Back to Journals » OncoTargets and Therapy » Volume 13
Connexin 43 Modulates the Cellular Resistance to Paclitaxel via Targeting β-Tubulin in Triple-Negative Breast Cancer
Authors Fu Y, Sun X, Gu Z, Zhuang Z
Received 29 August 2019
Accepted for publication 19 May 2020
Published 10 June 2020 Volume 2020:13 Pages 5323—5335
DOI https://doi.org/10.2147/OTT.S229076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Yun Fu, Xiaoyin Sun, Zhangyuan Gu, Zhigang Zhuang
Department of Breast Surgery, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
Correspondence: Zhigang Zhuang
Shanghai First Maternity and Infant Hospital, No. 2699, West Gaoke Road, Shanghai 201204, People’s Republic of China
Tel +86 21 20261056
Fax +86 21 20261477
Email [email protected]
Background: Triple-negative breast cancer has become an intricate part and hotspot in the clinical and experimental research. Connexins, serving as functional proteins in gap junctions, play an important role in tumorigenesis, cell proliferation and metastasis.
Methods: We constructed and employed the Connexin 43 (Cx43) overexpression lentiviral vectors and Cx43 siRNA in paclitaxel-treated MDA-MB-231 cells. We performed the experiments of clonal formation and flow cytometry to gauge the effect of paclitaxel on cellular behaviors and immunofluorescence and subsequent quantitative RT-PCR and Western blot to examine the expression of genes and corresponding proteins. Experiments of scrape loading/dye transfer were utilized to explore the gap junctions. The targets of Cx43 were identified via the experiments of co-immunoprecipitation (Co-IP), GST pull-down assays and proximal ligation assay (PLA).
Results: The results showed that Cx43 hindered cell proliferation and promoted apoptosis in the paclitaxel-treated MDA-MB-231 cells. Overexpressed Cx43 suppressed the expression of resistance genes such as BRCP, Txr-1, α-tubulin and β-tubulin and promoted the expression of apoptosis gene as TSP-1 and Bcl-2. Cx43 was also positively related to ITGα 9 and negatively related to ITGαV and ITGα 11. The gap junctions altered magnificently under different expressions of Cx43, which indicated that Cx43 could promote the number of intercellular gap junctions. The immunofluorescent experiment revealed that both of Cx43 and β-tubulin were mainly localized in the cytoplasm. The assays of Co-IP and GST pull-down demonstrated that there existed a direct interaction between Cx43 and β-tubulin. Furthermore, the result of PLA also showed that Cx43 interacts with β-tubulin in MDA-MB-231 cells.
Conclusion: Overexpression of Cx43 could modulate the cellular resistance to paclitaxel via targeting β-tubulin in triple-negative breast cancer.
Keywords: Cx43, β-tubulin, paclitaxel, chemoresistance, gap junctions
Introduction
Triple-negative breast cancer (TNBC) is defined as breast cancer that lacks the expression of estrogen, progesterone, and human epithelial receptor 2 (HER2).1 TNBC is a representative of more aggressive cancers with lower cellular differentiation and minimized survival, which constitutes 15% of breast cancer cases in the world.2 The therapeutic method of TNBC is confined to chemotherapy. Meanwhile, paclitaxel remained as the main component in the combinational chemotherapeutic modalities in TNBC.3,4 However, the therapeutic efficacy is often limited due to the development of drug resistance in tumor cells.5
Gap junctions (GJs) are membrane-spanning channels that allow for the passive movement of second messengers from cell to cell. The gap junctional intercellular communications (GJICs) are formed by the docking of two connexins. Loss of GJIC leads to a breach in the intercellular connection of epithelial barrier.6 Moreover, dislocation of connexins and interaction with down-stream pathways can impact on the intercellular communication. It revealed that the carboxyl-terminus of connexins could be bound to proteins such as tubulin, Src, ZO-1, and CDK1.7 Aberrant expression of connexins is well correlated with the extravasation, metastasis, and prognosis in breast cancer.8–10 Connexin 43 (Cx43) is a crucial component of GJs complex. The expression of Cx43 is either up-regulated and down-regulated according to various studies.11,12 However, dislocation of Cx43, that is, being localized in the cytoplasm of cancer cells rather than being localized in the cellular membrane of healthy breast epithelial cells remains to reach the consensus.12,13 Connexins are also found to be associated with chemotherapy response in cancer such as glioblastoma cells.14,15 It remains unclear that connexin could attenuate the chemotherapy effect in breast cancer, and the appropriate mechanism is yet to be elucidated.
Cx43 was reported to mediate TGF-β/BMP signaling pathway to promote cartilage differentiation of bone marrow mesenchymal stem cells and inhibit osteoblast differentiation.16 Chen et al reported that Cx43 regulated reduce expression of FN, ICAM-1 and TGF-β1, ultimately attenuating renal fibrosis in diabetes.17 As the TGF family is involved in the biological process of cell adhesion and migration, we hypothesized that Cx43 could mediate the cellular response to paclitaxel via TGF signaling pathway.
Materials and Methods
Cell Culture
The human breast cancer cell line of MDA-MB-231 was obtained from the organization of the American Type Culture Collection (ATCC; Manassas, VA, USA). The cell was then cultivated and maintained in the Dulbecco’s modified Eagle’s media (DMEM; Life Technology, Shanghai, China) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Beijing, China) and 1% antibiotics (penicillin/streptomycin, Invitrogen, Carlsbad, CA, USA). The cells were then cultured in a humidified thermostatic container with a constant flow of 5% CO2 at 37°C.
Construction of Cx43(+) Lentiviral Vectors
The whole coding sequence (CDS) of Cx43 was cloned and synthesized by the manufacturer (Synbio Technology Co. Ltd), HindIII and XhoI were set as the restriction enzyme cutting sites in the empty lentiviral vectors. After being dissected with the restriction enzyme, the synthesized Cx43 was constructed into the vectors. The vectors (pSuper: Cx43) were validated with sequencing, and endotoxin was removed and screened (concentration of endotoxin<100EU/mL). The vectors were also labeled with the gene of a green fluorescent protein (GFP) to facilitate the visibility in the following experiments.
Construction of Expression Plasmids for β-Tubulin and Cx43
The whole cDNA cloned from the cell line was used as the template. The genes β-tubulin were amplified using the following primers (Table S1), and the cutting site of restriction endonucleases for each gene was provided along with the primers respectively. After being dissected explicitly with the restriction enzyme, the cloned sequences were then connected with the empty vectors of pCMV-Myc. The Cx43 gene sequence was amplified, dissected, and connected with the empty vector of pGEX-6p-1 simultaneously. The expression plasmids were named as pCMV-Myc-Tubulin and pGEX-6p-1-Cx43 in respective.
Cell Transfection with the Lentiviral Vector pSuper Cx43 or Cx43 siRNA
The preparation process of Cx43(+) lentiviral vectors was described as before (refer to the section “Construction of Cx43(+) lentiviral vectors”). The process of transduction was done in 50mL polypropylene basins overnight at 37°C. Thirty-thousand to fifty-thousand number of cells were implanted into the basin before transduction when the cells reach 30% confluence. After transduction, the cells were placed and cultured for several days. Moreover, the efficiency of transduction was determined by the luminosity emitted by the GFP under fluorescence microscope.
MDA-MB-231 cells transfected with the vectors and plasmids were set as experimental group and the cells transfected with empty vectors were set as control. Meanwhile, Cx43 siRNA (5ʹ- GCAUCAAGAUUCCACUCAAUU-3ʹ, 5ʹ- UUGAGUGGAAUCUUGAUGCUU-3ʹ) and the negative control (5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ, 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ) were transfected into MDA-MB-231 cells as well. The transfection agents used for plasmids and siRNA was lipofectamine 3000 (Thermo scientific Co. ltd) with the ratio of plasmids/siRNA against the agent equal to 1:1. A series of experiments were carried out after treatment of 10 nmol/L paclitaxel.
Determining Cell Viability via Clonal Formation Assay After Treatment of Paclitaxel
The experiment of clonal formation was utilized to examine the ability of viability following the treatment of paclitaxel. In brief, monolayer cells were trypsinized into individual cells and suspended in the culture media with 10% bovine fetal serum. The cells were then titrated according to the gradient, and generally to 50, 100 and 200 per Petri dish in a set of dishes The cells were then incubated appropriately for 2–3 weeks, and the incubation was canceled immediately when the clonal formation was visible to naked eyes. The cells were then fixed with formalin solution and stained with Giemsa dye, and the mount of clonal clusters was counted under a low-power microscope.
Flow Cytometry to Examine the Apoptosis Rate and Cell Proliferation
In order to examine the rate of apoptosis, the cells with Cx43 overexpression or Cx43 siRNA and healthy cells as control were examined via the usage of Annexin V-fluorescein isothiocyanate/propidium iodide (PI) assay kit after being treated with 10 nmol/L paclitaxel. In brief, the cells (2×105 per well) were seeded in a 6-well plate and then incubated with paclitaxel for 24 hours. Following incubation, the cells were harvested with trypsin/EDTA, fixed, permeabilized, stained with propidium iodide, and tested by flow cytometry.
RNA Purification and Real-Time Quantitative PCR (qRT-PCR)
The total RNA was extracted with Trizol reagent (Invitrogen) according to the manufacturer’s protocol. After the reaction of reverse transcription, qRT-PCR was performed by LightCycler® 480II system (Roche) utilizing TB Green® Premix Ex Taq™ II M (Takara, Dalian, China) according to the manufacturer’s instructions. All amplifications were normalized by GADPH, and the data were analyzed via the comparison Ct (2-ΔΔt) method and described as the fold changes comparing to the control in respective. All experiments were performed three times in duplicate. The primers used in these experiments were provided in Table S2.
Western Blot
Cells were lysed with lysis buffer for Western blot analysis. The sample was titrated to the same concentration of protein according to the protocol of the BCA quantitative method and stored at −20°C. 50ug of total protein sample was resolved in SDS-PAGE and electrotransferred onto polyvinylidene fluoride membranes. The primary antibodies used here were anti- P-gp (abcam, ab216656), anti-BCRP (abcam, ab24115), anti-Txr1 (proteintech, 14,101-1-AP), anti-α-tubulin (abcam, ab18251), anti- β-tubulin (abcam, ab21057), anti-TSP1 (abcam, ab1823), anti-Bcl-2 (proteintech, 12,789-1-AP), anti-Cx43 (abcam, ab11370), anti-ITGαV (abcam, ab226816), anti-ITGα9 (abcam, ab240116), and anti-ITGα11 (abcam, ab198826). GAPDH (abcam, ab8245) was set as an internal control in the analysis. The primary antibodies were diluted at the ratio of 1:1000, and the secondary rabbit-anti-mouse antibodies conjugated with Horseradish Peroxidase (HRP) were diluted to 1:5000. The antigen-antibody complexes were visualized via the enhanced chemiluminescence detection system (ECL) by the instruction of manufacturers. Immunoreactive bands were analyzed in triplicates by normalizing the intensity of bands to GAPDH on scanned films with BIO-RAD Image Lab Software. To further determine the expression differences of the proteins above, semi-quantitative calculation of each protein was also carried out via densitometric analysis.
Determining the Function of Gap Junction via the Experiment of Scrape-Loading Dye Transfer After Treatment of Paclitaxel
The cells were treated with 10 nmol/L paclitaxel for 24 hours, and scrape-loading dye transfer was carried out to examine the function of the gap junction. Briefly, the cells in good condition treated with paclitaxel, as described above, were removed from the container and rinsed with Ca-Mg PBS for three times after the culture media being gently poured out and discarded. Sufficient lucifer yellow solution used as loading dye was added to cover the cell monolayer. The cells were then cut randomly with a scalpel. The plates containing cells would be placed in an undisturbed environment with minimal illumination for 3 to 6 mins, which would enable the dye to travel through the intercellular junction. Then the cells were fixed with 10% formalin solution, viewed and imaged immediately under an inverted fluorescent microscope.
Detection and Localization of Cx43 and β-Tubulin by Immunofluorescent Assays
Coverslips were rinsed up with 75% ethanol and applied onto a 24-well cell plate. The cells were then gently implanted into the flasks with culture media. When the cells were grown to 60–80% confluence, the same volume of 4% Paraformaldehyde was added in the petri dish after removal of culture media for 300ul to incubate for 20min at 37°C. The supernatant was then discarded, and 200ul of the permeable solution containing Triton-100 was added to each well for 5min. Then bovine serum albumin was added to each well and left for 1 hour at 37°C. The primary antibodies Cx43 and β-tubulin were diluted to 1:200 with 1% BSA and applied onto the coverslip at 37°C for overnight. And secondary antibodies labeled with fluorophore were added for 1 hour at 37°C in a moist, dark environmental box. Observation of immunofluorescent results was carried out with a Leica confocal microscope system.
Co-Immunoprecipitation
In order to explore the protein-protein interaction of Cx43, Co-Immunoprecipitation (Co-IP) was carried out on the foundation of basic methods with some modification. Initially, the cells were transiently transfected with the expression plasmids pCMV-Myc-Tubulin described above. The cell lysates were incubated with Protein G beads and then primary antibodies, including anti-Myc (abcam, ab9132) and anti-Cx43 (abcam, ab11370) right after being centrifuged. After incubation, the binding proteins were eluted from the beads with 1×SDS sample buffer (containing 62.5 mmol/L Tris pH 6.8, 2% SDS, 10% glycerol, 0.1 mol/L DTT, 0.005% bromophenol blue, 2% 2-mercaptoethanol) and electrotransferred to a PVDF membrane. The analysis of Western blot was carried out in subsequence.
GST Pull-Down Assays
GST-pEGX-6p-1-Cx43 was transformed into colibacillus BL21(DE3), and induced for expression by Isopropyl β-D-Thiogalactoside (IPTG). Then the bacteria were lysed with lysis buffer (PBS+1% Triton-100+PMSF) and stored at −80°C after being centrifuged. The expression plasmid pCMV-Myc-Tubulin was transfected into the MDA-MB-231 cells in respective. Proper dosage of GST-Cx43 was melted on ice and purified by Glutathione Sepharose 4B with other GST proteins abided by the manufacturer’s instructions. After purification, the GST proteins were then incubated with the cell lysates (MDA-MB-231 cells transfected with the proteins corresponding to the plasmids above) for four h at 4 °C with constant shaking. The beads were washed three times with cold PBS (pH 7.4) and then cold PBS buffer containing 1% Triton-100. Then the samples were subjected to subsequent SDS-PAGE electrophoresis and Western blot analysis.
Proximal Ligation Assay
MDA-MB-231 cells (3×105) were seeded in a 6 cm collagen-coated glass-bottom dish. 2 mL 10% Paraformaldehyde was added in the dish after removal of culture media to incubate for 10 min at room temperature (RT) under agitation. Stop the reaction with 1 mL of 1 M glycine and mix by rotation. After washing the cells by adding 1xPBS, 2 mL of the permeable solution containing 0.1% Triton-100 was added to the dish for for 15 min at RT under agitation. Then blocking solution (Sigma-Aldrich, DUO82014) was added to the dish and incubated for 30 min at 37°C in a humidity chamber. The blocking solution was then discarded, and the primary antibodies in 1x PBS (β-tubulin mouse antibody: 1/500, Cx43 rabbit antibody: 1/100) was added to the dish for incubating overnight in a humidity chamber at 4°C. Wash the slides two times using Tris Buffered Saline with 0.01% Tween-20 (TBS-T), and then added the diluted proximity ligation assay probe solution anti-rabbit PLUS (Sigma-Aldrich, DUO82029); anti-mouse MINUS (Sigma-Aldrich, DUO92004) and incubated the dishes in a pre-heated humidity chamber for 1 hr at 37°C. 100 ul ligase diluted in the 1x ligation solution (Sigma-Aldrich, DUO82016) 1:40 was added to the dish and incubated in a pre-heated humidity chamber for 30 min at 37°C. After washing with TBS-T, 100 ul polymerase was diluted with 1:80 in the 1x amplification solution (Sigma-Aldrich, DUO82019) and added to the dish in a pre-heated humidity chamber for 100 min at 37°C. Wash the slides in 1x SSC washing buffer for 2 min. Let the dishes dry at RT in the dark. The cells were stained with 500 ul medium containing DAPI for approximately 15 min before analyzing using confocal microscope.
Statistical Analysis
Data are presented as Mean ± SD. Statistical analyses were performed by GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). Two-way ANOVA was used to compare the mRNA and protein levels, followed by the least significant difference post hoc test. Tests were considered as significant at p<0.05.
Results
Cx43 Could Enhance the Sensitivity to Paclitaxel in MDA-MB-231 Cells
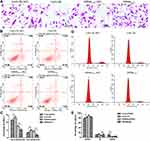
To investigate the effect of Cx43 on the cellular proliferation after being subjected to paclitaxel, the experiment of clonal formation was carried out. The results suggested that the number of cells and clonal clusters in the group of Cx43 overexpression decreased more significantly than that in Cx43-OE (NC) groups, and the cells in Cx43-siRNA group were increased compared to Cx43-siRNA (NC) group (Figure 1A). The results from Annexin-V staining examined by flow cytometry indicated that the rate of apoptosis in the group of Cx43 overexpression was significantly higher than that in control and the results in Cx43-siRNA group was opposite (Figure 1B and C).
Meanwhile, tests on the cell phase distribution via PI staining revealed that fewer cells stayed in the phase of G2 in Cx43-OE group in comparison to that the number of G2 phase cells in Cx43-OE (NC) group. In addition, more cells stayed in Cx43-siRNA group of S phase compared to that the number of S phase cells in Cx43-siRNA (NC) group (Figure 1D and E).
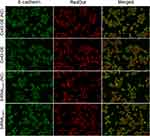
In order to evaluate the effect of Cx43 on a epithelial-mesenchymal transition (EMT) model based on MDA-MB-231 cells, the marker E-cadherin as a cost-effective and convenient means of evaluating EMT were selected for immunofluorescent assays in the cells. The results showed that the expression of E-cadherin in Cx43-OE group and Cx43-siRNA group unchanged compared to the control group, respectively (Figure 2).
Taken together, the above results suggested that Cx43 could demolish the cell clones and hinder cell proliferation. Also, it could promote apoptosis in the MDA-MB-231 cells subjected to paclitaxel.
Cx43 Was Associated with the Expression of Genes Relevant to Resistance and Apoptosis
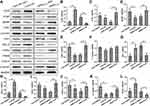
Intending for investigating the effect of Cx43 on regulation of the gene expression, the experiment of quantitative RT-PCR (Figure 3A–K) and Western blotting (Figure 4A) were carried out. In the group with overexpressed or downexpressed Cx43, the expression of genes relevant to paclitaxel resistance altered comparing to that in the corresponding control group. The expression of ATP binding cassette subfamily G member 2 (ABCG2, also known as BRCP), transcription factor Txr-1, α-tubulin and β-tubulin decreased significantly in Cx43-OE group, but increased in Cx43-siRNA group while the expression of ATP binding cassette subfamily B member (ABCB1, also known as P-gp) slightly altered. The genes described above have been proven to be well correlated with the cellular resistance to paclitaxel.18–21 Overexpressed Cx43 could also induce a significant surge on the expression of tumor suppressor region 1 (TSP-1) and B-cell lymphoma-2 (Bcl-2). However, the expression of TSP-1 and Bcl-2 were downexpressed in Cx43-siRNA group, in which the latter two genes are associated with apoptosis in breast cancer.22,23 Furthermore, Cx43 was positively related to the expression of ITGα9 and negatively related to the expression of ITGαV and ITGα11. Semi-quantitative analysis on the expression level also confirmed the above results concomitantly (Figure 4B–L).
The Effect of Cx43 on the Intercellular Gap Junction Altered Magnificently Subjected to Paclitaxel
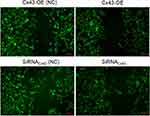
The cells in the group of Cx43 overexpression or Cx43 siRNA were subjected to the treatment of paclitaxel. Each groups underwent the experiment of scape loading/dye transfer via the usage of lucifer yellow. It revealed that the number of gap junctions increased more magnificently in the group of Cx43 overexpression in comparison to Cx43-OE (NC) group. To the opposite, the number of gap junctions in Cx43-siRNA group decreased significantly compared with the corresponding control group (Figure 5). They indicated that Cx43 could promote the number of intercellular gap junctions.
Cx43 and β-Tubulin Were Both Located in the Cytoplasm by the Immunofluorescent Assay
The immunofluorescent assay was carried out to explore the location of Cx43 and β-tubulin in the MDA-MB-231 cells. They were both mainly localized in the cytoplasm as revealed. In comparison to the control group, the expression of Cx43 in Cx43-OE group increased significantly (Figure 6), while the expression of β-tubulin decreased slightly, judged from the luminosity intensity in the films (Figure 7).
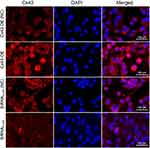 |
Figure 6 Immunofluorescence staining analysis of Cx43 in the MDA-MB-231 cells. The cell nucleus was stained with DAPI. A merged staining image is also shown. |
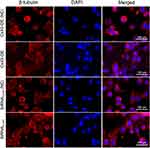 |
Figure 7 Immunofluorescence staining analysis of β-tubulin in the MDA-MB-231 cells. The cell nucleus was stained with DAPI. A merged staining image is also shown. |
Cx43 Directly Interacted with β-Tubulin by Co-IP, GST Pull-Down Assays and PLA
Based on the location of Cx43 and tubulins in the cytoplasm as described above, we aimed to explore whether Cx43 could interact directly with members of TGF-signaling pathways. The data from the intensity of immunoreactive bands within the experiment of Co-IP (Figure 8A) and further pull-down assays (Figure 8B) demonstrated that Cx43 could directly bind with the proteins of β-tubulin. Moreover, we also performed the proximal ligation assay to further determine whether the Cx43 could directly bind to the β-tubulin (Figure 8C). The results of PLA also demonstrate that Cx43 is directly bound to β-tubulin.
Discussion
Connexins, as representative proteins serving for intercellular gap junction, have long been viewed as the connecting proteins until recent findings. Recent data suggest that they are well linked with tumorigenesis, metastasis, and prognosis.24 Murphy et al reported that connexin inhibition could increase the cellular sensitivity to temozolomide in chemoresistant glioma cells.14 Nevertheless, inhibition of Cx43 could attenuate the cytotoxicity effect of platinum,25 which indicated a complicated role of Cx43 in chemoresistance. In our data, paclitaxel was utilized to test the role of Cx43 in triple-negative breast cancer cells. Overexpressed Cx43 suppressed the clonal clusters and induced apoptosis in the MDA-MB-231 cells subjected to paclitaxel. Via qRT-PCR and Western blot assays, overexpressed Cx43 suppressed the expression of resistance genes such as BRCP, Txr-1, α-tubulin and β-tubulin, and promoted the expression of apoptosis gene as TSP-1 and Bcl-2. In consistent with previous results,26 the expression of Cx43 was localized in the cytoplasm rather than on the surface of the cell membrane in breast cancer cells in our experiments, which indicated that Cx43 might have a GJIC-independent way to modulate the cellular sensitivity and fragility to the treatment of paclitaxel.
ITGαV, ITGα9 and ITGα11 are a cluster of glycoprotein receptors that participate in the process of intercellular adhesion and cellular interaction with the extracellular matrix. They have been revealed to be well correlated with the biological behaviors of cancer cells such as metastasis, angiogenesis, and invasion in breast cancer.27 Members of integrins are also involved in the process of chemotherapeutic resistance such as resistance to gemcitabine and paclitaxel.28,29 Menedez et al showed that functional blockade of the αvβ3 integrin receptor in CYR61-expressing MCF-7 cells led to sensitization in taxol-induced apoptosis.30 Loessner et al reported that combined KLK4-7 expression by ovarian cancer cells promoted reduced expression of α5β1/αvβ3 integrins to decrease adhesion and paclitaxel-induced chemoresistance.31 In our data, Cx43 was positively related to ITGα9 and negatively related to ITGαV and ITGα11 subjected to paclitaxel.
Members of the TGF-β family have long been considered as critical regulators in the pathogenesis and metastasis of breast cancer.32,33 As a component of the TGF-β family, β-tubulin is established as a significant predictor for the efficacy of taxane chemotherapy.34 Tubulins are also involved in cellular biological events such as microtentacle formation, adhesion, and invasive migration.35 It is known that tubulins participate in the microtube assembly via its colchicine binding site within the cytoplasm. Tubulins are involved in a consensus meta-adhesome, including integrins mainly composed of α5, αV or β1 integrins.36 Moreover, recent literature reported TGF-β signaling pathway has crosstalk with integrins in cancer.37,38 Loss of tubulin expression could lead to down-regulation of integrins in breast cancer metastasis.39 Combined with the experiment of Co-IP, GST pull-down assay and PLA, we discovered that Cx43 could interact directly with β-tubulin in MDA-MB-231 cells. Hence, we proposed that Cx43 could mediate the resistance to paclitaxel via directly binding to β-tubulin.
Conclusion
In summary, with the usage of lentiviral vectors, we proved that overexpression of Cx43 promoted the cellular sensitivity and fragility to the treatment of paclitaxel via directly binding to β-tubulin in triple-negative breast cancer, thus leading to the development of new therapeutic direction targeting on connexins. Further research on the function of Cx43 and development of new compounds that regulates the expression of Cx43 could be a novel adjuvant for chemotherapeutic modalities based on paclitaxel.
Acknowledgment
This work was supported by Shanghai Municipal Health Bureau (201640084), and the Fundamental Research Funds for the Central Universities (22120180407).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–269. doi:10.1007/s00404-015-3859-y
2. Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park). 2008;22(11):
3. Caparica R, Lambertini M, de Azambuja E. How I treat metastatic triple-negative breast cancer. ESMO Open. 2019;4(Suppl 2):e000504. doi:10.1136/esmoopen-2019-000504
4. Kumari S, Mohan MG, Shailender G, Badana AK, Malla RR. Synergistic enhancement of apoptosis by coralyne and paclitaxel in combination on MDA-MB-231 a triple-negative breast cancer cell line. J Cell Biochem. 2019;120(10):18104–18116. doi:10.1002/jcb.29114
5. Yao H, He G, Yan S, et al. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017;8(1):1913–1924. doi:10.18632/oncotarget.12284
6. Mao XY, Li QQ, Gao YF, Zhou HH, Liu ZQ, Jin WL. Gap junction as an intercellular glue: emerging roles in cancer EMT and metastasis. Cancer Lett. 2016;381(1):133–137. doi:10.1016/j.canlet.2016.07.037
7. Tabernero A, Gangoso E, Jaraiz-Rodriguez M, Medina JM. The role of connexin43-Src interaction in astrocytomas: a molecular puzzle. Neuroscience. 2016;323:183–194. doi:10.1016/j.neuroscience.2015.02.029
8. Rhett JM, Yeh ES. The potential for connexin hemichannels to drive breast cancer progression through regulation of the inflammatory response. Int J Mol Sci. 2018;19:4. doi:10.3390/ijms19041043
9. Viot J, Bachour M, Meurisse A, Pivot X, Fiteni F. Follow-up of patients with localized breast cancer and first indicators of advanced breast cancer recurrence: a retrospective study. Breast. 2017;34:53–57. doi:10.1016/j.breast.2017.05.005
10. Chasampalioti M, Green AR, Ellis IO, et al. Connexin 43 is an independent predictor of patient outcome in breast cancer patients. Breast Cancer Res Treat. 2019;174(1):93–102. doi:10.1007/s10549-018-5063-9
11. Kanczuga-Koda L, Sulkowski S, Lenczewski A, et al. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J Clin Pathol. 2006;59(4):429–433. doi:10.1136/jcp.2005.029272
12. Kanczuga-Koda L, Sulkowska M, Koda M, et al. Expression of connexin 43 in breast cancer in comparison with mammary dysplasia and the normal mammary gland. Folia Morphol (Warsz). 2003;62(4):439–442.
13. Jamieson S, Going JJ, D’Arcy R, George WD. Expression of gap junction proteins connexin 26 and connexin 43 in normal human breast and in breast tumours. J Pathol. 1998;184(1):37–43. doi:10.1002/(sici)1096-9896(199801)184:1<37::Aid-path966>3.0.Co;2-d
14. Murphy SF, Varghese RT, Lamouille S, et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 2016;76(1):139–149. doi:10.1158/0008-5472.Can-15-1286
15. Gielen PR, Aftab Q, Ma N, et al. Connexin43 confers temozolomide resistance in human glioma cells by modulating the mitochondrial apoptosis pathway. Neuropharmacology. 2013;75:539–548. doi:10.1016/j.neuropharm.2013.05.002
16. Zhang YD, Zhao SC, Zhu ZS, et al. Cx43- and Smad-mediated TGF-beta/BMP signaling pathway promotes cartilage differentiation of bone marrow mesenchymal stem cells and inhibits osteoblast differentiation. Cell Physiol Biochem. 2017;42(4):1277–1293. doi:10.1159/000478957
17. Chen Z, Xie X, Huang J, et al. Connexin43 regulates high glucose-induced expression of fibronectin, ICAM-1 and TGF-beta1 via Nrf2/ARE pathway in glomerular mesangial cells. Free Radic Biol Med. 2017;102:77–86. doi:10.1016/j.freeradbiomed.2016.11.015
18. Zhao M, Lei C, Yang Y, et al. Abraxane, the nanoparticle formulation of paclitaxel can induce drug resistance by up-regulation of P-gp. PLoS One. 2015;10(7):e0131429. doi:10.1371/journal.pone.0131429
19. Li W, Zhai B, Zhi H, et al. Association of ABCB1, β tubulin I, and III with multidrug resistance of MCF7/DOC subline from breast cancer cell line MCF7. Tumour Biol. 2014;35(9):8883–8891. doi:10.1007/s13277-014-2101-z
20. Boichuk S, Galembikova A, Sitenkov A, et al. Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance. Oncol Lett. 2017;14(4):5039–5045. doi:10.3892/ol.2017.6795
21. Yu B, Tian X, Zhang L, Feng R. Hematopoietic PBX-interaction protein promotes breast cancer sensitivity to paclitaxel through a microtubule-dependent mechanism. DNA Cell Biol. 2016;35(11):740–745. doi:10.1089/dna.2016.3318
22. Montenegro MF, Gonzalez-Guerrero R, Sanchez-del-Campo L, Pinero-Madrona A, Cabezas-Herrera J, Rodriguez-Lopez JN. Targeting the epigenetics of the DNA damage response in breast cancer. Cell Death Dis. 2016;7:e2180. doi:10.1038/cddis.2016.85
23. Loginov VI, Pronina IV, Burdennyi AM, et al. Role of methylation in the regulation of apoptosis genes APAF1, DAPK1, and BCL2 in breast cancer. Bull Exp Biol Med. 2017;162(6):797–800. doi:10.1007/s10517-017-3716-z
24. Graham SV, Jiang JX, Mesnil M. Connexins and pannexins: important players in tumorigenesis, metastasis and potential therapeutics. Int J Mol Sci. 2018;19:6. doi:10.3390/ijms19061645
25. Zhang Q, Su M. Sufentanil attenuates oxaliplatin cytotoxicity via inhibiting connexin 43composed gap junction function. Mol Med Rep. 2017;16(1):943–948. doi:10.3892/mmr.2017.6669
26. Bodenstine TM, Vaidya KS, Ismail A, et al. Homotypic gap junctional communication associated with metastasis suppression increases with PKA activity and is unaffected by PI3K inhibition. Cancer Res. 2010;70(23):10002–10011. doi:10.1158/0008-5472.CAN-10-2606
27. Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234–240. doi:10.1016/j.tcb.2014.12.006
28. Ahmed N, Yang P, Chen H, et al. Characterization of inter-sertoli cell tight and gap junctions in the testis of turtle: protect the developing germ cells from an immune response. Microb Pathog. 2018;123:60–67. doi:10.1016/j.micpath.2018.06.037
29. Naik A, Al-Yahyaee A, Abdullah N, et al. Neuropilin-1 promotes the oncogenic tenascin-C/integrin beta3 pathway and modulates chemoresistance in breast cancer cells. BMC Cancer. 2018;18(1):533. doi:10.1186/s12885-018-4446-y
30. Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;24(5):761–779. doi:10.1038/sj.onc.1208238
31. Loessner D, Quent VM, Kraemer J, et al. Combined expression of KLK4, KLK5, KLK6, and KLK7 by ovarian cancer cells leads to decreased adhesion and paclitaxel-induced chemoresistance. Gynecol Oncol. 2012;127(3):569–578. doi:10.1016/j.ygyno.2012.09.001
32. Busch S, Acar A, Magnusson Y, Gregersson P, Ryden L, Landberg G. TGF-beta receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene. 2015;34(1):27–38. doi:10.1038/onc.2013.527
33. Wu J, Zhou XJ, Sun X, et al. RBM38 is involved in TGF-beta-induced epithelial-to-mesenchymal transition by stabilising zonula occludens-1 mRNA in breast cancer. Br J Cancer. 2017;117(5):675–684. doi:10.1038/bjc.2017.204
34. Xiang Y, Yang Y, Guo G, et al. beta3-tubulin is a good predictor of sensitivity to taxane-based neoadjuvant chemotherapy in primary breast cancer. Clin Exp Med. 2016;16(3):391–397. doi:10.1007/s10238-015-0371-4
35. Boggs AE, Vitolo MI, Whipple RA, et al. alpha-tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015;75(1):203–215. doi:10.1158/0008-5472.Can-13-3563
36. Horton ER, Byron A, Askari JA, et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17(12):1577–1587. doi:10.1038/ncb3257
37. Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11(2):97–105. doi:10.1038/embor.2009.276
38. Ruan JS, Zhou H, Yang L, et al. Ursolic acid attenuates TGF-beta1-induced epithelial-mesenchymal transition in NSCLC by targeting integrin alphaVbeta5/MMPs signaling. Oncol Res. 2019;27(5):593–600. doi:10.3727/096504017X15051723858706
39. Kanojia D, Morshed RA, Zhang L, et al. BetaIII-tubulin regulates breast cancer metastases to the brain. Mol Cancer Ther. 2015;14(5):1152–1161. doi:10.1158/1535-7163.Mct-14-0950
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.