4 for the knee with optimal lead placements being at L3 and L4.231 While the 2019 Neurostimulation Appropriateness Consensus Committee (NACC) guidelines suggest that two leads may be sufficient for the majority of patients, therapy should be individualized to each patient and may require addition of more leads to depending on the patients’ response to the trial. Overall, DRG is a safe, efficacious and proven therapy for treatment of chronic post-surgical pain, including knee pain.232
Consensus Points for Dorsal Root Ganglion Stimulation
- DRG is a safe and effective treatment option for chronic post-surgical and focal neuropathic pain of the knee (ie, CRPS); Level I, Grade A, Consensus Strong
Recommendations Regarding Arthroscopy
Knee arthroscopy was presented to the scientific public in 1912 by Swedish Physician Dr Nordentoft at the 41st Congress of the German Society of Surgeons in Berlin.233,234 This early description involved applying the use of the lapro-thoroscope in examining the internal workings of the knee primarily in the diagnosis of tubercular disease of the knee for early treatment. By 1925, early US surgeons had started to pioneer/advocate for use of fluid distended arthroscopy of the knee joint to aid in meniscal pathology diagnosis. Dr Phillip Kreuscher published his paper, “Semilunar cartilage disease – a plea for the early recognition by means of the arthroscope” in 1925 and despite early frustration with technical limitations, laid the foundations for expansive growth in use later in the 20th century.235
Arthroscopy was re-introduced in the 1960s to North America after Dr Masaki Watanabe returned from WWII to Japan and drastically improved the technology involved in arthroscopy.235 Widely considered the “Father of Modern Arthroscopy”, his arthroscopic advancements helped usher in rapid adaptation of arthroscopic techniques culminating in 1982 with the formation of a separate Orthopedic Society (Arthroscopy Association of North America) aimed at advancing the scope and outcomes of this type of surgery. Currently, the advent of arthroscopy can be considered one of the three most significant advancements of orthopedic surgery in the last two centuries along with total joint replacement and internal fixation of fractures.235,236
The indications for arthroscopy of the knee have grown tremendously since its first adaptations in the United States back in the early 1920s and 1930s.233 As technology has improved, techniques have followed suit allowing increased diagnosis and treatment options available arthroscopically with a focus on limiting procedural morbidity.233,237 Currently, arthroscopy has become the standard of care for diagnosis of acute internal derangement of the knee and as such is one of the most performed orthopedic surgeries worldwide.238 Despite success in treatment and minimizing morbidity, there are clear contraindications to arthroscopic management. Several randomized controlled trials and subsequent meta analyses have demonstrated that arthroscopic management of degenerative joint disease has limited midterm benefits and does not represent a significant benefit versus conservative management.239,240 Specifically in middle-aged or older individuals with symptomatic degenerative knee joint disease, arthroscopic management has been shown to have minimal pain improvement and physical function improvement up to 3 months with no difference at 2 years compared to conservative management.239,240
Perioperative Interventions
As technology has increased and the indications for arthroscopic treatment of knee joint pathology have expanded, perioperative interventions aimed at decreasing morbidity have similarly expanded. In terms of specific interventions, deep venous thromboembolism (DVT) prophylaxis, antibiotic therapy, and choice of anesthesia are all considerations.241,242
DVT is a known complication that can occur after knee arthroscopy. Estimated rates of DVT after arthroscopic intervention of the knee range from 0.5% to 41% in the reported literature.243,244 Level IV evidence demonstrates that rates of proximal DVT can be reduced with use of low-molecular-weight heparin (LMWH).243 Other literature has recommended routine chemical prophylaxis in patients with increased venous thrombotic risk including patients with a clotting disorder, history of venous thromboembolism or malignancy, or two or more classic risk factors.245 The 2020 Cochrane review recommendations state low certainty evidence for reduction of pulmonary embolism and symptomatic DVT risk in the healthy adult population with LMWH and no moderate-to-low certainty evidence of no difference in asymptomatic DVT rates with use of LMWH, aspirin, or rivaroxaban.246
In terms of antibiotic prophylaxis, preoperative administration of antibiotic therapy has been shown to drastically reduce rates of local and systemic postoperative infection across orthopedic procedures.247 Some recent literature has questioned the need for antibiotic prophylaxis in the setting of simple knee arthroscopy secondary to such low reported local infection rates; however, consensus statements demonstrate decreased rates of septic arthritis and systemic infection and as such antibiotic prophylaxis is routinely used.247,248
In terms of anesthetic considerations, options between general, epidural, spinal, and regional anesthetics have been considered in knee arthroscopy. Literature results in knee arthroscopy suggest that general anesthesia outperforms neuraxial anesthesia in terms of recovery and satisfaction with decreased rates of post-operative admission.249,250 While Level 1 data are sparce for administration of peripheral anesthetics in ambulatory knee arthroscopy, limited literature suggests no difference in pain reduction or decreased opioid consumption over placebo.251 In contrast, robust literature exists documenting successful and safe use of local anesthesia with IV sedation in reducing recovery time, adequately controlling patients’ pain, and reducing cost.252–256
Surgical Considerations
Surgical considerations during all arthroscopic surgery of the knee currently include tourniquet use, and tranexamic acid use. Traditionally, tourniquet use has been debated as proponents have advocated better visualization during the procedure, but complications such as neurologic injuries, vascular injuries, increased pain, post-operative stiffness, and functional weakness have been reported.257 A large meta-analysis comparing tourniquet use for arthroscopic ACL reconstruction demonstrated that the tourniquet group experienced a fewer visualization difficulty events during the procedure; however, all other measured outcomes were no different.257 A more recent systematic review and meta-analysis suggested that the visualization benefit was more historic and that when directly compared, the use of a no tourniquet for simple knee arthroscopy resulted in decreased post-operative opioid requirement and less post-operative blood loss with no difference in operative time, post-operative pain score between groups, or post-operative functional strength.258
Tranexamic acid has been found to reduce post-operative swelling, hemarthrosis incidence, and improve early post-operative function in total joint arthroplasty.259 As such its use in knee arthroscopy has increased in use as a mechanism to both improve visualization and decrease symptomatic post-operative hemarthrosis. Recent literature suggests that routine use of tranexamic acid (TXA) for simple and complex knee arthroscopy can result not only in decreased rates of symptomatic post-operative hemarthrosis but also result in creased early patient-reported outcomes.259 The safety of systemic and local administration of TXA has been well documented260; however, some concerns regarding biologic injury to native adult human cartilage have been raised with routine TXA use for arthroscopic procedures based on in vitro toxicity.261 However, studies have demonstrated no difference in decreased hemarthrosis and improved post-operative functional scores with local or systemic administration of TXA and as such increasingly surgeons are routinely using TXA in knee arthroscopy.262
Outcomes
When correctly indicated knee arthroscopy can be a highly successful operation with good patient-reported outcomes and a low rate (<5%) of clinical complications with decreased morbidity compared to equivalent open techniques.263 However, certain preoperative patient factors and post-operative interventions have demonstrated an effect on overall outcomes. Smoking, or specifically clinically relevant levels of nicotine, places patients at increased risk of post-operative complication.264
Historic literature has estimated that the majority of patients who undergo simple knee arthroscopy demonstrate no knee-related activity restrictions by 4 weeks.265 As such, the routine use of physical therapy after routine simple knee arthroscopy has been controversial with no clear consensus across the largest reviews.266,267 Data for the use of physical therapy in the setting of complex arthroscopic reconstructions are heterogeneous.
Cryotherapy has been shown to be of benefit in reducing post-operative pain after arthroscopic ACL reconstruction while not demonstrating a difference in range of motion.268 The data are less clear ion the effect that cryotherapy has on patient-reported functional outcomes.269 For simple arthroscopic procedures, cryotherapy post-operatively has been shown to decrease 24-h opioid consumption and pain scores compared to placebo.270
Consensus Points for Knee Arthroscopy
- Arthroscopic knee surgery is a safe and effective treatment option for repairing soft tissue injuries and minor bony pathologies that cannot be rectified via conservative measures; Level I, Grade A, Consensus Strong
- Arthroscopic knee surgery is effective for the treatment of knee OA; Level II-2, Grade C, Consensus Weak
Recommendations Regarding Joint Preservation Techniques
With increased life expectancy, the concern surrounding articular cartilage damage continues to increase. Increasing obesity rates, changing lifestyles and an increasingly aging population have contributed to a double prevalence of OA over the 1999–2014 time period.271 It is now estimated that 31 million, or roughly >13% of the United States adult population suffers symptomatic, painful, functionally limiting arthritis representing a significantly morbid clinical and economic burden for the healthcare system.271,272 This trend holds at a regional, national, and global analytical level as well.
The intrinsic biomechanical property of cartilage itself makes it specifically at risk for progressive injury. Articular cartilage (type II hyaline cartilage) is on average 2–4 mm thick with a notable absence of blood vessels or innervation, relying on diffusion as a primary source of obtaining nutrients.273 As such, injury through this complex structure represents a significant challenge for healing and a compromise to the entire structure. Attempts at regeneration of type II cartilage are ongoing, but nevertheless, treatment of isolated articular lesions remains a clinical focus for improvement in the orthopedic community.274
Surgeons have struggled with developing techniques to combat the progression of symptomatic isolated cartilage lesions into arthritis since the advent of arthroscopy. Several reviews have estimated that the rate of Grade II of greater cartilage lesions in patients with symptomatic knee pain exceeds 60% based upon arthroscopic diagnosis.275,276 The techniques traditionally employed to treat these lesions have included marrow stimulation, autologous chondrocyte implantation, chondral transplantation (either autogenous or allograft), soft tissue procedures such as meniscal transplantation, and alignment surgeries such as osteotomies.274
Marrow Stimulation
Marrow stimulation which encompasses abrasive sub-chondroplasty, microfracture, or subchondral drilling aims at brining bleeding through the subchondral plate in the area of cartilage injury with the end goal of relocating mesenchymal stem cells into the area of injury.277–279 This migration coalesces with formation of a clot in the cartilage defect and eventual development of Type I fibrocartilage to replace the Type II hyaline defect.278 Historically microfracture has shown good short-to-midterm outcomes specifically for younger patients with smaller (~1 cm2) isolated lesions, but more recent reviews have called into question failure rates, arthritic progression, and return to sport rates.280,281 The biomechanical deterioration of fibrocartilage at 2 years when placed under the cyclic load experience at the joint surface is the impotence behind this concern.277
Autologous Chondrocyte Implantation
In an attempt to improve rates of Type II collagen in areas of traumatic cartilage loss, Brittberg et al in 1987 proposed the harvest and culture of autologous chondrocytes and reimplantation into the defect behind a periosteal membrane (matrix autologous chondrocyte implantation; MACI).274,282 In vivo animal studies in rabbit and equine models had previously been promising with second-look histological samples demonstrating >74% Type II collagen.274,282 Current indications for MACI include young active patients who have failed conservative management with isolated cartilage lesions ≥2 cm2 with no subchondral bone involvement, BMI <36 kg/m2, and no mechanical malalignment.283–285 The majority of failures happen early with this treatment (<24 months) and the overall complication rates are low with arthrofibrosis from open implantation the most commonly reported complication.283 Despite this, good short-to-midterm results have been reported with >70% of the grafts intact at long-term follow-up in some series.286,287
Osteochondral Autograft Transplantation (Mosaicplasty)
In addition to a two-stage chondrocyte culture and re-implantation, bulk allograft or autograft osteochondral transplantation has been proposed as a mechanism of restoring type II hyaline cartilage to a focally painful defect.274 Osteochondral autograft transplantation is the concept of taking a plug(s) of articular cartilage and subchondral bone from a donor site of decreased utility and implanting this into the focally painful defect to restore motion arc and encourage Type II collagen ingrowth.288 The advantages include a single-stage procedure, ability to treat small to medium sized articular lesions with multiple “plugs” and restoration of Type II collagen at the joint surface. The disadvantages primarily include donor site morbidity and contour matching.289,290 Good Level I evidence suggests that for medium sized defects, 2–5 cm2 in patients 18–50 years old, osteochondral autograft transplantation results in better clinically relevant outcomes compared to microfracture at 2, 5, and 10 years.290 Despite these results, concern for donor cite morbidity with ongoing pain rates from 5% to 13% have driven treating physicians to continue searching for optimal joint preservation techniques.289,291,292
Osteochondral Allograft Transplantation
For larger lesions, autograft osteochondral harvesting carries to great of risk of morbidity and as such osteochondral allograft transplantation (OATs) has traditionally been used.274 Larger lesions, uncontained lesions, multiple lesions, and revision situations have traditionally been treated with OATs to allow restoration of joint architecture in addition to Type II cartilage ingrowth. The concerns for OATs have traditionally been the maintenance of chondrocyte viability from donor tissue and potential transmission of infectious diseases from the deceased host.274 Laboratory studies demonstrate superior chondrocyte viability with fresh allografts; however, good results and chondrocyte viability have still been demonstrated with frozen grafts implanted within 28 days from harvest.293,294 Additionally, results for OATs are difficult to standardize as satisfaction, survivorship, and functional scores are dependent upon patient factors and treatment area.295 In general, increasing age, obesity, malalignment, salvage use, bipolar cartilage lesions, or use in conjunction with meniscal transplant have portended worse outcomes.274,295 Added clinical caution is warranted as arthroplasty literature suggests that patients converted from OATs to a total knee arthroplasty represent an increased technical demand during the procedure and can expect higher rates of revision surgery.296
Meniscal Transplantation
Other procedures designed at joint preservation include restoration of joint contact pressures, lubrication, and stability with meniscal allograft transplantation.274,297 Since its first use in the 1980s, meniscal transplantation has gained in popularity for the correctly indicated patient.298 Typically, these patients are young (<50 years), with symptomatic meniscal deficiency, without arthritis, and with normal mechanical alignment and ligamentous stability.298 Technical considerations with meniscal transplantation revolve around graft incorporation to the native bone and joint capsule. While proponents of bone fixation will argue that bone-to-bone healing generates decreased rates of meniscal extrusion on MRI, recent reviews have demonstrated no difference in clinical outcomes with fixation method.299 Current experience suggests that meniscal allograft transplantation provides good pain relief and clinical results at short- and midterm follow-up but a high complication rate (up to 29%) and survivorship of 50% at 16 years.299,300 Longer term follow-up studies and higher-quality literature are still needed in this field.
Distal Femoral and Proximal Tibial Osteotomies
The final tool in the orthopedic armamentarium for joint preservation about the knee has been mechanical alignment procedures. Patients with greater than 5 degrees of mechanical malalignment have been shown to be at increased risk of progressive arthritic deformity in the overloaded knee compartment.274 This poses a difficult problem as younger patients with symptomatic unicompartmental arthritic changes are at increased risk of failure and dissatisfaction with arthroplasty procedures.301 As such, distal femoral and proximal tibial osteotomies have been proposed to neutralize alignment and treat unicompartmental pain. For varus deformities, a closing wedge lateral tibial/femoral osteotomy or opening medial femoral/tibial osteotomy is typically performed. Conversely, for valgus deformities, an opening wedge lateral tibial/femoral osteotomy or closing wedge medial femoral/tibial osteotomy can be performed. Comparison of opening and closing wedge osteotomies is not definitive and advantages/risks exist with either treatment method. Closing wedge osteotomy proponents advocate stability of bone-on-bone apposition for healing, while opening wedge osteotomy proponents argue that correction is less reliant upon preoperative planning and there is greater ability to augment correction intraoperatively.302 In addition, specific anatomic concerns with each approach make this surgeon dependent. Despite this, literature suggests that young patients (<60 years of age), with isolated unicompartmental arthritis, good preoperative range of motion and ligamentous stability benefit clinically from distal femoral osteotomy or high tibial osteotomy.274,301–304 There are concerns however as survivorship at 10 years is somewhere between 51% and 90%, and conversion from an osteotomy to an arthroplasty is technically demanding.305
Consensus Points for Joint Preservation Techniques
- Marrow stimulation is an effective treatment for younger patients with small, isolated hyaline defects; Level II-2, Grade C, Consensus Moderate
- Autologous chondrocyte implantation is an effective treatment for young patients with small, isolated cartilage lesions ≥2 cm2 who have tried and failed conservative care; Level II-2, Grade C, Consensus Moderate
- Mosaicplasty is an effective long-term treatment option for patients 18–50 years old with hyaline cartilage lesions 2–5 cm2; Level I, Grade A, Consensus Moderate
- OAT is an effective for knee joint preservation technique; Level II-2, Grade C, Consensus Weak
- Meniscal transplantation is an effective treatment option for patients with symptomatic meniscal deficiency; Level II-2, Grade B, Consensus Moderate
- Distal femoral and high tibial osteotomy are effective treatment options that can delay, or even prevent, the need for a partial or total knee replacement by preserving damaged joint tissue; Level I, Grade A, Consensus Strong
Knee Joint Arthroplasty
Total knee arthroplasty (TKA) is an incredibly popular solution for painful end-stage knee arthritis that has developed significantly over the last 50+ years. As early at the mid-19th century, physicians discussed soft tissue interposition grafting to alleviate knee pain but with little success.306–309 The idea of artificial replacement of the tibia and condyles came into focus in the 1950s, likely first attempted by Dr McKeever.306 The 1970s saw several biomechanical versions of knee replacement including condylar replacement, hinged replacement and resurfacing all with high component failure rates and high rates of infection.310 Modern implant designs come from further focus on knee kinematics after replacement. Dr Insall proposed adding a cam-post to knee replacement to aid in femoral roll back and allow an increased degree of flexion.310 Continued improvement of metallurgy, polyethylene wear rates. and kinematic designs have yielded high functioning prosthesis, and currently total knee arthroplasty is among the most performed orthopedic procedure worldwide.
Total knee arthroplasty is a well-described treatment for painful knee arthritis that has failed appropriate conservative therapy. The goal of arthroplasty is to alleviate pain and improve function secondary to pain alleviation.311 Correction of limb deformity secondary to structural progression of arthritis is also a relative indication for joint replacement surgery.311,312 Appropriate conservative therapy can include non-steroidal anti-inflammatories, weight loss, activity modification, bracing, physical therapy, walking aids, and intraarticular injections.311 Outside of the above indications, age, weight, or other medical cutoffs are debated across the literature.312 Contraindications include active sepsis, active local joint infection, or a medically unstable patient, while relative contraindications include neuropathic joint, morbid obesity, active smoking, or severe peripheral vascular disease.312
Perioperative Interventions
In addition to implant design improvement, technical improvement, and enhanced recovery protocols, patient modifiable risk factors also play an important role in optimizing outcomes after total joint arthroplasty.313 In the United States in particular, an increasingly aged population in combination with a longer lifespan is predicted to increase arthroplasty rates significantly over the next decades.313 While non-modifiable risk factors include age, race, gender, and stable chronic disease, modifiable risk factors such as cardiovascular disease, morbid obesity, poorly controlled diabetes, opioid use, tobacco use, deconditioning, poor dentition, preoperative anemia and Staphylococcus aureus colonization have been areas of focus to reduce rates of complications and failure.314
Specific focus has been on improving obesity rates as literature suggests that joint arthroplasty does not result in weight loss for patients, and in addition, obese patients have increased operative time, more frequent rates of infection, greater malpositioning of implants, and increased rates of early failure.314,315 This has led to a current work group recommendation to delay patients with BMI >40 kg/m2.314 In terms of diabetic control, there is debate in the literature on optimal cut-off points for elective total joint surgery. However, the general consensus seems to be that with a hemoglobin A1c >7.7% patients are at an increased risk of periprosthetic joint infection.316 Smoking, or smokeless tobacco use, has also been reported as the single most important factor in wound healing problems and current smoking has been associated with increased risk of implant loosening, readmission, and mortality as well as wound complications.317,318 Current protocols for cessation or tobacco testing are also still debated, but literature does suggest that preoperative smoking cessation interventions may be effective and decrease the risk of these complications.319
Literature has consistently demonstrated that the largest predictor of prolonged opioid use after surgery is opioid use prior to surgery and the amount of opioid use is correlated with increased length of stay, complications, and even 90-day mortality after elective total joint arthroplasty.320,321 Clear patient expectations for post-operative prescribing patterns are recommended, but exact cut-offs are varied. In terms of dentition, the current recommendation from the American Dental Association is to maintain oral hygiene prior to elective total joint replacement; however, recommendations on dental clearance are not uniformly recommended.322 Anemia is another strong predictor of increased post-operative cardiovascular and infectious complications.323 Recommendations for screening and intervention are mixed, and some experts have recommended anemia work up for Hgb <11 g/dL, but the majority of literature has focused on minimizing perioperative blood loss as a means of preventing symptomatic post-operative acute surgical blood loss anemia.314,324
In summary, modifiable risk factors exist in the arthroplasty population that warrant further work on evidence-based protocols for clearance for these elective procedures. It is estimated that >43% of the patients undergoing revision total knee surgery had at least one modifiable risk factor unoptimized at the time of index surgery.325 While cut-offs for denial of elective joint arthroplasty have demonstrated the ability to decrease complication and revision rates (HA1c <7%, Hgb >11 g/dL, BMI <35 kg/m2, albumin >3.5 g/L) in some studies, specific recommendations are still group/patient/practitioner dependent.326
Surgical Considerations
As arthroplasty and patient selection have evolved, surgical technique and implant design have similarly improved. Specifically, varied approaches, metallurgy, and implant biomechanics have been compared with some consensus.
The standard extensile anterior medial parapatellar approach to the knee historically provides excellent access to the knee joint, ability to correctly position implants and correct deformity.327 In the 1990s minimally invasive or quadriceps sparing approaches to the knee joint including the sub/mid vastus approaches were reported to offer improved early post-operative pain and function compared to the standard paramedian approach.328 More recent literature suggests that objective differences between the approaches may not be as clinically relevant as earlier reported and the choice of approach should be based more upon patient-specific anatomy and surgeon familiarity.329
With the evolution of polyethylene components in total knee arthroplasty minimizing ultimate poly failure and wear rates, attention has turned to the metallurgy of the implants in volved. Reports of metal hypersensitivity to nickel containing implant alloys remain a relevant controversy.330 While modern implants are made with significantly less nickel in their alloys, reports of hypersensitivity reactions range up to 10% in varying degrees.331,332 Standard metal allergy screening is not a consensus recommendation; however, nonmetal containing implants do exist for select use at the discretion of the treating surgeon and the patient.330
When selecting an implant design, total knee implants come on a spectrum of varying degrees of constraint ranging from a cruciate sacrificing implant all the way to a fully constrained hinge.333 Traditionally primary total knee arthroplasty has been divided into cruciate retaining implants (posterior cruciate retaining) and posterior stabilized designs. There are proponents of both designs as they have differencing biomechanical mechanisms contributing to flexion and posterior femoral rollback.333 However, the majority of direct comparisons have not demonstrated a significant clinical difference in function, satisfaction, or revision-free survivorship.334,335
After design has been selected, fixation strategy is another perioperative surgical consideration which has returned to controversy in the modern arthroplasty world. Traditional designs rely upon cement fixation to grout implants to the biologic bone surface. With the success of biologic bone ingrowth fixation in other joints, comparison between cemented and uncemented total knee arthroplasty has received increased interest. Currently, short- and midterm outcomes with modern implants demonstrate equivalent survivorship and functional outcomes.336–339
Outcomes/Revision Surgery
Large database and population-based studies have demonstrated total knee arthroplasty is a safe and common procedure which results in marked improvements in quality of life, pain relief, and function.340 Factors that have been associated with improved patient outcome scores and implant survival outside of patient modifiable risk factors include age, sex, socioeconomic status and surgeon experience/volume.341–343
However, even when these factors are controlled for, revision total knee arthroplasty surgeries historically have not demonstrated the same degree of improvements subjectively or objectively as their primary counterparts.341 Despite an almost 9% decrease in patient-reported outcomes with revision TKA compared to primary TKA, good results are still obtainable with modern bone augmentation and fixation techniques.341,344
Consensus Points for Knee Joint Arthroplasty
- Knee joint arthroplasty is an effective surgical option for treating symptomatic knee OA that fails conservative treatment options; Level I, Grade A, Consensus Strong
Conclusion
The diagnosis and care of knee pain is an evolving area of medicine that is rife with innovation and emerging treatments. Considering the commonality of this malady in the aging and injured population, it is imperative to have a consistent treatment algorithm that is recognized and followed across the various specialties of medicine that encounter these patients. While the current paradigm still emphasizes palliative treatments as a means of prolonging or avoiding the need for surgical intervention, there is no consistency or clear agreement on which treatments should be provided at the various stages in the patient journey.
The guidance provided in this document is intended to delineate which treatments are proven to be the most efficacious and suggest the order in which they should be offered to a particular patient based on current peer reviewed evidence supplemented with expert opinion by a heterogeneous group of well-experienced clinicians. As newer modalities continue to enter the space, there will be an even greater need for guidance and grading of the evidence such that clinicians will be able to offer the right therapy to the right patient at the right time. These processes will change rapidly going forward and ASPN is committed to a living documented that is updated at regular intervals to guide best practices in the international community.
Abbreviations
ACL, Anterior cruciate ligament; ACR, American College of Radiology OR American College of Rheumatology; AD, Assistive device; ADLs, Activities of daily living; AEs, Adverse events; AFO, Ankle foot orthosis; AKSS, American Knee Society Score; AMUC, Amniotic membrane/umbilical cord particulate; ASA, Amniotic suspension allograft; ASPN, American Society for Pain and Neuroscience; BMAC, Bone marrow aspirate concentrate; BMI, Body mass index; BMLs, Bone marrow lesions; CDC, Centers for Disease Control; COX-2, Cyclooxygenase-2; CRPS, Complex regional pain syndrome; CT, Computed tomography; DHEP, Diclofenac hydroxyethylpyrrolidine; DME, Durable medical equipment; DRG, Dorsal root ganglion; DSG, Diclofenac sodium gel; DVT, Deep venous thromboembolism; EMG, Electromyography; EULAR, European League Against Rheumatism; GNA, Genicular nerve ablation; GPI, Global perceived improvement; HA, Hyaluronic acid; HAFCs, Human amniotic fluid-derived cells; HAM, Human amniotic membrane; IAC, IACS, Intra-articular corticosteroid; IAHA, Intra-articular hyaluronic acid; IAS, Intra-articular steroid; ICOAP, Intermittent and constant osteoarthritis pain index; IDEA‐033, Ultra‐deformable carrier loaded with ketoprofen for epicutaneous application; IKDC, International Knee Documentation Committee; IL, Inferior lateral; IM, Inferior medial; IPB, Infrapatellar branch of the saphenous nerve; IPM, Interventional Pain Management—Quality Appraisal of Reliability and Risk of Bias Assessment; ISK, Index of Severity for Osteoarthritis for the Knee; IT, Iliotibial; IV, Intravenous; JOA, Japan Orthopaedic Association; KOOS, Knee Osteoarthritis Outcome Score; LCL, Lateral collateral ligament; LMWH, Low-molecular-weight heparin; LR, Lateral retinacular nerve; MACI, Matrix autologous chondrocyte implantation; MCL, Medial collateral ligament; MODEMS, Musculoskeletal Outcomes Data Evaluation and Management System; MR, Medial retinacular nerve; MRI, Magnetic resonance imaging; MSCs, Mesenchymal stem cells; NACC, Neurostimulation Appropriateness Consensus Committee; NEATS, National Guideline Clearinghouse Extent Adherence to Trustworthy Standards; NRS, Numeric rating scale; NSAIDs, Non-steroidal anti-inflammatory drugs; OA, Osteoarthritis; OARSI, OsteoArthritis Research Society International; OAT, Osteochondral allograft transplantation; OKS, Oxford Knee Score; OMERACT, Outcome Measures in Rheumatology; PCL, Posterior cruciate ligament OR Posterior collateral ligament; PFL, Popliteal fibular ligament; PFPS, Patellofemoral pain syndrome; PGA, Patient global assessment; PGA, Patient global assessment; PGIC, Patient global impression of change; PNfS, Peripheral nerve field stimulation; PNS, Peripheral nerve stimulation; PROs, Patient-reported outcomes; PRP, Platelet-rich-plasma; PT, Physical therapy; PVNS, Pigmented villonodular synovitis; QALYs, Quality-adjusted life years; QAREL, Quality Appraisal of Reliability Studies; QOL, Quality of life; QOL, Quality of life; RCT, Randomized controlled trial; RFA, Radiofrequency ablation; RFN, Recurrent fibular nerve; ROM, Range of motion; SANE, Single assessment numeric evaluation; SF-12, −36, Short-Form Health Survey 12- or 36-item; SL, Superior lateral; SM, Superior medial; SPB, Suprapatellar branch of the saphenous nerve; STEP, Consensus Guidelines on Interventional Therapies for Knee Pain; TCA, Tricyclic antidepressants; TENS, Transcutaneous electrical nerve stimulation; TKA, Total knee arthroplasty; TKR, Total knee replacement; TRFA, Thermal RFA; TXA, Tranexamic acid; USPSTF, US Preventive Services Task Force; VAS, Visual analog scale; VMO, Vastus medialis oblique; WOMAC, The Western Ontario and McMaster Universities Osteoarthritis Index.
Acknowledgments
The authors thank Allison Foster, PhD, for editing services in the preparation of this manuscript.
Disclosure
CWH is a consultant for Abbott, Averitas, Biotronik, Boston Scientific, Mainstay, Nalu, PainTEQ, Saluda, SKK, Vivex. Funded Research by Abbott, Boston Scientific, Discgenics, Mesoblast, Saluda, TissueGene, Vivex. Grants from Saluda, PainTEQ, and Mainstay, outside the submitted work. TRD is a consultant/Research Investigator for Abbott, Avanos, Medtronic, Boston Scientific, Saluda, Nalu, Cornorloc, PainTEQ, Spinal Simplicity, Mainstay Medical, Ethos, Spinethera, SPR Therapeutic, Tissue Tech and Vertos Medical. Funded Research by Abbott, Boston Scientific, Nalu and PainTEQ. In addition, Dr TDR has a patent Abbott pending to DRG Surgical Leads and stock options from Vertos Medical, SpineThera, Saluda Medical, Nalu Medical, Cornerloc, SPR Therapeutic, PainTEQ and Spinal Simplicity; Common stock in Ethos. TD is a consultant for Abbott and Vivex. Funded Research by Discgenics, Mesoblast, TissueGene, Vivex. Research Support, OA Knee Study from Biostar, Kolon Tissuegene and Xalud, outside the submitted work. MFE is a consultant for Abbott, Boston Scientific, Flowonix, Medtronic, Nevro and Stimwave, outside the submitted work. JHG is a consultant for Abbott and Saluda, reports personal fees from Abbott, Stratus Medical, Research Support from SPR Therapeutics and Mainstay Medical, outside the submitted work. Funded Research for Saluda. SP is a consultant for Abbott. JSW is a consultant for Abbott and reports personal fees from Medtronic, Saluda, Biotronik, and SI Bone, during the conduct of the study. AC reports consultant from Arthrex and Zimmer Biomet, outside the submitted work. DB is a consultant for Discgenics, Mesoblast, Vivex, Medtronic, Spineology, Merit Medical, Johnson and Johnson, IZI, Techlamed, Peterson Enterprises, Medical Metrics, Radius Pharmaceuticals, Avanos, Boston Scientific, Sollis Pharmaceuticals, Simplify Medical, Stryker, Lenoss Medical, Spine BioPharma, Piramal, ReGelTec, Nanofuse, Spinal Simplicity, Pain Theory, Spark Biomedical, Micron Medical Corp, Bronx Medical, Smart Soft, Tissue Tech, Kahtnu Surgical, RayShield, Stayble, Thermaquil, Stratus Medical, Genesys, Abbott, Eliquence, SetBone Medical, Amber Implants, Cerapedics, Neurovasis, outside the submitted work. DS is a consultant for Boston Scientific, Neuralace, Nevro, PainTEQ, and Saluda. Funded Research by Neuralace, Nevro, PainTEQ, and Saluda. NS is a consultant for Abbott, Nimbus, Saluda, and Nevro. The authors report no other conflicts of interest in this work.
References
1. Nguyen USDT, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155(11):725–732. doi:10.7326/0003-4819-155-11-201112060-00004
2. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi:10.1136/ard.60.2.91
3. Zeni JA, Axe MJ, Snyder-Mackler L. Clinical predictors of elective total joint replacement in persons with end-stage knee osteoarthritis. BMC Musculoskelet Disord. 2010;11(1):86. doi:10.1186/1471-2474-11-86
4. Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain. 2002;100(1–2):55–64. doi:10.1016/s0304-3959(02
5. Gillan B. Top 10 Demographics & Interests Facts About Americans Age 50+. American Association of Retired Persons; 2014. Available from: https://blog.aarp.org/notebook/top-10-demographics-interests-facts-about-americans-age-50.
6. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020; 29-30:100587. doi:10.1016/j.eclinm.2020.100587
7. Wylde V, Beswick A, Bruce J, Blom A, Howells N, Gooberman-Hill R. Chronic pain after total knee arthroplasty. EFORT Open Rev. 2018;3(8):461–470. doi:10.1302/2058-5241.3.180004
8. Antony AB, Schultheis BC, Jolly SM, Bates D, Hunter CW, Levy RM. Neuromodulation of the Dorsal Root Ganglion for Chronic Postsurgical Pain. Pain Medicine. 2019;20(Suppl 1):S41–S46. doi:10.1093/pm/pnz072
9. Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehabil. 2006;85(11Suppl):S2–11. doi:10.1097/01.phm.0000245568.69434.1a
10. Lee S, Kim S-J. Prevalence of knee osteoarthritis, risk factors, and quality of life: the Fifth Korean National Health And Nutrition Examination Survey. Int J Rheum Dis. 2017;20(7):809–817. doi:10.1111/1756-185X.12795
11. Hiligsmann M, Cooper C, Arden N, et al. Health economics in the field of osteoarthritis: an Expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2013;43(3):303–313. doi:10.1016/j.semarthrit.2013.07.003
12. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling Hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology. 2005;44(12):1531–1537. doi:10.1093/rheumatology/kei049
13. Steiner C, Andrews R, Barrett M, Weiss A HCUP Projections: mobility/Orthopedic Procedures 2003 to 2012. U.S. Agency for Healthcare Research and Quality; 2012. Available from: http://www.hcup-us.ahrq.gov/reports/projections/2012-03.pdf.
14. Bedenbaugh AV, Bonafede M, Marchlewicz EH, Lee V, Tambiah J. Real-world health care resource utilization and costs among US patients with knee osteoarthritis compared with controls. Clinicoeconomics Outcomes Res. 2021;13:421–435. doi:10.2147/CEOR.S302289
15. Laires PA, Canhão H, Rodrigues AM, Eusébio M, Gouveia M, Branco JC. The impact of osteoarthritis on early exit from work: results from a population-based study. BMC Public Health. 2018;18(1):472. doi:10.1186/s12889-018-5381-1
16. Abbott JH, Usiskin IM, Wilson R, Hansen P, Losina E, Lammi MJ. The quality-of-life burden of knee osteoarthritis in New Zealand adults: a model-based evaluation. PLoS One. 2017;12(10):e0185676. doi:10.1371/journal.pone.0185676
17. Manchikanti L. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;2s;11(3;2s):S63–88. doi:10.36076/ppj.2008/11/S63
18. DeMik DE, Bedard NA, Dowdle SB, Burnett RA, McHugh MA, Callaghan JJ. Are we still prescribing opioids for osteoarthritis? J Arthroplasty. 2017;32(12):3578–3582.e1. doi:10.1016/j.arth.2017.07.030
19. Ivers N, Dhalla IA, Allan GM. Opioids for osteoarthritis pain: benefits and risks. Can Fam Physician. 2012;58(12):e708.
20. Nüesch E, Rutjes AW, Husni E, Welch V, Jüni P. Oral or transdermal opioids for osteoarthritis of the knee or Hip. Cochrane Database Syst Rev. 2009;4:CD003115. doi:10.1002/14651858.CD003115.pub3
21. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265–269. doi:10.15585/mmwr.mm6610a1
22. Overdose Death Rates. National Institute on Drug Abuse; 2021. Available from: https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates.
23. Force USPST. Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. U.S. Department of Health and Human Services, Office of Public Health and Science, Office of Disease Prevention and Health Promotion; 1996.
24. Manchikanti L, Hirsch JA, Cohen SP, et al. Assessment of methodologic quality of randomized trials of interventional techniques: development of an interventional pain management specific instrument. Pain Physician. 2014;17(3):E263–290.
25. Lucas NP, Macaskill P, Irwig L, Bogduk N. The development of a quality appraisal tool for studies of diagnostic reliability (QAREL). J Clin Epidemiol. 2010;63(8):854–861. doi:10.1016/j.jclinepi.2009.10.002
26. Furlan AD, Malmivaara A, Chou R, et al. 2015 Updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine. 2015;40(21):1660–1673. doi:10.1097/BRS.0000000000001061
27. Manchikanti L, Falco FJE, Benyamin RM, Kaye AD, Boswell MV, Hirsch JA. A modified approach to grading of evidence. Pain Physician. 2014;17(3):E319–325.
28. Jue JJ, Cunningham S, Lohr K, et al. Developing and Testing the Agency for Healthcare Research and Quality’s National Guideline Clearinghouse Extent of Adherence to Trustworthy Standards (NEATS) Instrument. Ann Intern Med. 2019;170(7):480–487. doi:10.7326/M18-2950
29. Examination and Special Tests Of The Knee — orthopaedicPrinciples.com. Available from: https://orthopaedicprinciples.com/2017/10/examination-and-special-tests-of-The-knee/.
30. Rossi R, Dettoni F, Bruzzone M, Cottino U, D’Elicio DG, Bonasia DE. Clinical examination of the knee: know your tools for diagnosis of knee injuries. Sports Med Arthroscopy Rehabilitation Therapy Technol. 2011;3:25. doi:10.1186/1758-2555-3-25
31. Physical Exam and History for Osteoarthritis. Available from: https://wa.kaiserpermanente.org/kbase/topic.jhtml?docId=hw125475.
32. Fox MG, Chang EY, Amini B, et al. ACR Appropriateness Criteria(®) Chronic Knee Pain. J Am Coll Radiol. 2018;15(11S):S302–S312. doi:10.1016/j.jacr.2018.09.016
33. Hayashi D, Roemer FW, Guermazi A. Imaging for osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):161–169. doi:10.1016/j.rehab.2015.12.003
34. Krasnokutsky S, Belitskaya-Lévy I, Bencardino J, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63(10):2983–2991. doi:10.1002/art.30471
35. Grando H, Chang EY, Chen KC, Chung CB. MR imaging of extrasynovial inflammation and impingement about the knee. Magn Reson Imaging Clin N Am. 2014;22(4):725–741. doi:10.1016/j.mric.2014.07.011
36. Wick MC, Kastlunger M, Weiss RJ. Clinical imaging assessments of knee osteoarthritis in the elderly: a mini-review. Gerontology. 2014;60(5):386–394. doi:10.1159/000357756
37. Keen HI, Hensor EMA, Wakefield RJ, Mease PJ. Bingham CO 3rd, Conaghan PG. Ultrasound assessment of response to intra-articular therapy in osteoarthritis of the knee. Rheumatology. 2015;54(8):1385–1391. doi:10.1093/rheumatology/keu529
38. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi:10.1136/ard.16.4.494
39. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–1893. doi:10.1007/s11999-016-4732-4
40. Kaeding CC, Léger-St-Jean B, Magnussen RA. Epidemiology and diagnosis of anterior cruciate ligament injuries. Clin Sports Med. 2017;36(1):1–8. doi:10.1016/j.csm.2016.08.001
41. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi:10.1177/0363546504269591
42. Verhulst FV, MacDonald P. Diagnosing PCL injuries: history, physical examination, imaging studies, arthroscopic evaluation. Sports Med Arthrosc Rev. 2020;28(1):2–7. doi:10.1097/JSA.0000000000000251
43. Edson CJ. Conservative and postoperative rehabilitation of isolated and combined injuries of the medial collateral ligament. Sports Med Arthrosc. 2006;14(2):105–110. doi:10.1097/01.jsa.0000212308.32076.f2
44. Grawe B, Schroeder AJ, Kakazu R, Messer MS. Lateral collateral ligament injury about the knee: anatomy, evaluation, and management. J Am Acad Orthop Surg. 2018;26(6):e120–e127. doi:10.5435/JAAOS-D-16-00028
45. Kopf S, Beaufils P, Hirschmann MT, et al. Management of traumatic meniscus tears: the 2019 ESSKA meniscus consensus. Knee Surg Sports Traumatol. 2020;28(4):1177–1194. doi:10.1007/s00167-020-05847-3
46. Beaufils P, Pujol N. Management of traumatic meniscal tear and degenerative meniscal lesions. Save the meniscus. Orthopaedics Traumatology Surgery Res. 2017;103(8S):S237–S244. doi:10.1016/j.otsr.2017.08.003
47. Cardoso TB, Pizzari T, Kinsella R, Hope D, Cook JL. Current trends in tendinopathy management. Best Pract Res Clin Rheumatol. 2019;33(1):122–140. doi:10.1016/j.berh.2019.02.001
48. Hodgson RJ, O’Connor PJ, Grainger AJ. Tendon and ligament imaging. Br J Radiol. 2012;85(1016):1157–1172. doi:10.1259/bjr/34786470
49. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7(5):415–420. doi:10.1177/1941738114568775
50. Neph A, Onishi K, Wang JHC. Myths and facts of in-office regenerative procedures for tendinopathy. Am J Phys Med Rehabil. 2019;98(6):500–511. doi:10.1097/PHM.0000000000001097
51. Jiménez Díaz F, Gitto S, Sconfienza LM, Draghi F. Ultrasound of iliotibial band syndrome. J Ultrasound. 2020;23(3):379–385. doi:10.1007/s40477-020-00478-3
52. Frederico TN, Peng P editors. Ultrasound-Guided Knee Intervention. Ultrasound for Interventional Pain Management: An Illustrated Procedural Guide.
53. Brown OS, Smith TO, Parsons T, Benjamin M, Hing CB. Management of septic and aseptic prepatellar bursitis: a systematic review. Arch Orthop Trauma Surg. 2021. doi:10.1007/s00402-021-03853-9
54. Baumbach SF, Lobo CM, Badyine I, Mutschler W, Kanz KG. Prepatellar and olecranon bursitis: literature review and development of a treatment algorithm. Arch Orthop Trauma Surg. 2014;134(3):359–370. doi:10.1007/s00402-013-1882-7
55. OA as a Serious Disease; 2016. Available from: https://oarsi.org/research/oa-serious-disease.
56. Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi:10.1001/jama.2020.22171
57. Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–59. doi:10.1056/NEJMcp1903768
58. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–e33. doi:10.1016/S0140-6736(17
59. Wolff DG, Christophersen C, Brown SM, Mulcahey MK. Topical nonsteroidal anti-inflammatory drugs in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Phys Sportsmed. 2021;1:1–11. doi:10.1080/00913847.2021.1886573
60. Osani MC, Lohmander LS, Bannuru RR. Is there any role for opioids in the management of knee and hip osteoarthritis? A systematic review and meta-analysis. Arthritis Care Res. 2015;1:54. doi:10.1002/acr.24363
61. Richards MM, Maxwell JS, Weng L, Angelos MG, Golzarian J. Intra-articular treatment of knee osteoarthritis: from anti-inflammatories to products of regenerative medicine. Phys Sportsmed. 2016;44(2):101–108. doi:10.1080/00913847.2016.1168272
62. Shim H, Rose J, Halle S, Shekane P. Complex regional pain syndrome: a narrative review for the practising clinician. Br J Anaesth. 2019;123(2):e424–e433. doi:10.1016/j.bja.2019.03.030
63. Harden RN, Bruehl S, Perez RSGM, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain. 2010;150(2):268–274. doi:10.1016/j.pain.2010.04.030
64. Kessler A, Yoo M, Calisoff R. Complex regional pain syndrome: an updated comprehensive review. NeuroRehabilitation. 2020;47(3):253–264. doi:10.3233/NRE-208001
65. Neumeister MW, Romanelli MR. Complex regional pain syndrome. Clin Plast Surg. 2020;47(2):305–310. doi:10.1016/j.cps.2019.12.009
66. Yucel I, Demiraran Y, Ozturan K, Degirmenci E. Complex regional pain syndrome type I: efficacy of stellate ganglion blockade. J Orthopaedics Traumatol. 2009;10(4):179–183. doi:10.1007/s10195-009-0071-5
67. Urits I, Shen AH, Jones MR, Viswanath O, Kaye AD. Complex regional pain syndrome, current concepts and treatment options. Curr Pain Headache Rep. 2018;22(2):10. doi:10.1007/s11916-018-0667-7
68. Macmull S, Jaiswal PK, Bentley G, Skinner JA, Carrington RWJ, Briggs TWR. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int Orthop. 2012;36(7):1371–1377. doi:10.1007/s00264-011-1465-6
69. Hong E, Kraft MC. Evaluating anterior knee pain. Med Clin North Am. 2014;98(4):697–717. doi:10.1016/j.mcna.2014.03.001
70. Aksahin E, Aktekin CN, Kocadal O, et al. Sagittal plane tilting deformity of the patellofemoral joint: a new concept in patients with chondromalacia patella. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3038–3045. doi:10.1007/s00167-016-4083-4
71. Tuna BK, Semiz-Oysu A, Pekar B, Bukte Y, Hayirlioglu A. The association of patellofemoral joint morphology with chondromalacia patella: a quantitative MRI analysis. Clin Imaging. 2014;38(4):495–498. doi:10.1016/j.clinimag.2014.01.012
72. Lack S, Barton C, Vicenzino B, Morrissey D. Outcome predictors for conservative patellofemoral pain management: a systematic review and meta-analysis. Sports Med. 2014;44(12):1703–1716. doi:10.1007/s40279-014-0231-5
73. von Keudell A, Han R, Bryant T, Minas T. Autologous chondrocyte implantation to isolated patella cartilage defects. Cartilage. 2017;8(2):146–154. doi:10.1177/1947603516654944
74. Winter AR, Collins JE, Katz JN. The likelihood of total knee arthroplasty following arthroscopic surgery for osteoarthritis: a systematic review. BMC Musculoskelet Disord. 2017;18(1):408. doi:10.1186/s12891-017-1765-0
75. Chawla H, van der List JP, Christ AB, Sobrero MR, Zuiderbaan HA, Pearle AD. Annual revision rates of partial versus total knee arthroplasty: a comparative meta-analysis. The Knee. 2017;24(2):179–190. doi:10.1016/j.knee.2016.11.006
76. Flierl MA, Sobh AH, Culp BM, Baker EA, Sporer SM. Evaluation of the painful total knee arthroplasty. JAAOS. 2019;27(20):743–751. doi:10.5435/JAAOS-D-18-00083
77. Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. BJA. 2015;114(4):551–561. doi:10.1093/bja/aeu441
78. Li J, Ma Y, Xiao L. Postoperative pain management in total knee arthroplasty. Orthop Surg. 2019;11(5):755–761. doi:10.1111/os.12535
79. Yu S, Dundon J, Solovyova O, Bosco J, Iorio R. Can multimodal pain management in TKA eliminate patient-controlled analgesia and femoral nerve blocks? Clin Orthop Relat Res. 2018;476(1):101–109. doi:10.1007/s11999.0000000000000018
80. Koh IJ, Kim MS, Sohn S, Song KY, Choi NY, In Y. Duloxetine reduces pain and improves quality of recovery following total knee arthroplasty in centrally sensitized patients: a prospective, randomized controlled study. JBJS. 2019;101(1):64–73. doi:10.2106/JBJS.18.00347
81. Rakel BA, Zimmerman BM, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo-controlled trial. Pain. 2014;155(12):2599–2611. doi:10.1016/j.pain.2014.09.025
82. Balanescu AR, Feist E, Wolfram G, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or Hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis. 2014;73(9):1665–1672. doi:10.1136/annrheumdis-2012-203164
83. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi:10.1056/NEJMoa052771
84. Schnitzer TJ, Ekman EF, Spierings ELH, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or Hip osteoarthritis pain. Ann Rheum Dis. 2015;74(6):1202–1211. doi:10.1136/annrheumdis-2013-204905
85. Ekman EF, Gimbel JS, Bello AE, et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol. 2014;41(11):2249–2259. doi:10.3899/jrheum.131294
86. Ohtori S, Inoue G, Orita S, et al. Efficacy of combination of meloxicam and pregabalin for pain in knee osteoarthritis. Yonsei Med J. 2013;54(5):1253–1258. doi:10.3349/ymj.2013.54.5.1253
87. Simon LS, Grierson LM, Naseer Z, Bookman AAM, Shainhouse ZJ. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143(3):238–245. doi:10.1016/j.pain.2009.03.008
88. Yoo WH, Yoo HG, Park SH, et al. Efficacy and safety of PG201 (Layla(®)) and celecoxib in the treatment of symptomatic knee osteoarthritis: a double-blinded, randomized, multi-center, active drug comparative, parallel-group, non-inferiority, phase III study. Rheumatol Int. 2014;34(10):1369–1378. doi:10.1007/s00296-014-2964-8
89. DeLemos BP, Xiang J, Benson C, et al. Tramadol hydrochloride extended-release once-daily in the treatment of osteoarthritis of the knee and/or Hip: a double-blind, randomized, dose-ranging trial. Am J Ther. 2011;18(3):216–226. doi:10.1097/MJT.0b013e3181cec307
90. Levy R, Khokhlov A, Kopenkin S, et al. Efficacy and safety of flavocoxid compared with naproxen in subjects with osteoarthritis of the knee- a subset analysis. Adv Ther. 2010;27(12):953–962. doi:10.1007/s12325-010-0083-9
91. Pareek A, Chandurkar N, Gupta A, et al. Efficacy and safety of aceclofenac-cr and aceclofenac in the treatment of knee osteoarthritis: a 6-week, comparative, randomized, multicentric, double-blind study. J Pain. 2011;12(5):546–553. doi:10.1016/j.jpain.2010.10.013
92. Chiranthanut N, Hanprasertpong N, Teekachunhatean S. Thai massage, and Thai herbal compress versus oral ibuprofen in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. Biomed Res Int. 2014;2014:490512. doi:10.1155/2014/490512
93. Bianchi M, Broggini M, Balzarini P, Franchi S, Sacerdote P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract. 2007;61(8):1270–1277. doi:10.1111/j.1742-1241.2007.01453.x
94. Meunier A, Lisander B, Good L. Effects of celecoxib on blood loss, pain, and recovery of function after total knee replacement: a randomized placebo-controlled trial. Acta Orthop. 2007;78(5):661–667. doi:10.1080/17453670710014365
95. Zhou TJ, Tang J, White PF. Propacetamol versus ketorolac for treatment of acute postoperative pain after total Hip or knee replacement. Anesth Analg. 2001;92(6):1569–1575. doi:10.1097/00000539-200106000-00044
96. Munteanu AM, Cionac Florescu S, Anastase DM, Stoica CI. Is there any analgesic benefit from preoperative vs. postoperative administration of etoricoxib in total knee arthroplasty under spinal anaesthesia?: a randomised double-blind placebo-controlled trial. Eur J Anaesthesiol. 2016;33(11):840–845. doi:10.1097/EJA.0000000000000521
97. Gong L, Dong JY, Li ZR. Effects of combined application of muscle relaxants and celecoxib administration after total knee arthroplasty (TKA) on early recovery: a randomized, double-blind, controlled study. J Arthroplasty. 2013;28(8):1301–1305. doi:10.1016/j.arth.2012.10.002
98. Alexander R, El-Moalem HE, Gan TJ. Comparison of the morphine-sparing effects of diclofenac sodium and ketorolac tromethamine after major orthopedic surgery. J Clin Anesth. 2002;14(3):187–192. doi:10.1016/s0952-8180(01
99. Reynolds LW, Hoo RK, Brill RJ, North J, Recker DP, Verburg KM. The COX-2 specific inhibitor, valdecoxib, is an effective, opioid-sparing analgesic in patients undergoing total knee arthroplasty. J Pain Symptom Manage. 2003;25(2):133–141. doi:10.1016/s0885-3924(02)00637-1
100. Schroer WC, Diesfeld PJ, LeMarr AR, Reedy ME. Benefits of prolonged postoperative cyclooxygenase-2 inhibitor administration on total knee arthroplasty recovery: a double-blind, placebo-controlled study. J Arthroplasty. 2011;26(6):2–7. doi:10.1016/j.arth.2011.04.007
101. Zhuang Q, Tao L, Lin J, et al. Postoperative intravenous parecoxib sodium followed by oral celecoxib post total knee arthroplasty in osteoarthritis patients (PIPFORCE): a multicentre, double-blind, randomised, placebo-controlled trial. BMJ Open. 2020;10(1):e030501. doi:10.1136/bmjopen-2019-030501
102. Suter E, Herzog W, Souza K, Bray R. Inhibition of the quadriceps muscles in patients with anterior knee pain. J Appl Biomech. 1998;14(4):360–373. doi:10.1123/JAB.14.4.360
103. Underwood M, Ashby D, Carnes D, et al. Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study. Health Technol Assess. 2008;12(22):
104. Baraf HSB, Gloth FM, Barthel HR, Gold MS, Altman RD. Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Drugs Aging. 2011;28(1):27–40. doi:10.2165/11584880-000000000-00000
105. Roth SH, Shainhouse JZ. Efficacy and safety of a topical diclofenac solution (Pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164(18):2017–2023. doi:10.1001/archinte.164.18.2017
106. Niethard FU, Gold MS, Solomon GS, et al. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J Rheumatol. 2005;32(12):2384–2392.
107. Bookman AAM. Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trial. CMAJ. 2004;171(4):333–338. doi:10.1503/cmaj.1031793
108. Barthel HR, Haselwood D, Longley S, Gold MS, Altman RD. Randomized controlled trial of diclofenac sodium gel in knee osteoarthritis. Semin Arthritis Rheum. 2009;39(3):203–212. doi:10.1016/j.semarthrit.2009.09.002
109. Conaghan PG, Dickson J, Bolten W, Cevc G, Rother M. A multicentre, randomized, placebo- and active-controlled trial comparing the efficacy and safety of topical ketoprofen in Transfersome gel (IDEA-033) with ketoprofen-free vehicle (TDT 064) and oral celecoxib for knee pain associated with osteoarthritis. Rheumatology. 2013;52(7):1303–1312. doi:10.1093/rheumatology/ket133
110. Rother M, Lavins BJ, Kneer W, Lehnhardt K, Seidel EJ, Mazgareanu S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis. 2007;66(9):1178–1183. doi:10.1136/ard.2006.065128
111. Brühlmann P, Michel BA. Topical diclofenac patch in patients with knee osteoarthritis: a randomized, double-blind, controlled clinical trial. Clin Exp Rheumatol. 2003;21(2):193–198.
112. Yataba I, Otsuka N, Matsushita I, Matsumoto H, Hoshino Y. Efficacy of S-flurbiprofen plaster in knee osteoarthritis treatment: results from a phase III, randomized, active-controlled, adequate, and well-controlled trial. Mod Rheumatol. 2017;27(1):130–136. doi:10.1080/14397595.2016.1176624
113. Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial [ISRCTN53366886]. BMC Musculoskelet Disord. 2005;6(1):44. doi:10.1186/1471-2474-6-44
114. Kneer W, Rother M, Mazgareanu S, Seidel EJ. A 12-week randomized study of topical therapy with three dosages of ketoprofen in Transfersome® gel (IDEA-033) compared with the ketoprofen-free vehicle (TDT 064), in patients with osteoarthritis of the knee. J Pain Res. 2013;6:743–753. doi:10.2147/JPR.S51054
115. Varadi G. Randomized clinical trial evaluating transdermal ibuprofen for moderate to severe knee osteoarthritis. Pain Physician. 2013;6;16(6;11):E749–762. doi:10.36076/ppj.2013/16/E749
116. Wadsworth LT, Kent JD, Holt RJ. Efficacy and safety of diclofenac sodium 2% topical solution for osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled, 4 week study. Curr Med Res Opin. 2016;32(2):241–250. doi:10.1185/03007995.2015.1113400
117. Baraf HSB, Gold MS, Clark MB, Altman RD. Safety and efficacy of topical diclofenac sodium 1% gel in knee osteoarthritis: a randomized controlled trial. Phys Sportsmed. 2010;38(2):19–28. doi:10.3810/psm.2010.06.1779
118. Tiso RL, Tong-Ngork S, Fredlund KL. Oral versus topical Ibuprofen for chronic knee pain: a prospective randomized pilot study. Pain Physician. 2010;13(5):457–467.
119. Rother M, Conaghan PG. A randomized, double-blind, phase III trial in moderate osteoarthritis knee pain comparing topical ketoprofen gel with ketoprofen-free gel. J Rheumatol. 2013;40(10):1742–1748. doi:10.3899/jrheum.130192
120. Welsch P, Petzke F, Klose P, Häuser W. Opioids for chronic osteoarthritis pain: an updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks double-blind duration. Eur J Pain. 2020;24(4):685–703. doi:10.1002/ejp.1522
121. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872–882. doi:10.1001/jama.2018.0899
122. Catchpool M, Knight J, Young JT, et al. Opioid use prior to elective surgery is strongly associated with persistent use following surgery: an analysis of 14 354 Medicare patients. ANZ J Surg. 2019;89(11):1410–1416. doi:10.1111/ans.15492
123. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total Hip arthroplasty. Pain. 2016;157(6):1259–1265. doi:10.1097/j.pain.0000000000000516
124. Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460. doi:10.1001/jama.2018.18472
125. Vergne-Salle P. Management of neuropathic pain after knee surgery. Joint Bone Spine. 2016;83(6):657–663. doi:10.1016/j.jbspin.2016.06.001
126. Hudson B, Williman JA, Stamp LK, et al. Nortriptyline for pain in knee osteoarthritis: a double-blind randomised controlled trial in New Zealand general practice. Br J Gen Pract. 2021;71(708):e538–e546. doi:10.3399/BJGP.2020.0797
127. Kerrick JM, Fine PG, Lipman AG, Love G. Low-dose amitriptyline as an adjunct to opioids for postoperative orthopedic pain: a placebo-controlled trial. Pain. 1993;52(3):325–330. doi:10.1016/0304-3959(93)90166-M
128. Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010;65(5):500–504. doi:10.1111/j.1365-2044.2010.06310.x
129. Cho C-H, Song K-S, Min B-W, et al. Multimodal approach to postoperative pain control in patients undergoing rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1744–1748. doi:10.1007/s00167-010-1294-y
130. Wluka AE, Urquhart DM, Teichtahl AJ, et al. Effect of low-dose amitriptyline on reducing pain in clinical knee osteoarthritis compared to benztropine: study protocol of a randomised, double blind, placebo-controlled trial. BMC Musculoskelet Disord. 2021;22(1):826. doi:10.1186/s12891-021-04690-y
131. Zhou L, Kwoh CK, Ran D, Ashbeck EL, Lo-Ciganic W-H. Lack of evidence that beta blocker use reduces knee pain, areas of joint pain, or analgesic use among individuals with symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2020;28(1):53–61. doi:10.1016/j.joca.2019.08.008
132. Li M, Zeng Y, Nie Y, et al. The effects of different antihypertensive drugs on pain and joint space width of knee osteoarthritis – a comparative study with data from Osteoarthritis Initiative. J Clin Hypertens. 2021;23(11):2009–2015. doi:10.1111/jch.14362
133. Phillips BB, Muller BA. Severe neuromuscular complications possibly associated with amlodipine. Ann Pharmacother. 1998;32(11):1165–1167. doi:10.1345/aph.18082
134. Smith KM. Arthralgia associated with calcium-channel blockers. Am J Health Syst Pharm. 2000;57(1):55–57. doi:10.1093/ajhp/57.1.55
135. Kaplan N, Yilmaz I, Karaarslan N, Kaya YE, Sirin DY, Ozbek H. Does nimodipine, a selective calcium channel blocker, impair chondrocyte proliferation or damage extracellular matrix structures? Curr Pharm Biotechnol. 2019;20(6):517–524. doi:10.2174/1389201020666190506124548
136. Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66(3):622–636. doi:10.1002/art.38290
137. Smart KM, Wand BM, O’Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Pain, Palliative and Supportive Care Group editor. Cochrane Database of Systematic Reviews. 2016;1:86. doi:10.1002/14651858.CD010853.pub2
138. Brown CK, Southerst D, Côté P, et al. The effectiveness of exercise on recovery and clinical outcomes in patients with soft tissue injuries of the hip, thigh, or knee: a systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) collaboration. J Manipulative Physiol Ther. 2016;39(2):110–120.e1. doi:10.1016/j.jmpt.2016.01.003
139. Baron R, Backonja MM, Eldridge P, et al. Refractory Chronic Pain Screening Tool (RCPST): a feasibility study to assess practicality and validity of identifying potential neurostimulation candidates. Pain Medicine. 2014;15(2):281–291. doi:10.1111/pme.12272
140. Choosing the right knee brace. BetterBraces. Available from: https://www.betterbraces.com/choosing-The-right-knee-brace.
141. Gueugnon M, Fournel I, Soilly A-L, et al. Effectiveness, safety, and cost–utility of a knee brace in medial knee osteoarthritis: the ERGONOMIE randomized controlled trial. Osteoarthritis Cartilage. 2021;29(4):491–501. doi:10.1016/j.joca.2020.11.009
142. van Egmond N, van Grinsven S, van Loon CJ. Is there a difference in outcome between two types of valgus unloading braces? A randomized controlled trial. Acta Orthop Belg. 2017;83(4):690–699.
143. Duivenvoorden T, Brouwer RW, van Raaij TM, Verhagen AP, Verhaar JAN, Bierma-Zeinstra SMA. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Sys Rev. 2015;3:CD004020. doi:10.1002/14651858.CD004020.pub3
144. Robert‐Lachaine X, Dessery Y, Belzile ÉL, Turmel S, Corbeil P. Three-month efficacy of three knee braces in the treatment of medial knee osteoarthritis in a randomized crossover trial. J Orthop Res. 2020;38(10):2262–2271. doi:10.1002/jor.24634
145. Yu SP, Williams M, Eyles JP, Chen JS, Makovey J, Hunter DJ. Effectiveness of knee bracing in osteoarthritis: pragmatic trial in a multidisciplinary clinic. Int J Rheum Dis. 2016;19(3):279–286. doi:10.1111/1756-185X.12796
146. Petersen W, Ellermann A, Henning J, et al. Non-operative treatment of unicompartmental osteoarthritis of the knee: a prospective randomized trial with two different braces—ankle–foot orthosis versus knee unloader brace. Arch Orthop Trauma Surg. 2019;139(2):155–166. doi:10.1007/s00402-018-3040-8
147. Cudejko T, van der Esch M, Schrijvers J, et al. The immediate effect of a soft knee brace on dynamic knee instability in persons with knee osteoarthritis. Rheumatology. 2018;57(10):1735–1742. doi:10.1093/rheumatology/key162
148. Cudejko T, van der Esch M, van den Noort JC, et al. Decreased pain and improved dynamic knee instability mediate the beneficial effect of wearing a soft knee brace on activity limitations in patients with knee osteoarthritis. Arthritis Care Res. 2019;71(8):1036–1043. doi:10.1002/acr.23722
149. Yang X-G, Feng J-T, He X, Wang F, Hu Y-C. The effect of knee bracing on the knee function and stability following anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomized controlled trials. Orthop Traumatol Surg Res. 2019;105(6):1107–1114. doi:10.1016/j.otsr.2019.04.015
150. Kölle T, Alt W, Wagner D. Immediate effects of an elastic patellar brace on pain, neuromuscular activity and knee kinematics in subjects with patellofemoral pain. Arch Orthop Trauma Surg. 2020;140(7):905–912. doi:10.1007/s00402-020-03378-7
151. Added MAN, Added C, Kasawara KT, Rotta VP, de Freitas DG. Effects of a knee brace with a patellar hole versus without a patellar hole in patients with knee osteoarthritis: a double-blind, randomized controlled trial. Eval Health Prof. 2018;41(4):512–523. doi:10.1177/0163278717714307
152. Priore LB, Lack S, Garcia C, Azevedo FM, De oliveira silva D. Two weeks of wearing a knee brace compared with minimal intervention on kinesiophobia at 2 and 6 weeks in people with patellofemoral pain: a randomized controlled trial. Arch Phys Med Rehabil. 2020;101(4):613–623. doi:10.1016/j.apmr.2019.10.190
153. Luque-Suarez A, Martinez-Calderon J, Falla D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: a systematic review. Br J Sports Med. 2019;53(9):554–559. doi:10.1136/bjsports-2017-098673
154. Hewlett J, Kenney J. Innovations in functional and rehabilitative knee bracing. Ann Transl Med. 2019;7(Suppl S7):S248. doi:10.21037/atm.2019.03.34
155. Parkes MJ, Maricar N, Lunt M, et al. Lateral wedge insoles as a conservative treatment for pain in patients with medial knee osteoarthritis: a meta-analysis. JAMA. 2013;310(7):722–730. doi:10.1001/jama.2013.243229
156. Lu Z, Li X, Chen R, Guo C. Kinesio taping improves pain and function in patients with knee osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;59:27–35. doi:10.1016/j.ijsu.2018.09.015
157. Jones A, Silva PG, Silva AC, et al. Impact of cane use on pain, function, general health and energy expenditure during gait in patients with knee osteoarthritis: a randomised controlled trial. Ann Rheum Dis. 2012;71(2):172–179. doi:10.1136/ard.2010.140178
158. Van Ginckel A, Hinman RS, Wrigley TV, et al. Impact of cane use on bone marrow lesion volume in people with medial knee osteoarthritis (Cuba Trial). Phys Ther. 2017;97(5):537–549. doi:10.1093/ptj/pzx015
159. Hollander JL. Intra-articular hydrocortisone in arthritis and allied conditions; a summary of two years’ clinical experience. J Bone Joint Surg Am. 1953;35(4):983–990. doi:10.2106/00004623-195335040-00017
160. Miller JH, White J, Norton TH. The value of intra-articular injections in osteoarthritis of the knee. J Bone Joint Surg Br. 1958;40-B(4):636–643. doi:10.1302/0301-620X.40B4.636
161. Jüni P, Hari R, Rutjes AWS, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(10):CD005328. doi:10.1002/14651858.CD005328.pub3
162. Saltychev M, Mattie R, McCormick Z, Laimi K. The magnitude and duration of the effect of intra-articular corticosteroid injections on pain severity in knee osteoarthritis: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2020;99(7):617–625. doi:10.1097/PHM.0000000000001384
163. Dai W-L, Lin Z-M, Guo D-H, Shi Z-J, Wang J. Efficacy and safety of hylan versus hyaluronic acid in the treatment of knee osteoarthritis. The Journal of Knee Surgery. 2019;32(3):259–268. doi:10.1055/s-0038-1641142
164. Ran J, Yang X, Ren Z, Wang J, Dong H. Comparison of intra-articular hyaluronic acid and methylprednisolone for pain management in knee osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surgery. 2018;53:103–110. doi:10.1016/j.ijsu.2018.02.065
165. Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290(23):3115–3121. doi:10.1001/jama.290.23.3115
166. Miller LE, Fredericson M, Altman RD. Hyaluronic acid injections or oral nonsteroidal anti-inflammatory drugs for knee osteoarthritis: systematic review and meta-analysis of randomized trials. Orthop JSports Med. 2020;8(1):2325967119897909. doi:10.1177/2325967119897909
167. Vincent P. Intra-articular hyaluronic acid in the symptomatic treatment of knee osteoarthritis: a meta-analysis of single-injection products. Curr Ther Res Clin Exp. 2019;90:39–51. doi:10.1016/j.curtheres.2019.02.003
168. McElheny K, Toresdahl B, Ling D, Mages K, Asif I. Comparative effectiveness of alternative dosing regimens of hyaluronic acid injections for knee osteoarthritis: a systematic review. Sports Health. 2019;11(5):461–466. doi:10.1177/1941738119861545
169. Bronstone A, Neary JT, Lambert TH, Dasa V. Supartz (sodium hyaluronate) for the treatment of knee osteoarthritis: a review of efficacy and safety. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2019;12:1179544119835221. doi:10.1177/1179544119835221
170. Sarı S, Aydın ON, Turan Y, Özlülerden P, Efe U, Kurt Ömürlü İ. Which one is more effective for the clinical treatment of chronic pain in knee osteoarthritis: radiofrequency neurotomy of the genicular nerves or intra-articular injection? Int J Rheum Dis. 2018;21(10):1772–1778. doi:10.1111/1756-185X.12925
171. Santana Pineda MM, Vanlinthout LE, Moreno Martín A, van Zundert J, Rodriguez Huertas F, Novalbos Ruiz JP. Analgesic effect and functional improvement caused by radiofrequency treatment of genicular nerves in patients with advanced osteoarthritis of the knee until 1 year following treatment. Reg Anesth Pain Med. 2017;42(1):62–68. doi:10.1097/AAP.0000000000000510
172. Kirdemir P, Çatav S, Alkaya Solmaz F. The genicular nerve: radiofrequency lesion application for chronic knee pain. Turkish J Med Sci. 2017;47(1):268–272. doi:10.3906/sag-1601-171
173. Gupta A. Comparative effectiveness review of cooled versus pulsed radiofrequency ablation for the treatment of knee osteoarthritis: a systematic review. Pain Physician. 2017;3(20;3):155–171. doi:10.36076/ppj.2017.171
174. Davis T, Loudermilk E, DePalma M, et al. Prospective, multicenter, randomized, crossover clinical trial comparing the safety and effectiveness of cooled radiofrequency ablation with corticosteroid injection in the management of knee pain from osteoarthritis. Reg Anesth Pain Med. 2018;43(1):84–91. doi:10.1097/AAP.0000000000000690
175. Jamison DE, Cohen SP. Radiofrequency techniques to treat chronic knee pain: a comprehensive review of anatomy, effectiveness, treatment parameters, and patient selection. J Pain Res. 2018;11:1879–1888. doi:10.2147/JPR.S144633
176. Alcidi L, Beneforti E, Maresca M, Santosuosso U, Zoppi M. Low power radiofrequency electromagnetic radiation for the treatment of pain due to osteoarthritis of the knee. Reumatismo. 2007;59(2):140–145. doi:10.4081/reumatismo.2007.140
177. Choi W-J, Hwang S-J, Song J-G, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. PAIN. 2011;152(3):481–487. doi:10.1016/j.pain.2010.09.029
178. El-Hakeim EH, Elawamy A, Kamel EZ, et al. Fluoroscopic guided radiofrequency of genicular nerves for pain alleviation in chronic knee osteoarthritis: a single-blind randomized controlled trial. Pain Physician. 2018;21(2):169–177.
179. Qudsi-Sinclair S, Borrás-Rubio E, Abellan-Guillén JF, Padilla Del Rey ML, Ruiz-Merino G. A comparison of genicular nerve treatment using either radiofrequency or analgesic block with corticosteroid for pain after a total knee arthroplasty: a double-blind, randomized clinical study. Pain Practice. 2017;17(5):578–588. doi:10.1111/papr.12481
180. Hunter C, Davis T, Loudermilk E, Kapural L, DePalma M. Cooled radiofrequency ablation treatment of the genicular nerves in the treatment of osteoarthritic knee pain: 18- and 24-month results. Pain Practice. 2020;20(3):238–246. doi:10.1111/papr.12844
181. Ikeuchi M, Ushida T, Izumi M, Tani T. Percutaneous radiofrequency treatment for refractory anteromedial pain of osteoarthritic knees. Pain Med. 2011;12(4):546–551. doi:10.1111/j.1526-4637.2011.01086.x
182. Gulec E. Bipolar versus unipolar intraarticular pulsed radiofrequency thermocoagulation in chronic knee pain treatment: a prospective randomized trial. Pain Physician. 2017;3(20;3):197–206. doi:10.36076/ppj.2017.206
183. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008;14(3):249–258. doi:10.1089/ten.teb.2008.0062
184. Hannink M, Donoghue DJ. Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim Biophys Acta. 1989;989(1):1–10. doi:10.1016/0304-419x(89)90031-0
185. Chen CPC, Cheng C-H, Hsu -C-C, Lin H-C, Tsai Y-R, Chen J-L. The influence of platelet rich plasma on synovial fluid volumes, protein concentrations, and severity of pain in patients with knee osteoarthritis. Exp Gerontol. 2017;93:68–72. doi:10.1016/j.exger.2017.04.004
186. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–1975. doi:10.1001/jama.2017.5283
187. Zeng C, Lane NE, Hunter DJ, et al. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27(6):855–862. doi:10.1016/j.joca.2019.01.007
188. Trams E, Kulinski K, Kozar-Kaminska K, Pomianowski S, Kaminski R. The clinical use of platelet-rich plasma in knee disorders and surgery—a systematic review and meta-analysis. Life. 2020;10(6):94. doi:10.3390/life10060094
189. Dai W-L, Zhou A-G, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–670.e1. doi:10.1016/j.arthro.2016.09.024
190. Chen Z, Wang C, You D, Zhao S, Zhu Z, Xu M. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: a meta-analysis. Medicine. 2020;99(11):e19388. doi:10.1097/MD.0000000000019388
191. Han Y, Huang H, Pan J, et al. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20(7):1418–1429. doi:10.1093/pm/pnz011
192. Hohmann E, Tetsworth K, Glatt V. Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur J Orthop Surg Traumatol. 2020;30(6):955–967. doi:10.1007/s00590-020-02623-4
193. Joshi Jubert N, Rodríguez L, Reverté-Vinaixa MM, Navarro A. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthop J Sports Med. 2017;5(2):2325967116689386. doi:10.1177/2325967116689386
194. Uslu Güvendi E, Aşkin A, Güvendi G, Koçyiğit H. Comparison of efficiency between corticosteroid and platelet rich plasma injection therapies in patients with knee osteoarthritis. Arch Rheumatol. 2018;33(3):273–281. doi:10.5606/ArchRheumatol.2018.6608
195. Bennell KL, Paterson KL, Metcalf BR, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021;326(20):2021–2030. doi:10.1001/jama.2021.19415
196. Chu J, Duan W, Yu Z, et al. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2022. doi:10.1007/s00167-022-06887-7
197. Vilchez-Cavazos F, Millán-Alanís JM, Blázquez-Saldaña J, et al. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthopaedic J Sports Med. 2019;7(12):2325967119887116. doi:10.1177/2325967119887116
198. Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. 2018;37(5):1341–1350. doi:10.1007/s10067-018-3985-6
199. Sánchez M, Delgado D, Sánchez P, et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: a pilot study. Biomed Res Int. 2016;2016:4868613. doi:10.1155/2016/4868613
200. Sánchez M, Delgado D, Pompei O, et al. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: an observational study. Cartilage. 2019;10(2):245–253. doi:10.1177/1947603518756462
201. Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42(3):610–618. doi:10.1177/0363546513518416
202. Zayni R, Thaunat M, Fayard JM, et al. Platelet-rich plasma as a treatment for chronic patellar tendinopathy: comparison of a single versus two consecutive injections. Muscles Ligaments Tendons J. 2015;5(2):92–98.
203. Rowicki K, Płomiński J, Bachta A. Evaluation of the effectiveness of platelet rich plasma in treatment of chronic pes anserinus pain syndrome. Ortop Traumatol Rehabil. 2014;16(3):307–318. doi:10.5604/15093492.1112532
204. Sharaki F, Esfahani MP, Sajjadi MM, Salehi S, Yekta AA, Hasabi M. Determination of Effect of platelet rich plasma injection on improving pain and function in young healthy athletes with isolated grade 2 or 3 knee medial collateral ligament sprains. Novelty Biomed. 2019;7(3):147–157. doi:10.22037/nbm.v7i3.23862
205. Urzen JM, Fullerton BD. Nonsurgical resolution of a bucket handle meniscal tear: a case report. j Injury Function Rehabilitation. 2016;8(11):1115–1118. doi:10.1016/j.pmrj.2016.05.011
206. Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535–1541. doi:10.1097/TP.0b013e318291a2da
207. Prodromos C, Finkle S, Rumschlag T, Lotus J. Autologous mesenchymal stem cell treatment is consistently effective for the treatment of knee osteoarthritis: the results of a systematic review of treatment and comparison to a placebo group. Medicines. 2020;7(8):42. doi:10.3390/medicines7080042
208. Migliorini F, Rath B, Colarossi G, et al. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature. Arch Orthop Trauma Surg. 2020;140(7):853–868. doi:10.1007/s00402-019-03267-8
209. Tan SHS, Kwan YT, Neo WJ, et al. Intra-articular injections of mesenchymal stem cells without adjuvant therapies for knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2021:363546520981704. doi:10.1177/0363546520981704
210. Dai W, Leng X, Wang J, et al. Intra-articular mesenchymal stromal cell injections are no different from placebo in the treatment of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Arthroscopy. 2021;37(1):340–358. doi:10.1016/j.arthro.2020.10.016
211. Kim SH, Ha CW, Park YB, Nam E, Lee JE, Lee HJ. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971–980. doi:10.1007/s00402-019-03140-8
212. Ha CW, Park YB, Kim SH, Lee HJ. Intra-articular mesenchymal stem cells in osteoarthritis of the knee: a systematic review of clinical outcomes and evidence of cartilage repair. Arthroscopy. 2019;35(1):277–288.e2. doi:10.1016/j.arthro.2018.07.028
213. Jaibaji M, Jaibaji R, Volpin A. Mesenchymal stem cells in the treatment of cartilage defects of the knee: a systematic review of the clinical outcomes. Am J Sports Med. 2021;363546520986812. doi:10.1177/0363546520986812
214. Ma W, Liu C, Wang S, Xu H, Sun H, Fan X. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis. Medicine. 2020;99(49):e23343. doi:10.1097/MD.0000000000023343
215. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(06):443–450. doi:10.1055/s-0035-1569481
216. Castellanos R, Tighe S. Injectable amniotic membrane/umbilical cord particulate for knee osteoarthritis: a prospective, single-center pilot study. Pain Med. 2019;20(11):2283–2291. doi:10.1093/pm/pnz143
217. Mead OG, Mead LP. Intra-articular injection of amniotic membrane and umbilical cord particulate for the management of moderate to severe knee osteoarthritis. Orthop Res Rev. 2020;12:161–170. doi:10.2147/ORR.S272980
218. Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC, Group AS. A randomized controlled single-blind study demonstrating superiority of amniotic suspension allograft injection over hyaluronic acid and saline control for modification of knee osteoarthritis symptoms. J Knee Surg. 2019;32(11):1143–1154. doi:10.1055/s-0039-1696672
219. Barolat G. Techniques for subcutaneous peripheral nerve field stimulation for intractable pain. In: Neuromodulation.
220. Deer TR, Pope JE, McRoberts WP, Verrills P, Bowman R. Peripheral Nerve Stimulation for the Treatment of Knee Pain. In: Deer TR, Pope JE editors. Atlas of Implantable Therapies for Pain Management. Springer; 2016:185–190. doi:10.1007/978-1-4939-2110-2_27.
221. Deer T, Pope J, Benyamin R, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation. 2016;19(1):91–100. doi:10.1111/ner.12381
222. Ilfeld BM, Gilmore CA, Grant SA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res. 2017;12(1):4. doi:10.1186/s13018-016-0506-7
223. Lin CP, Chang KV, Wu WT, Özçakar L. Ultrasound-guided peripheral nerve stimulation for knee pain: a mini-review of the neuroanatomy and the evidence from clinical studies. Pain Med. 2020;21(Supplement_1):S56–S63. doi:10.1093/pm/pnz318
224. Tran J, Peng PWH, Lam K, Baig E, Agur AMR, Gofeld M. Anatomical study of the innervation of anterior knee joint capsule: implication for image-guided intervention. Reg Anesth Pain Med. 2018;43(4):407–414. doi:10.1097/AAP.0000000000000778
225. Tran J, Peng PWH, Gofeld M, Chan V, Agur AMR. Anatomical study of the innervation of posterior knee joint capsule: implication for image-guided intervention. Reg Anesth Pain Med. 2019;44(2):234–238. doi:10.1136/rapm-2018-000015
226. Harrison C, Epton S, Bojanic S, Green AL, FitzGerald JJ. The efficacy and safety of dorsal root ganglion stimulation as a treatment for neuropathic pain: a literature review. Neuromodulation. 2018;21(3):225–233. doi:10.1111/ner.12685
227. Martin SC, Macey AR, Raghu A, et al. Dorsal root ganglion stimulation for the treatment of chronic neuropathic knee pain. World Neurosurg. 2020;143:e303–e308. doi:10.1016/j.wneu.2020.07.102
228. Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019;22(1):61–79. doi:10.1111/ner.12796
229. Morgalla MH, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation (DRGS) for the treatment of chronic neuropathic pain: a single-center study with long-term prospective results in 62 cases. Pain Physician. 2018;21(4):E377–E387.
230. Hunter CW, Yang A, Davis T. Selective radiofrequency stimulation of the dorsal root ganglion (DRG) as a method for predicting targets for neuromodulation in patients with post amputation pain: a case series. Neuromodulation. 2017;20(7):708–718. doi:10.1111/ner.12595
231. Zuidema X, Breel J, Wille F. Paresthesia mapping: a practical workup for successful implantation of the dorsal root ganglion stimulator in refractory groin pain. Neuromodulation. 2014;17(7):665–669. doi:10.1111/ner.12113
232. Deer TR, Pope JE, Lamer TJ, et al. The neuromodulation appropriateness consensus committee on best practices for dorsal root ganglion stimulation. Neuromodulation. 2019;22(1):1–35. doi:10.1111/ner.12845
233. Jackson RW. A history of arthroscopy. Arthroscopy. 2010;26(1):91–103. doi:10.1016/j.arthro.2009.10.005
234. Kieser CW, Jackson RW. Severin Nordentoft: the first arthroscopist. Arthroscopy. 2001;17(5):532–535. doi:10.1053/jars.2001.24058
235. Jackson RW. The introduction of arthroscopy to North America. Clin Orthopaedics Related Res. 2000;374:183–186.
236. THE ROLE OF ARTHROSCOPY IN THE MANAGEMENT OF DISORDERS OF THE KNEE. https://online.boneandjoint.org.uk/doi/epdf/10.1302/0301-620X.54B2.310.
237. Treuting R. Minimally invasive orthopedic surgery: arthroscopy. Ochsner J. 2000;2(3):158–163.
238. Jameson SS, Dowen D, James P, Serrano-Pedraza I, Reed MR, Deehan DJ. The burden of arthroscopy of the knee. J Bone Joint Surg Br. 2011;93-B(10):1327–1333. doi:10.1302/0301-620X.93B10.27078
239. Brignardello-Petersen R, Guyatt GH, Buchbinder R, et al. Knee arthroscopy versus conservative management in patients with degenerative knee disease: a systematic review. BMJ Open. 2017;7(5):e016114. doi:10.1136/bmjopen-2017-016114
240. Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747. doi:10.1136/bmj.h2747
241. Müller-Rath R, Ingenhoven E, Mumme T, Schumacher M, Miltner O. [Perioperative management in outpatient arthroscopy of the knee joint]. Z Orthop Unfall. 2010;148(3):282–287. doi:10.1055/s-0029-1240784
242. Brattwall M, Jacobson E, Forssblad M, Jakobsson J. Knee arthroscopy routines and practice. Knee Surg Sports Traumatol Arthrosc. 2010;18(12):1656–1660. doi:10.1007/s00167-010-1266-2
243. Sun Y, Chen D, Xu Z, et al. Deep venous thrombosis after knee arthroscopy: a systematic review and meta-analysis. Arthroscopy. 2014;30(3):406–412. doi:10.1016/j.arthro.2013.12.021
244. Ilahi OA, Reddy J, Ahmad I. Deep venous thrombosis after knee arthroscopy: a meta-analysis. Arthroscopy. 2005;21(6):727–730. doi:10.1016/j.arthro.2005.03.007
245. Krych AJ, Sousa PL, Morgan JA, Levy BA, Stuart MJ, Dahm DL. Incidence and risk factor analysis of symptomatic venous thromboembolism after knee arthroscopy. Arthroscopy. 2015;31(11):2112–2118. doi:10.1016/j.arthro.2015.04.091
246. Perrotta C, Chahla J, Badariotti G, Ramos J. Interventions for preventing venous thromboembolism in adults undergoing knee arthroscopy. Cochrane Database Sys Rev. 2020;(5). doi:10.1002/14651858.CD005259.pub4
247. Carney J, Heckmann N, Mayer EN, et al. Should antibiotics be administered before arthroscopic knee surgery? A systematic review of the literature. World J Orthop. 2018;9(11):262–270. doi:10.5312/wjo.v9.i11.262
248. Qi Y, Yang X, Pan Z, Wang H, Chen L. Value of antibiotic prophylaxis in routine knee arthroscopy. Orthopäde. 2018;47(3):246–253. doi:10.1007/s00132-017-3486-3
249. Azim DN. Comparison among spinal, epidural, and general anesthesia for knee arthroscopy: a study in a tertiary care hospital, Chattogram, Bangladesh. J Med. 2021;5.
250. Padwal JA, Burton BN, Fiallo AA, Swisher MW, Gabriel RA. The association of neuraxial versus general anesthesia with inpatient admission following arthroscopic knee surgery. J Clin Anesth. 2019;56:145–150. doi:10.1016/j.jclinane.2019.01.045
251. Sehmbi H, Brull R, Shah UJ, et al. Evidence basis for regional anesthesia in ambulatory arthroscopic knee surgery and anterior cruciate ligament reconstruction: part II: adductor canal nerve block—a systematic review and meta-analysis. Anesth Analg. 2019;128(2):223–238. doi:10.1213/ANE.0000000000002570
252. Jacobson E, Forssblad M, Rosenberg J, Westman L, Weidenhielm L. Can local anesthesia be recommended for routine use in elective knee arthroscopy? a comparison between local, spinal, and general anesthesia. Arthroscopy. 2000;16(2):183–190. doi:10.1016/S0749-8063(00
253. Trieshmann HW. Knee arthroscopy: a cost analysis of general and local anesthesia. Arthroscopy. 1996;12(1):60–63. doi:10.1016/S0749-8063(96
254. Møiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A systematic review of intra-articular local anesthesia for postoperative pain relief after arthroscopic knee surgery. Reg Anesth Pain Med. 1999;24(5):430–437. doi:10.1016/S1098-7339(99
255. Dahl MR, Dasta JF, Zuelzer W, McSweeney TD. Lidocaine local anesthesia for arthroscopic knee surgery. Anesth Analg. 1990;71(6):670–674. doi:10.1213/00000539-199012000-00016
256. Law BKY, Yung PSH, Ho EPY, et al. Review of knee arthroscopy performed under local anesthesia. BMC Sports Sci Med Rehabil. 2009;1(1):3. doi:10.1186/1758-2555-1-3
257. Smith TO, Hing CB. A meta-analysis of tourniquet assisted arthroscopic knee surgery. The Knee. 2009;16(5):317–321. doi:10.1016/j.knee.2009.01.004
258. Wang J, Xu W, Is LJ. It better to routinely use tourniquet for knee arthroscopic surgery: a systematic review and meta-analysis. J Knee Surg. 2020;33(9):866–874. doi:10.1055/s-0039-1688555
259. Belk JW, McCarty EC, Houck DA, Dragoo JL, Savoie FH, Thon SG. Tranexamic acid use in knee and shoulder arthroscopy leads to improved outcomes and fewer hemarthrosis-related complications: a systematic review of Level I and II studies. Arthroscopy. 2021;37(4):1323–1333. doi:10.1016/j.arthro.2020.11.051
260. Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Sys Rev. 2011;(1). doi:10.1002/14651858.CD001886.pub3
261. McLean M, McCall K, Smith IDM, et al. Tranexamic acid toxicity in human periarticular tissues. Bone Joint Res. 2019;8(1):11–18. doi:10.1302/2046-3758.81.BJR-2018-0181.R1
262. Ma R, Wu M, Li Y, et al. The comparative efficacies of intravenous administration and intra-articular injection of tranexamic acid during anterior cruciate ligament reconstruction for reducing postoperative hemarthrosis: a prospective randomized study. BMC Musculoskelet Disord. 2021;22(1):114. doi:10.1186/s12891-021-03990-7
263. Salzler MJ, Lin A, Miller CD, Herold S, Irrgang JJ, Harner CD. Complications after arthroscopic knee surgery. Am J Sports Med. 2014;42(2):292–296. doi:10.1177/0363546513510677
264. Heyer JH, Perim DA, Amdur RL, Pandarinath R. Impact of smoking on outcomes following knee and shoulder arthroscopy. Eur J Orthop Surg Traumatol. 2020;30(2):329–336. doi:10.1007/s00590-019-02577-2
265. Lubowitz JH, Ayala M, Appleby D. Return to activity after knee arthroscopy. Arthroscopy. 2008;24(1):58–61.e4. doi:10.1016/j.arthro.2007.07.026
266. Dias JM, Mazuquin BF, Mostagi FQRC, et al. The effectiveness of postoperative physical therapy treatment in patients who have undergone arthroscopic partial meniscectomy: systematic review with meta-analysis. J Orthop Sports Phys Ther. 2013;43(8):560–576. doi:10.2519/jospt.2013.4255
267. Goodwin PC, Morrissey MC, Omar RZ, Brown M, Southall K, McAuliffe TB. Effectiveness of supervised physical therapy in the early period after arthroscopic partial meniscectomy. Phys Ther. 2003;83(6):520–535. doi:10.1093/ptj/83.6.520
268. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction –a meta-analysis. J Knee Surg. 2005;18(2):123–129. doi:10.1055/s-0030-1248169
269. Martimbianco ALC. Effectiveness and safety of cryotherapy after arthroscopic anterior cruciate ligament reconstruction. A systematic review of the literature. Physical Therapy Sport. 2014;15(4):261–268. doi:10.1016/j.ptsp.2014.02.008
270. Gazendam A, Ekhtiari S, Horner NS, Nucci N, Dookie J, Ayeni OR. Perioperative nonopioid analgesia reduces postoperative opioid consumption in knee arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2021;29(6):1887–1903. doi:10.1007/s00167-020-06256-2
271. Zhao X, Shah D, Gandhi K, et al. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthritis Cartilage. 2019;27(11):1618–1626. doi:10.1016/j.joca.2019.07.002
272. Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819–828. doi:10.1136/annrheumdis-2019-216515
273. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468. doi:10.1177/1941738109350438
274. York PJ, Wydra FB, Belton ME, Vidal AF. Joint preservation techniques in orthopaedic surgery. Sports Health. 2017;9(6):545–554. doi:10.1177/1941738117712203
275. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. doi:10.1053/jars.2002.32839
276. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. The Knee. 2007;14(3):177–182. doi:10.1016/j.knee.2007.02.001
277. Gracitelli GC, Moraes VY, Franciozi CE, Luzo MV, Belloti JC. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Sys Rev. 2016;9:548.
278. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthopaedics Related Research. 2001;391:S362–S369.
279. Orth P, Gao L, Madry H. Microfracture for cartilage repair in the knee: a systematic review of the contemporary literature. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):670–706. doi:10.1007/s00167-019-05359-9
280. Mithoefer K, Williams RJI, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. JBJS. 2005;87(9):1911–1920. doi:10.2106/JBJS.D.02846
281. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. doi:10.1177/0363546508328414
282. Frenkel SR, Toolan B, Menche D, Pitman MI, Pachence JM. Chondrocyte transplantation using a collagen bilayer matrix for cartilage repair. J Bone Joint Surg Br. 1997;79(5):831–836.
283. Krill M, Early N, Everhart JS, Flanigan DC. Autologous chondrocyte implantation (ACI) for knee cartilage defects: a review of indications, technique, and outcomes. JBJS Reviews. 2018;6(2):e5. doi:10.2106/JBJS.RVW.17.00078
284. Demange MK, Minas T, Gomoll AH. Autologous Chondrocyte Implantation After Previous Treatment with Marrow Stimulation Techniques. In: Emans PJ, Peterson L editors. Developing Insights in Cartilage Repair. Springer; 2014:213–225. doi:10.1007/978-1-4471-5385-6_12.
285. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902–908. doi:10.1177/0363546508330137
286. Long-term Results of Autologous Chondrocyte Implantation in the Knee for Chronic Chondral and Osteochondral Defects. Available from: https://oce.ovid.com/article/00000475-201409000-00019.
287. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001;24(391 Suppl):S349–361. doi:10.1097/00003086-200110001-00032
288. Kizaki K, El-Khechen HA, Yamashita F, et al. Arthroscopic versus open osteochondral autograft transplantation (mosaicplasty) for cartilage damage of the knee: a systematic review. J Knee Surg. 2021;34(1):94–107. doi:10.1055/s-0039-1692999
289. Ajaadmin2016. The Mosaicplasty/OAT procedure: technique, Pearls and Pitfalls. Asian Journal of Arthroscopy; 2019 Available from: http://asianarthroscopy.com/the-mosaicplasty-oat-procedure-technique-pearls-and-pitfalls/.
290. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826–831. doi:10.1177/0363546517745281
291. Andrade R, Vasta S, Pereira R, et al. Knee donor-site morbidity after mosaicplasty – a systematic review. J EXP ORTOP. 2016;3(1):31. doi:10.1186/s40634-016-0066-0
292. Matricali G, Dereymaeker G, Luyten F. Donor site morbidity after articular cartilage repair procedures: a review. Acta Orthop Belg. 2010;76:669–674.
293. Pallante AL, Bae WC, Chen AC, Görtz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37°C than at 4 C for osteochondral grafts. Am J Sports Med. 2009;37(1_suppl):24–32. doi:10.1177/0363546509351496
294. Ball ST, Amiel D, Williams SK, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthopaedics Related Research. 2004;418:246–252.
295. Bugbee WD, Pallante-Kichura AL, Görtz S, Amiel D, Sah R. Osteochondral allograft transplantation in cartilage repair: graft storage paradigm, translational models, and clinical applications. J Orthopaedic Res. 2016;34(1):31–38. doi:10.1002/jor.22998
296. Morag G, Kulidjian A, Zalzal P, Shasha N, Gross AE, Backstein D. Total knee replacement in previous recipients of fresh osteochondral allograft transplants. JBJS. 2006;88(3):541–546. doi:10.2106/JBJS.D.02816
297. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. doi:10.1016/j.biomaterials.2011.06.037
298. Cavendish A. Meniscal allograft transplantation: a review of indications, techniques, and outcomes. Knee Surg Sports Traumatol Arthrosc. 2020;28(11):3539–3550. doi:10.1007/s00167-020-06058-6
299. Rosso F, Bisicchia S, Bonasia DE, Amendola A. Meniscal allograft transplantation: a systematic review. Am J Sports Med. 2015;43(4):998–1007. doi:10.1177/0363546514536021
300. van der Wal RJP, Thomassen BJW, van Arkel ERA. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134–2139. doi:10.1177/0363546509336725
301. Kahlenberg CA, Nwachukwu BU, Hamid KS, Steinhaus ME, Williams RJ. Analysis of outcomes for high tibial osteotomies performed with cartilage restoration techniques. Arthroscopy. 2017;33(2):486–492. doi:10.1016/j.arthro.2016.08.010
302. Amendola A, Bonasia DE. Results of high tibial osteotomy: review of the literature. International Orthopaedics. 2010;34(2):155–160. doi:10.1007/s00264-009-0889-8
303. Bode G, Schmal H, Pestka JM, Ogon P, Südkamp NP, Niemeyer P. A non-randomized controlled clinical trial on autologous chondrocyte implantation (ACI) in cartilage defects of the medial femoral condyle with or without high tibial osteotomy in patients with varus deformity of less than 5. Arch Orthop Trauma Surg. 2013;133(1):43–49. doi:10.1007/s00402-012-1637-x
304. Mitchell JJ, Dean CS, Chahla J, Moatshe G, Cram TR, LaPrade RF. Varus-producing lateral distal femoral opening-wedge osteotomy. Arthroscopy Techniques. 2016;5(4):e799–e807. doi:10.1016/j.eats.2016.03.009
305. Song SJ, Bae DK, Kim KI, Lee CH. Conversion Total knee arthroplasty after failed high tibial osteotomy. Knee Surg Relat Res. 2016;28(2):89–98. doi:10.5792/ksrr.2016.28.2.89
306. Fokter S. Recent Advances in Hip and Knee Arthroplasty. BoD – Books on Demand; 2012.
307. Papas PV, Cushner FD, Scuderi GR. The history of total knee arthroplasty. Techniques Orthopaedics. 2018;33(1):2–6. doi:10.1097/BTO.0000000000000286
308. Ranawat CS. History of total knee replacement. J South Orthop Assoc. 2002;11(4):218–226.
309. Ranawat AS, Ranawat CS. The history of total knee arthroplasty. In: Bonnin M, Amendola A, Bellemans J, MacDonald S, Ménétrey J editors. The Knee Joint: Surgical Techniques and Strategies. Springer; 2012:699–707. doi:10.1007/978-2-287-99353-4_63.
310. Kinov P. Arthroplasty: Update. BoD – Books on Demand; 2013.
311. Hsu H, Siwiec RM Knee Arthroplasty. StatPearls Publishing; 2021. Available from: http://www.ncbi.nlm.nih.gov/books/NBK507914/.
312. Adie S, Harris I, Chuan A, Lewis P, Naylor JM. Selecting and optimising patients for total knee arthroplasty. Med J Aust. 2019;210(3):135–141. doi:10.5694/mja2.12109
313. Bernstein DN, Liu TC, Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33(12):3642–3648. doi:10.1016/j.arth.2018.08.018
314. Edwards PK, Mears SC, Stambough JB, Foster SE, Barnes CL. Choices, compromises, and controversies in total knee and total hip arthroplasty modifiable risk factors: what you need to know. J Arthroplasty. 2018;33(10):3101–3106. doi:10.1016/j.arth.2018.02.066
315. Vasarhelyi EM, MacDonald SJ. Obesity and total joint arthroplasty. Semin Arthroplasty. 2012;23(1):10–12. doi:10.1053/j.sart.2011.12.002
316. Tarabichi M, Shohat N, Kheir MM, et al. Determining the threshold for HbA1c as a predictor for adverse outcomes after total joint arthroplasty: a multicenter, retrospective study. J Arthroplasty. 2017;32(9, Supplement):S263–S267.e1. doi:10.1016/j.arth.2017.04.065
317. Singh JA. Smoking and outcomes after knee and hip arthroplasty: a systematic review. J Rheumatol. 2011;38(9):1824–1834. doi:10.3899/jrheum.101221
318. Sahota S, Lovecchio F, Harold RE, Beal MD, Manning DW. The effect of smoking on thirty-day postoperative complications after total joint arthroplasty: a propensity score-matched analysis. J Arthroplasty. 2018;33(1):30–35. doi:10.1016/j.arth.2017.07.037
319. Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359(9301):114–117. doi:10.1016/S0140-6736(02
320. Namba RS, Inacio MCS, Pratt NL, Graves SE, Roughead EE, Paxton EW. Persistent opioid use following total knee arthroplasty: a signal for close surveillance. J Arthroplasty. 2018;33(2):331–336. doi:10.1016/j.arth.2017.09.001
321. Rozell JC, Courtney PM, Dattilo JR, Wu CH, Lee GC. Preoperative opiate use independently predicts narcotic consumption and complications after total joint arthroplasty. J Arthroplasty. 2017;32(9):2658–2662. doi:10.1016/j.arth.2017.04.002
322. Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: evidence-based clinical practice guideline for dental practitioners—a report of the American Dental Association Council on Scientific Affairs. J Am Dental Association. 2015;146(1):11–16.e8. doi:10.1016/j.adaj.2014.11.012
323. Greenky M, Gandhi K, Pulido L, Restrepo C, Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthopaedics Related Research. 2012;470(10):2695–2701. doi:10.1007/s11999-012-2435-z
324. Liu D, Dan M, Adivi N. Blood conservation strategies in total hip and knee arthroplasty. Reconstructive Rev. 2014;4(4):39. doi:10.15438/rr.4.4.85
325. Kee JR, Mears SC, Edwards PK, Barnes CL. Modifiable risk factors are common in early revision hip and knee arthroplasty. J Arthroplasty. 2017;32(12):3689–3692. doi:10.1016/j.arth.2017.07.005
326. Nussenbaum FD, Rodriguez-Quintana D, Fish SM, Green DM, Cahill CW. Implementation of preoperative screening criteria lowers infection and complication rates following elective total hip arthroplasty and total knee arthroplasty in a veteran population. J Arthroplasty. 2018;33(1):10–13. doi:10.1016/j.arth.2017.07.031
327. Barrack RL, Smith P, Munn B, Engh G, Rorabeck C. Comparison of surgical approaches in total knee arthroplasty. Clin Orthopaedics Related Res. 1998;356:16–21.
328. Dalury DF, Jiranek WA. A comparison of the midvastus and paramedian approaches for total knee arthroplasty. J Arthroplasty. 1999;14(1):33–37. doi:10.1016/S0883-5403(99
329. Bonutti PM, Zywiel MG, Ulrich SD, Stroh DA, Seyler TM, Mont MA. A comparison of subvastus and midvastus approaches in minimally invasive total knee arthroplasty. JBJS. 2010;92(3):575–582. doi:10.2106/JBJS.I.00268
330. Innocenti M, Carulli C, Matassi F, Carossino AM, Brandi ML, Civinini R. Total knee arthroplasty in patients with hypersensitivity to metals. International Orthopaedics. 2014;38(2):329–333. doi:10.1007/s00264-013-2229-2
331. Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. JBJS. 2001;83(3):428.
332. Merritt K, Rodrigo JJ. Immune response to synthetic materials: sensitization of patients receiving orthopaedic implants. Clin Orthopaedics Related Res. 1996;326:71–79.
333. Morgan H, Battista V, Leopold SS. Constraint in primary total knee arthroplasty. JAAOS. 2005;13(8):515–524.
334. Clark CR, Rorabeck CH, MacDonald S, MacDonald D, Swafford J, Cleland D. Posterior-stabilized and cruciate-retaining total knee replacement: a randomized study. Clin Orthop Relat Res. 2001;392:208–212. doi:10.1097/00003086-200111000-00025
335. Forster MC. Survival analysis of primary cemented total knee arthroplasty: which designs last? J Arthroplasty. 2003;18(3):265–270. doi:10.1054/arth.2003.50051
336. Rassir R, Nolte PA, van der Lugt JCT, Nelissen RGHH, Sierevelt IN, Verra WC. No differences in cost-effectiveness and short-term functional outcomes between cemented and uncemented total knee arthroplasty. BMC Musculoskelet Disord. 2020;21(1):448. doi:10.1186/s12891-020-03477-x
337. Brown TE, Harper BL, Bjorgul K. Comparison of cemented and uncemented fixation in total knee arthroplasty. Orthopedics. 2013;36(5):380–387. doi:10.3928/01477447-20130426-10
338. Dodd CA, Hungerford DS, Krackow KA. Total knee arthroplasty fixation. Comparison of the early results of paired cemented versus uncemented porous coated anatomic knee prostheses. Clin Orthop Relat Res. 1990; 260:66–70.
339. Manoli A, Markel JF, Pizzimenti NM, Markel DC. Early results of a modern uncemented total knee arthroplasty system. Orthopedics. 2019;42(6):355–360. doi:10.3928/01477447-20190906-04
340. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227–1236. doi:10.1001/2012.jama.11153
341. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615–620. doi:10.1016/j.arth.2010.04.026
342. Kreder HJ, Grosso P, Williams JI, et al. Provider volume and other predictors of outcome after total knee arthroplasty: a population study in Ontario. Can J Surg. 2003;46(1):15–22.
343. Birdsall PD, Hayes JH, Cleary R, Pinder IM, Moran CG, Sher JL. Health outcome after total knee replacement in the very elderly. J Bone Joint Surg Br. 1999;81-B(4):660–662. doi:10.1302/0301-620X.81B4.0810660
344. Rosso F, Cottino U, Dettoni F, Bruzzone M, Bonasia DE, Rossi R. Revision total knee arthroplasty (TKA): mid-term outcomes and bone loss/quality evaluation and treatment. J Orthop Surg Res. 2019;14(1):280. doi:10.1186/s13018-019-1328-1
345. Adatia A, Rainsford KD, Kean WF. Osteoarthritis of the knee and Hip. Part II: therapy with ibuprofen and a review of clinical trials. J Pharm Pharmacol. 2012;64(5):626–636. doi:10.1111/j.2042-7158.2012.01456.x
346. Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis Cartilage. 2016;24(6):962–972. doi:10.1016/j.joca.2016.01.135
347. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328(7444):869. doi:10.1136/bmj.38039.573970.7C
348. van Middelkoop M, Arden NK, Atchia I, et al. The OA Trial Bank: meta-analysis of individual patient data from knee and Hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage. 2016;24(7):1143–1152. doi:10.1016/j.joca.2016.01.983
349. Concoff A, Sancheti P, Niazi F, Shaw P, Rosen J. The efficacy of multiple versus single hyaluronic acid injections: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2017;18:542. doi:10.1186/s12891-017-1897-2
350. Richette P, Chevalier X, Ea HK, et al. Hyaluronan for knee osteoarthritis: an updated meta-analysis of trials with low risk of bias. RMD Open. 2015;1(1):e000071. doi:10.1136/rmdopen-2015-000071
351. Weegen W. No Difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: a randomized, controlled, double-blind trial. J Arthroplasty. 2015;30(5):754–757. doi:10.1016/j.arth.2014.12.012
352. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–1711. doi:10.1002/art.24925
353. Yilmaz E. The evaluation of the effectiveness of intra-articular steroid, tenoxicam, and combined steroid-tenoxicam injections in the treatment of patients with knee osteoarthritis. Clin Rheumatol. 2019;38(11):
354. Riis RGC, Henriksen M, Klokker L, et al. The effects of intra-articular glucocorticoids and exercise on pain and synovitis assessed on static and dynamic magnetic resonance imaging in knee osteoarthritis: exploratory outcomes from a randomized controlled trial. Osteoarthritis Cartilage. 2017;25(4):481–491. doi:10.1016/j.joca.2016.10.009
355. Bodick N, Lufkin J, Willwerth C, et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97(11):877–888. doi:10.2106/JBJS.N.00918
356. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2002;162(3):292–298. doi:10.1001/archinte.162.3.292
357. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;1(2):CD005321. doi:10.1002/14651858.CD005321.pub2
358. Stitik TP, Issac SM, Modi S, Nasir S, Kulinets I. Effectiveness of 3 weekly injections compared with 5 weekly injections of intra-articular sodium hyaluronate on pain relief of knee osteoarthritis or 3 weekly injections of other hyaluronan products: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98(5):1042–1050. doi:10.1016/j.apmr.2017.01.021
359. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(10):2036–2045.e14. doi:10.1016/j.arthro.2015.03.030
360. Navarro-Sarabia F, Coronel P, Collantes E, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70(11):1957–1962. doi:10.1136/ard.2011.152017
361. Strand V, Baraf HSB, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350–356. doi:10.1016/j.joca.2012.01.013
362. Berenbaum F, Grifka J, Cazzaniga S, et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann Rheum Dis. 2012;71(9):1454–1460. doi:10.1136/annrheumdis-2011-200972
363. Arden NK, Åkermark C, Andersson M, Todman MG, Altman RD. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr Med Res Opin. 2014;30(2):279–286. doi:10.1185/03007995.2013.855631
364. Shen WS, Xu XQ, Zhai NN, Zhou ZS, Shao J, Yu YH. Radiofrequency thermocoagulation in relieving refractory pain of knee osteoarthritis. Am J Ther. 2017;24(6):e693–e700. doi:10.1097/MJT.0000000000000393
365. Takahashi K, Hashimoto S, Kurosaki H, et al. A pilot study comparing the efficacy of radiofrequency and microwave diathermy in combination with intra-articular injection of hyaluronic acid in knee osteoarthritis. J Phys Ther Sci. 2016;28(2):525–529. doi:10.1589/jpts.28.525
366. McCormick ZL, Reddy R, Korn M, et al. A prospective randomized trial of prognostic genicular nerve blocks to determine the predictive value for the outcome of cooled radiofrequency ablation for chronic knee pain due to osteoarthritis. Pain Medicine. 2018;19(8):1628–1638. doi:10.1093/pm/pnx286
367. Cerza F, Carnì S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. doi:10.1177/0363546512461902
368. Filardo G, Kon E, Di martino A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:229. doi:10.1186/1471-2474-13-229
369. Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. doi:10.1097/PHM.0b013e3182aab72
370. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. doi:10.1177/0363546512471299
371. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339–346. doi:10.1177/0363546516665809
372. Filardo G, Di Matteo B, Di martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–1582. doi:10.1177/0363546515582027
373. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet- rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:1–8. doi:10.4137/CMAMD.S17894
374. Forogh B, Mianehsaz E, Shoaee S, Ahadi T, Raissi GR, Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56(7–8):901–908.
375. Lana JFSD, Weglein A, Sampson SE, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78.
376. Montañez-Heredia E, Irízar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritic knee pain: a randomized clinical trial in the context of the spanish national health care system. Int J Mol Sci. 2016;17(7):E1064. doi:10.3390/ijms17071064
377. Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: a double-blind, randomized controlled pilot study. BMC Musculoskelet Disord. 2016;17:67. doi:10.1186/s12891-016-0920-3
378. Simental-Mendía M, Vílchez-Cavazos JF, Peña-Martínez VM, Said-Fernández S, Lara-Arias J, Martínez-Rodríguez HG. Leukocyte-poor platelet-rich plasma is more effective than the conventional therapy with Acetaminophen for the treatment of early knee osteoarthritis. Arch Orthop Trauma Surg. 2016;136(12):1723–1732. doi:10.1007/s00402-016-2545-2
379. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884–891. doi:10.1177/0363546515624678
380. Duymus TM, Mutlu S, Dernek B, Komur B, Aydogmus S, Kesiktas FN. Choice of intra-articular injection in treatment of knee osteoarthritis: platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):485–492. doi:10.1007/s00167-016-4110-5
381. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958–965. doi:10.1007/s00167-015-3705-6
382. Lisi C, Perotti C, Scudeller L, et al. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018;32(3):330–339. doi:10.1177/0269215517724193
383. Ahmad HS, Farrag SE, Okasha AE, et al. Clinical outcomes are associated with changes in ultrasonographic structural appearance after platelet-rich plasma treatment for knee osteoarthritis. Int J Rheum Dis. 2018;21(5):960–966. doi:10.1111/1756-185X.13315
384. Angoorani H, Mazaherinezhad A, Marjomaki O, Younespour S. Treatment of knee osteoarthritis with platelet-rich plasma in comparison with transcutaneous electrical nerve stimulation plus exercise: a randomized clinical trial. Med J Islam Repub Iran. 2015;29:223.
385. Buendía-López D, Medina-Quirós M, Fernández-Villacañas Marín MÁ. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J Orthop Traumatol. 2018;19(1):3. doi:10.1186/s10195-018-0501-3
386. Di Martino A, Di Matteo B, Papio T, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347–354. doi:10.1177/0363546518814532
387. Gaballa NM, Mohammed YA, Kamel LM, Mahgoub HM. Therapeutic efficacy of intra-articular injection of platelet–rich plasma and ozone therapy in patients with primary knee osteoarthritis. Egyptian Rheumatologist. 2019;41(3):183–187. doi:10.1016/j.ejr.2018.07.005
388. Khan AF, Gillani S, Khan A. Role of intra-articular corticosteroid with xylocaine vs plate rich plasma for the treatment of early grade II knee osteoarthritis at Akhtar Saeed Teaching Hospital Lahore: a randomized controlled trial. Int J Med. 2018;12(4):1432–1435.
389. Lin KY, Yang CC, Hsu CJ, Yeh ML, Renn JH. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy. 2019;35(1):106–117. doi:10.1016/j.arthro.2018.06.035
390. Louis ML, Magalon J, Jouve E, et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: a randomized double blind noninferiority trial compared with viscosupplementation. Arthroscopy. 2018;34(5):1530–1540.e2. doi:10.1016/j.arthro.2017.11.035
391. Bahram NN, Abbas S, Mohsen MK, et al. Comparing the effectiveness of intra-articular platelet-rich plasma and corticosteroid injection under ultrasound guidance on pain control of knee osteoarthritis, Arthroscpy. 2018;20(3):5.
392. Rahimzadeh P, Imani F, Faiz SHR, Entezary SR, Zamanabadi MN, Alebouyeh MR. The effects of injecting intra-articular platelet-rich plasma or prolotherapy on pain score and function in knee osteoarthritis. Clin Interv Aging. 2018;13:73–79. doi:10.2147/CIA.S147757
393. Wu J, Zhou J, Liu C, et al. A prospective study comparing platelet-rich plasma and local anesthetic (LA)/corticosteroid in intra-articular injection for the treatment of lumbar facet joint syndrome. Pain Pract. 2017;17(7):914–924. doi:10.1111/papr.12544
394. Huang Y, Liu X, Xu X, Liu J. Intra-articular injections of platelet-rich plasma, hyaluronic acid or corticosteroids for knee osteoarthritis: a prospective randomized controlled study. Orthopade. 2019;48(3):239–247. doi:10.1007/s00132-018-03659-5
395. Anz AW, Hubbard R, Rendos NK, Everts PA, Andrews JR, Hackel JG. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: a prospective, randomized trial. Orthop J Sports Med. 2020;8(2):2325967119900958. doi:10.1177/2325967119900958
396. Elksniņš-Finogejevs A, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: a single-center prospective randomized controlled study with a 1-year follow up. J Orthop Surg Res. 2020;15(1):257. doi:10.1186/s13018-020-01753-z
397. Kesiktas FN, Dernek B, Sen EI, Albayrak HN, Aydin T, Yildiz M. Comparison of the short-term results of single-dose intra-articular peptide with hyaluronic acid and platelet-rich plasma injections in knee osteoarthritis: a randomized study. Clin Rheumatol. 2020;39(10):3057–3064. doi:10.1007/s10067-020-05121-4
398. Pishgahi A, Abolhasan R, Shakouri SK, et al. Effect of dextrose prolotherapy, platelet rich plasma and autologous conditioned serum on knee osteoarthritis: a randomized clinical trial. Iran J Allergy Asthma Immunol. 2020:243–252. doi:10.18502/ijaai.v19i3.3452
399. Raeissadat SA, Gharooee Ahangar A, Rayegani SM, Minator Sajjadi M, Ebrahimpour A, Yavari P. Platelet-rich plasma-derived growth factor vs hyaluronic acid injection in the individuals with knee osteoarthritis: a one year randomized clinical trial. J Pain Res. 2020;13:1699–1711. doi:10.2147/JPR.S210715
400. Reyes-Sosa R, Lugo-Radillo A, Cruz-Santiago L, Garcia-Cruz CR, Mendoza-Cano O. Clinical comparison of platelet-rich plasma injection and daily celecoxib administration in the treatment of early knee osteoarthritis: a randomized clinical trial. J Appl Biomed. 2020;18(2–3):41–45. doi:10.32725/jab.2020.012
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
