Back to Journals » Drug Design, Development and Therapy » Volume 11
Conjugation of metronidazole with dextran: a potential pharmaceutical strategy to control colonic distribution of the anti-amebic drug susceptible to metabolism by colonic microbes
Authors Kim W, Yang Y, Kim D, Jeong S, Yoo J , Yoon JH, Jung Y
Received 12 December 2016
Accepted for publication 14 January 2017
Published 14 February 2017 Volume 2017:11 Pages 419—429
DOI https://doi.org/10.2147/DDDT.S129922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Wooseong Kim, Yejin Yang, Dohoon Kim, Seongkeun Jeong, Jin-Wook Yoo, Jeong-Hyun Yoon, Yunjin Jung
Laboratory of Biomedicinal Chemistry, Department of Manufacturing Pharmacy, College of Pharmacy, Pusan National University, Busan, Republic of Korea
Abstract: Metronidazole (MTDZ), the drug of choice for the treatment of protozoal infections such as luminal amebiasis, is highly susceptible to colonic metabolism, which may hinder its conversion from a colon-specific prodrug to an effective anti-amebic agent targeting the entire large intestine. Thus, in an attempt to control the colonic distribution of the drug, a polymeric colon-specific prodrug, MTDZ conjugated to dextran via a succinate linker (Dex-SA-MTDZ), was designed. Upon treatment with dextranase for 8 h, the degree of Dex-SA-MTDZ depolymerization (%) with a degree of substitution (mg of MTDZ bound in 100 mg of Dex-SA-MTDZ) of 7, 17, and 30 was 72, 38, and 8, respectively, while that of dextran was 85. Depolymerization of Dex-SA-MTDZ was found to be necessary for the release of MTDZ, because dextranase pretreatment ensures that de-esterification occurs between MTDZ and the dextran backbone. In parallel, Dex-SA-MTDZ with a degree of substitution of 17 was found not to release MTDZ upon incubation with the contents of the small intestine and stomach of rats, but it released MTDZ when incubated with rat cecal contents (including microbial dextranases). Moreover, Dex-SA-MTDZ exhibited prolonged release of MTDZ, which contrasts with drug release by small molecular colon-specific prodrugs, MTDZ sulfate and N-nicotinoyl-2-{2-(2-methyl-5-nitroimidazol-1-yl)ethyloxy}-d,l-glycine. These prodrugs were eliminated very rapidly, and no MTDZ was detected in the cecal contents. Consistent with these in vitro results, we found that oral gavage of Dex-SA-MTDZ delivered MTDZ (as MTDZ conjugated to [depolymerized] dextran) to the distal colon. However, upon oral gavage of the small molecular prodrugs, no prodrugs were detected in the distal colon. Collectively, these data suggest that dextran conjugation is a potential pharmaceutical strategy to control the colonic distribution of drugs susceptible to colonic microbial metabolism.
Keywords: dextran–metronidazole conjugate, colon-specific prodrug, metronidazole, dextran, polymeric prodrug, controlled release
Introduction
Metronidazole (MTDZ), marketed under the brand name Flagyl, is an antibiotic and antiprotozoal medication. It is effective in treating bacterial infections and protozoan infections such as amebiasis.1 It is the drug of choice for luminal amebiasis and first-episode occurrences of mild-to-moderate Clostridium difficile colitis.1–3 MTDZ, available as an oral formulation, cream, and intravenous preparation, is on the World Health Organization’s List of Essential Medicines, which contains important medications necessary in a basic health system.
Despite the benefits of the drug, systemic MTDZ therapy causes common adverse drug reactions, including nausea, diarrhea, weight loss, abdominal pain, vomiting, headache, and dizziness, and has an unpleasant metallic taste. Moreover, long-term systemic treatment has been associated with the development of leukopenia, neutropenia, peripheral neuropathy, and central nervous system toxicity.4
Given the systemic adverse reactions associated with MTDZ, it is beneficial to develop a system that delivers the drug specifically to the large intestine for the treatment of luminal amebiasis and colitis, which would increase the drug’s effectiveness and reduce systemic side effects.5–7 For this purpose, two kinds of colon-specific prodrugs have been developed – MTDZ sulfate and N-nicotinoyl-2-{2-(2-methyl-5-nitroimidazol-1-yl)ethyloxy}-D,L-glycine (NMG).8,9 However, these colon-specific prodrugs do not seem to distribute MTDZ to the entire large intestine. Previous studies demonstrated that neither MTDZ nor the prodrugs were detected in the colon, but that the prodrugs were found in the cecum.8,9 It was suggested that for both the tested agents, almost the entire prodrug was converted to MTDZ in the proximal part of the large intestine, where it was metabolized rapidly before reaching the distal part. This is because MTDZ is highly susceptible to colonic metabolism carried out by microbial enzymes.8 For effective treatment of colonic diseases such as colitis and amebic infection, it is necessary to distribute the anti-amebic drug to the entire large intestine, including the distal portion, which may also show disease pathology.5
Dextran, a non-starch polysaccharide consisting of linear α−1,6-glucopyranose chains with α−1,3-glucopyranose branching, is nonimmunogenic and biocompatible and has been widely studied as a polymeric drug carrier.10 It is a non-digestible polysaccharide easily depolymerized by microbial endodextranases in the large intestine.11 Depolymerization enables chemical and/or enzymatic cleavage of the chemical linker between any drug molecule and dextran, which is protected by steric hindrance during transit through the stomach and small intestine.10,12 Based on this property, researchers investigated the potential of dextran as a colon-specific carrier.13,14 A number of studies of colon-specific prodrugs that use dextran as a polymeric carrier have been published.15–21
In this study, we investigated a feasible strategy for the control of colonic distribution of a drug susceptible to colonic metabolism. Based on the pharmaceutical property that a drug conjugated to a polymer generally exhibits a sustained release in biological environments,10 MTDZ was conjugated to dextran using a succinic acid linker molecule (Dex-SA-MTDZ), and its colonic distribution was examined and compared with that of small molecular colon-specific MTDZ prodrugs. Our data demonstrate that dextran acts as a colon-specific carrier and a polymer matrix that sustains MTDZ release over a prolonged period.
Methods
Materials and instruments
Dextran (MW 70,000), dextranase (Penicillium sp.), esterase, 2,4-dinitrosalicylicacid (DNS), succinic anhydride, carbonyldiimidazole (CDI), and MTDZ were obtained from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). Dimethyl sulfoxide (DMSO)-d6 was obtained from Cambridge Isotope Laboratories (Andover, MA, USA). All reagents and solvents for high-performance liquid chromatography (HPLC) were obtained from Merck (Darmstadt, Germany). All other chemicals used were reagent grade, commercially available products. Infrared (IR) spectra were recorded on a Fourier transform-infrared (FT-IR) spectrophotometer (Varian, Palo Alto, CA, USA). 1H-nuclear magnetic resonance (NMR) spectra were obtained using a Varian AS 500 spectrometer, and the chemical shifts were recorded in parts per million (ppm) downfield from tetramethylsilane. Small molecular colon-specific prodrugs of MTDZ, MTDZ sulfate, and NMG were synthesized as described in previous studies.8,9 Structures of the prodrugs are shown in Figure S1.
Animals
Male Sprague Dawley (SD) rats aged 6 weeks (Samtako Bio Korea, Kyeong-gi-do, South Korea) were housed in conventional cages and acclimatized for 3–7 days under standard light- and climate-controlled conditions with free access to food and water. The animal protocols used in this study were reviewed and approved by the Pusan National University–Institutional Animal Care and Use Committee (PNU-IACUC) and followed their ethical procedures and scientific care regulations and guidelines.
Synthesis of Dex-SA-MTDZ
To 1.2 g (11.7 mmol) of succinic anhydride benzene containing 0.1 mL of triethylamine (TEA), 2 g of MTDZ (11.7 mmol) dissolved in 90 mL of acetonitrile was added, and the mixture was stirred for 7 days at 25°C. After evaporation of the solvent, the residue was subjected to recrystallization using water/ethanol (1:1). MTDZ monosuccinate (MM) was obtained as a white powder (melting point: 107°C–109°C, IR [nujol]: 1,747 cm−1 [C=O, ester], 1,718 cm−1 [C=O, carboxylic]), and 1.36 g (5.0 mmol) of it was dissolved in 15 mL of DMSO and reacted with 1.78 g (11.0 mmol) of CDI for 1 h. The reaction mixture was added to 1 g of dextran (MW 70,000) dissolved in 200 mL of DMSO containing 17 mL of TEA and stirred for 24 h at 25°C. The reaction mixture was poured into an excess of an ethanol/ether mixture (1:5), and the resulting sticky precipitate was dissolved in DMSO, followed by addition to an excess of ethanol/ether. This procedure was repeated until a precipitate in powder form was obtained and until MTDZ and MM went undetected in thin-layer chromatography analysis (MeOH/chloroform [1:1]). Coupling of MTDZ to dextran was confirmed by IR and 1H-NMR data (Figure S2).
HPLC analysis and UV spectrophotometry
The HPLC system consisted of Model 305 and 306 pumps, a 117 variable UV detector, a Model 234 autoinjector, a Model 805 manometric module, and a Model 811C dynamic mixer from Gilson, Inc. (Middleton, WI, USA). The mobile phase consisted of 10% acetonitrile in 0.067 M, pH 4.5 phosphate buffer, passed through a 0.45-μm membrane filter before use. A symmetry C18 column (250×4.6; Waters [Milford, MA, USA]) was eluted with the mobile phase at a flow rate of 1 mL/min. The column eluent was monitored at 319 nm with a sensitivity of 0.01 absorbance units full scale. For the analysis of MTDZ by UV spectrophotometry, a standard calibration curve was constructed from the absorbance at 319 nm of standard MTDZ solutions (1–20 ppm) in a pH 6.8 isotonic phosphate buffer.
Degree of substitution (DS) and pH stability
DS is defined as the weight (in mg) of MTDZ bound in 100 mg of Dex-SA-MTDZ. Dex-SA-MTDZ was completely hydrolyzed by reacting 100 mg of the sample with 100 mL of 0.1 M NaOH solution at 60°C for 1 h. The quantity of MTDZ was analyzed by UV spectrophotometry at 319 nm. For pH stability test, one solution of Dex-SA-MTDZ (DS 17) in pH 1.2 hydrochloric acid buffer and another in pH 6.8 phosphate-buffered saline (PBS; 1 g/50 mL) were prepared and incubated for 6 h at 37°C. At predetermined time intervals, 20 μL aliquots of the supernatant were subjected to HPLC analysis.
DNS colorimetric assay
Depolymerization of Dex-SA-MTDZ by dextranases was analyzed by colorimetric determination of reducing sugars generated by cleavage of the glycosidic bonds of dextran.22 DNS reagent solution was prepared by dissolving DNS (5 g) in 2 M NaOH (100 mL) and distilled water (250 mL). In this solution, potassium sodium tartrate tetrahydrate (150 g) was dissolved and the volume was adjusted to 500 mL with distilled water. Maltose (0.093–0.75 mg/mL) was dissolved in pH 5.4, 0.1 M acetate buffer, and 200 μL of MTDZ solutions of different concentrations were each mixed with 600 μL of DNS reagent solution, boiled for 5 min, and cooled for 10 min. Subsequently, absorbance at 540 nm was measured using a UV spectrophotometer. A calibration curve was obtained based on the results. A portion of the incubated sample was treated with DNS reagent solution using the same procedure, and the quantity of the terminal reducing sugar present was determined using the calibration curve.
Depolymerization of Dex-SA-MTDZ by dextranase
To Dex-SA-MTDZ (DS 7, 17, and 30, equivalent to 2.5 mg/mL of dextran) dissolved in pH 5.4 isotonic acetate buffer, dextranase (15 dose unit [U]/mL) was added, and the mixture was incubated at 37°C. In all, 1 mL of the solution was collected at each hour following solution preparation and subsequently incubated in boiled water for 1 min to deactivate the enzyme. The samples were centrifuged for 5 min at 10,000× g. A 200 μL sample of the supernatant and 600 μL of the DNS reagent were boiled together in a microtube for 5 min and then cooled for 10 min. Absorbance at 540 nm was measured using a UV spectrophotometer. The quantity of terminal reducing sugar present was determined using the calibration curve (obtained from the DNS assay).
Release of MTDZ from Dex-SA-MTDZ upon incubation with esterase and/or dextranase
Dextran-SA-MTDZ (DS 17) in pH 5.4, 0.1 M acetate buffer (0.6 mg/mL) was incubated in a microtube with dextranase (6 U/mL) at 37°C. The pH of the reaction solution was adjusted to 8 using 0.01 M NaOH 3 h later, followed by addition of esterase (6 U/mL). At appropriate time intervals, small quantities of the solution were placed in boiling water to inactivate the enzymes and then centrifuged. Free MTDZ in the supernatants was measured by HPLC analysis. In separate experiments, dextran-SA-MTDZ was incubated with dextranases or esterase alone under the same experimental conditions, and free MTDZ was analyzed as previously described.
Incubation with the contents of stomach and small intestine of rats
Male SD rats were sacrificed by CO2 asphyxiation, and a midline incision was made. The small intestine was divided into proximal small intestine (PSI) and distal small intestine (DSI). The luminal contents were diluted to half of the original concentration using pH 6.8 PBS. A 200-μL sample of the contents was incubated in a microtube with Dex-SA-MTDZ (DS 17, equivalent to 100 μg/mL of MTDZ) dissolved in pH 6.8 PBS at 37°C. The same experiment was performed with the contents of stomach diluted with isotonic acetate buffer (pH 4.5).23 At appropriate time intervals, the mixture was centrifuged at 3,000× g for 3 min. To a 100-μL sample of the supernatant, 900 μL of methanol was added. The mixture was vortexed for 2 min and centrifuged for 5 min at 10,000× g. Of the supernatant obtained, 20 μL of the obtained supernatant was used for HPLC analysis.
Incubation with the cecal contents of rats
The cecal segment of the intestine was cut open, and the contents were collected separately in a glove box in which air had been previously displaced by nitrogen. The cecal contents were suspended in pH 6.8 PBS to make 2.5%, 5%, and 10% suspensions. A 500-μL sample of each suspension was incubated with 500 μL of Dex-SA-MTDZ (DS 17, equivalent to 100 μg/mL of MTDZ) dissolved in pH 6.8 PBS in microtubes at 37°C under nitrogen atmosphere. Samples obtained at appropriate time intervals were subjected to HPLC analysis. The same experiment was performed with small molecular colon-specific MTDZ prodrugs in 5% suspensions of rat cecal contents.
Oral gavage of Dex-SA-MTDZ and small molecular colon-specific MTDZ prodrugs
Male SD rats were starved for 24 h prior to use in these experiments but were allowed free access to water. Dex-SA-MTDZ, MTDZ sulfate, or NMS (equivalent to 16 mg MTDZ/kg) was administered to separate groups of rats via oral gavage. After appropriate time intervals, the rats were sacrificed by CO2 asphyxiation, and a midline incision was made. The contents of the cecum, proximal colon, and distal colon were collected and diluted ten times with pH 6.8 PBS followed by centrifugation at 10,000× g. The levels of free MTDZ, the small molecular prodrugs, and (depolymerized) dextran-conjugated MTDZ in the supernatants were determined by HPLC analysis of samples. To obtain total MTDZ (free MTDZ + conjugated MTDZ), 1 M NaOH (20 μL) was added to the supernatants (180 μL) for 1 h at 60°C. The mixture was then neutralized using 0.1 M HCl. A 20-μL portion of each resulting sample was used in HPLC analysis.
Results
Preparation and physicochemical properties of Dex-SA-MTDZ
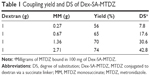
Synthesis of Dex-SA-MTDZ was achieved in two steps as shown in Figure 1. MM was reacted with CDI to form the imidazolide of MM, which was then reacted with dextran in the presence of TEA as a catalyst. Dex-SA-MTDZ with varied DS was prepared by changing the ratio in which dextran and MM were reacted (Table 1). The IR spectrum of Dex-SA-MTDZ showed a broad carbonyl band between 1,643 and 1,734 cm−1, with peaks originating from MM and dextran. Signals ascribed to dextran and MM were observed in the 1H-NMR spectrum of Dex-SA-MTDZ, as shown in Figure S2.
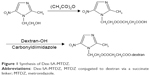  | Figure 1 Synthesis of Dex-SA-MTDZ. |
(Bio)chemical stability in the stomach and small intestine
To be delivered specifically to the large intestine, a prodrug should pass the stomach and small intestine without biochemical conversion to its parent drug.5 To examine the chemical stability of Dex-SA-MTDZ during transit through the stomach and small intestine, Dex-SA-MTDZ samples were placed in a buffer solution of pH 1.2 or 6.8 at 37°C and incubated for 6 h. Release of MTDZ was not detected, suggesting that Dex-SA-MTDZ is not deconjugated under these conditions.
Additionally, Dex-SA-MTDZ was incubated with the contents of the small intestine and stomach and MTDZ release was analyzed by HPLC. MTDZ was not detected in the intestinal and gastric contents throughout the experimental period. In addition, MTDZ was stable in the small intestinal and gastric contents for up to 8 h (Figure S3). These results suggest that Dex-SA-MTDZ administered orally can deliver MTDZ to the large intestine without significant loss of the drug in the upper intestine.
Depolymerization of Dex-SA-MTDZ is required for the release of MTDZ
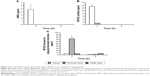
A dextran–drug conjugate reaching the large intestine needs to be depolymerized by colonic dextranases in order to release its parent drug.24 Such depolymerization abates the steric protection provided by the polymer, thus exposing susceptible ester bonds between the drug and dextran, and consequently leading to the release of the drug.25 While dextranases originating from microbes exist only in the large intestine, esterases are abundant throughout the digestive tract.26 We tested whether the modified dextrans were susceptible to dextranase. Dex-SA-MTDZ samples of various DSs were incubated with dextranase, and depolymerization was determined by the DNS method. As shown in Figure 2A, Dex-SA-MTDZ with DS of 7, 17, and 30 was depolymerized by up to 72%, 38%, and 4% following 8 h of incubation and 74%, 50%, and 12% following 24 h of incubation. Dextran was depolymerized by up to 85% following 24 h of incubation. To examine whether depolymerization could indeed lead to cleavage of ester bonds between dextran and MTDZ (refer Figure 1 for the structure of Dex-SA-MTDZ), Dex-SA-MTDZ (DS 17) was incubated with esterase in the presence and absence of dextranase. As shown in Figure 2B, neither esterase nor dextranase alone led to the release of MTDZ, but ~18% of the drug was released following concurrent action by both enzymes.
Dex-SA-MTDZ released MTDZ into the cecal contents in a prolonged manner
We examined whether the conjugation of MTDZ with dextran in Dex-SA-MTDZ affects the release property of the drug. Dex-SA-MTDZ was incubated in 5% cecal contents followed by HPLC analysis of MTDZ. For comparison, the same experiment was performed using small molecular colon-specific prodrugs of our previous studies.8,9 As shown in Figure 3A, the concentration of the small molecular prodrugs was reduced very fast without any detection of MTDZ occurring. On the contrary, as shown in Figure 3B, Dex-SA-MTDZ released MTDZ in a prolonged manner, with a gradual decline in the concentration over 24 h of incubation. To verify this sustained release of MTDZ from Dex-SA-MTDZ into cecal contents, Dex-SA-MTDZ was incubated with lower concentrations of cecal contents, in which case MTDZ metabolism was found to be retarded (Figure S3). As concentration of cecal contents increased, concentration of MTDZ reached its peak earlier, following which its concentration steadily declined. The rate of MTDZ decline was greater in a 5% suspension than in a 2.5% suspension of cecal contents (Figure 3B). In a 1.25% cecal content suspension, where MTDZ metabolism was not substantial (Figure S3), a steady level of MTDZ was maintained up to 24 h (Figure 3B). These observations lend credence to the hypothesis that MTDZ release from Dex-SA-MTDZ is sustained in the large intestine.
Dex-SA-MTDZ administered orally delivers MTDZ to the distal part of the large intestine
The in vitro results strongly suggest that, unlike the small molecular prodrugs, Dex-SA-MTDZ would ensure prolonged release of MTDZ in the large intestine, thus delivering MTDZ to the distal part of the large intestine. To verify this, Dex-SA-MTDZ was administered to rats by oral gavage. The levels of both free MTDZ and MTDZ bound to (depolymerized) dextran were measured in the cecum and the proximal and distal colon at appropriate time intervals. For comparison, the same experiment was performed using small molecular prodrugs. As shown in Figure 4A–C, in corroboration with the in vitro results, small molecular prodrugs, NMG (Figure 4A), and MTDZ sulfate (Figure 4B) were observed in the cecum but not in the distal part of the colon, while MTDZ bound to (depolymerized) dextran was detected in the cecum and in both parts of the colon. Moreover, the conjugated MTDZ was detected in the colon 4 and 8 h following oral gavage (Figure 4C). Regardless of the prodrug carriers used, no MTDZ was observed in the large intestine.
Discussion
Our data demonstrate that Dex-SA-MTDZ is chemically and enzymatically stable in the upper intestine, while the conjugate is depolymerized and releases MTDZ into the cecal contents, a process dependent on the action of microbial enzyme(s). Unlike small molecular colon-specific MTDZ prodrugs that disappear rapidly and result in no detection of MTDZ in the cecal contents, Dex-SA-MTDZ ensured sustained release of MTDZ during the experimental period. In agreement with this in vitro result, oral gavage of the small molecular and polymeric MTDZ prodrugs induces delivery of MTDZ conjugated to (depolymerized) dextran to the distal colon. However, small molecular prodrugs were not detected there.
In agreement with previous studies,5,12 dextran was found to act as a colon-specific polymeric carrier. Dex-SA-MTDZ liberated MTDZ into the cecal contents. By contrast, MTDZ was not detected in pH 1.2 and 6.8 buffers or in the small intestine homogenates. Moreover, a substantial amount of (depolymerized) dextran-bound MTDZ was detected in the large intestine upon oral gavage. These in vitro and in vivo results suggest that Dex-SA-MTDZ is a polymeric colon-specific prodrug.
We confirmed that the dextran-based colon-specific prodrug was activated by microbial enzymes.5 The release of MTDZ is dependent on them as evidenced by the non-release of the prodrug into autoclaved cecal contents (data not shown).27 In addition, MTDZ release by de-esterification required prior depolymerization of the dextran backbone, which reduces steric hindrance, thus exposing ester bonds between dextran and MTDZ. Incubation of Dex-SA-MTDZ with both dextranase and esterase liberated MTDZ, whereas esterase or dextranase alone both failed to release MTDZ from the dextran conjugate. These results confirm that dextran-conjugated drugs are protected during transit from esterases, which are found abundantly in the upper intestine.5,28,29
Data showing that MTDZ was liberated from Dex-SA-MTDZ upon incubation in cecal contents strongly suggest that the conjugate should release MTDZ upon delivery to the large intestine. However, we did not detect MTDZ in the large intestine following oral gavage of Dex-SA-MTDZ. This is understandable given that MTDZ is very susceptible to reductive metabolism by colonic microbes.8 MTDZ is metabolized rapidly following liberation from Dex-SA-MTDZ in the large intestine, where microbial metabolism may be much greater than that seen in the 5% suspension of cecal contents, resulting in MTDZ levels falling below the detection limit. On the other hand, this MTDZ metabolism is likely associated with the anti-amebic effect of MTDZ,9 since the anti-amebic and anti-bacterial activities of MTDZ result from its reductive conversion to toxic metabolites in colonic microbes.30,31
Drugs susceptible to colonic microbial metabolism do not reach the distal colon if the drugs are liberated from their colon-specific prodrugs in the first part of the large intestine.5 Therefore, an additional pharmaceutical strategy (along with colon-specific delivery) to sustain release of such drugs is required, especially for efficient treatment of colonic diseases where pathological lesions exist in the distal portion.32,33 Our data showing that MTDZ conjugated to (depolymerized) dextran was detected in the proximal colon and in the distal colon upon oral gavage suggest that dextran conjugation may provide another pharmaceutical benefit in the form of controlled distribution. The beneficial property of dextran is likely due to the drug release mechanism of dextran-conjugated MTDZ. Unlike small molecular MTDZ prodrugs where the linkers between the drug and carriers are directly cleaved in the large intestine leading to generation of MTDZ that is immediately exposed to colonic metabolism, dextran conjugation protects MTDZ from metabolism until depolymerization of the modified dextran occurs sufficiently to eliminate steric hindrance imposed by the dextran backbone. Such a release process may allow for differences in the rate of release of MTDZ depending on where MTDZ is bound in the dextran backbone. MTDZ bound to dextran may exhibit differing ease of depolymerization and subsequent de-esterification in various environments, resulting in differing release rates. This hypothesis is partly supported by data showing that the rate of MTDZ release from Dex-SA-MTDZ is dependent on the DS. It is likely that MTDZ may not be evenly distributed in a dextran backbone with a particular DS value, meaning that the DS may be different in different environments.
Collectively, our data suggest that dextran conjugation is a feasible pharmaceutical strategy to control colonic distribution of drugs susceptible to colonic microbial metabolism.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP; No 2009-0083538).
Disclosure
The authors report no conflicts of interest in this work.
References
Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs. 1997;54(5):679–708. | ||
Petri WA Jr. Therapy of intestinal protozoa. Trends Parasitol. 2003; 19(11):523–526. | ||
Sack DM, Peppercorn MA. Drug therapy of inflammatory bowel disease. Pharmacotherapy. 1983;3(3):158–176. | ||
Kapoor K, Chandra M, Nag D, Paliwal JK, Gupta RC, Saxena RC. Evaluation of metronidazole toxicity: a prospective study. Int J Clin Pharmacol Res. 1999;19(3):83–88. | ||
Jung Y, Kim YM. What should be considered on design of a colon-specific prodrug? Expert Opin Drug Deliv. 2010;7(2):245–258. | ||
Amidon S, Brown JE, Dave VS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 2015;16(4):731–741. | ||
Patel M, Shah T, Amin A. Therapeutic opportunities in colon-specific drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 2007;24(2):147–202. | ||
Kim D, Hong S, Jung S, Jung Y, Kim YM. Synthesis and evaluation of N-nicotinoyl-2-{2-(2-methyl-5-nitroimidazol-1-yl)ethyloxy}-D,L-glycine as a colon-specific prodrug of metronidazole. J Pharm Sci. 2009;98(11):4161–4169. | ||
Kim H, Lee Y, Yoo H, et al. Synthesis and evaluation of sulfate conjugated metronidazole as a colon-specific prodrug of metronidazole. J Drug Target. 2012;20(3):255–263. | ||
Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release. 2000;69(1):1–25. | ||
Sery TW, Hehre EJ. Degradation of dextrans by enzymes of intestinal bacteria. J Bacteriol. 1956;71(3):373–380. | ||
Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm. 2001;224(1–2):19–38. | ||
Shah N, Shah T, Amin A. Polysaccharides: a targeting strategy for colonic drug delivery. Expert Opin Drug Deliv. 2011;8(6):779–796. | ||
Kosaraju SL. Colon targeted delivery systems: review of polysaccharides for encapsulation and delivery. Crit Rev Food Sci Nutr. 2005;45(4):251–258. | ||
Lee Y, Kim IH, Kim J, et al. Evaluation of dextran-flufenamic acid ester as a polymeric colon-specific prodrug of flufenamic acid, an anti-inflammatory drug, for chronotherapy. J Drug Target. 2011;19(5):336–343. | ||
Varshosaz J, Emami J, Tavakoli N, et al. Synthesis and evaluation of dextran-budesonide conjugates as colon specific prodrugs for treatment of ulcerative colitis. Int J Pharm. 2009;365(1–2):69–76. | ||
McLeod AD, Tolentino L, Tozer TN. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: steady-state pharmacokinetics in the rat. Biopharm Drug Dispos. 1994;15(2):151–161. | ||
Lee Y, Kim J, Kim W, et al. Celecoxib coupled to dextran via a glutamic acid linker yields a polymeric prodrug suitable for colonic delivery. Drug Des Devel Ther. 2015;9:4105–4113. | ||
Lee JS, Jung YJ, Doh MJ, Kim YM. Synthesis and properties of dextran-nalidixic acid ester as a colon-specific prodrug of nalidixic acid. Drug Dev Ind Pharm. 2001;27(4):331–336. | ||
Nam J, Kim W, Lee S, et al. Dextran-5-(4-ethoxycarbonylphenylazo)salicylic acid ester, a polymeric colon-specific prodrug releasing 5-aminosalicylic acid and benzocaine, ameliorates TNBS-induced rat colitis. J Drug Target. 2016;24(5):468–474. | ||
Jung YJ, Lee JS, Kim HH, Kim YT, Kim YM. Synthesis and properties of dextran-5-aminosalicylic acid ester as a potential colon-specific prodrug of 5-aminosalicylic acid. Arch Pharm Res. 1998;21(2):179–186. | ||
Gusakov AV, Kondratyeva EG, Sinitsyn AP. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem. 2011;2011:283658. | ||
Jung YJ, Lee JS, Kim YM. Synthesis and in vitro/in vivo evaluation of 5-aminosalicyl-glycine as a colon-specific prodrug of 5-aminosalicylic acid. J Pharm Sci. 2000;89(5):594–602. | ||
Chourasia MK, Jain SK. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004;11(2):129–148. | ||
Larsen C, Harboe E, Johansen M, Olesen HP. Macromolecular prodrugs. XVI. Colon-targeted delivery – comparison of the rate of release of naproxen from dextran ester prodrugs in homogenates of various segments of the pig gastrointestinal (GI) tract. Pharm Res. 1989;6(12):995–999. | ||
Inoue M, Morikawa M, Tsuboi M, Sugiura M. Species difference and characterization of intestinal esterase on the hydrolizing activity of ester-type drugs. Jpn J Pharmacol. 1979;29(1):9–16. | ||
Kim H, Kong H, Choi B, et al. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm Res. 2005;22(9):1499–1509. | ||
Nielsen LS, Weibel H, Johansen M, Larsen C. Macromolecular prodrugs. XX. Factors influencing model dextranase-mediated depolymerization of dextran derivatives in vitro. Acta Pharm Nord. 1992;4(1):23–30. | ||
Harboe E, Larsen C, Johansen M, Olesen HP. Macromolecular prodrugs. XV. Colon-targeted delivery – bioavailability of naproxen from orally administered dextran-naproxen ester prodrugs varying in molecular size in the pig. Pharm Res. 1989;6(11):919–923. | ||
Leitsch D, Kolarich D, Wilson IB, Altmann F, Duchene M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 2007;5(8):e211. | ||
Ludlum DB, Colinas RJ, Kirk MC, Mehta JR. Reaction of reduced metronidazole with guanosine to form an unstable adduct. Carcinogenesis. 1988;9(4):593–596. | ||
Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991; 325(13):928–937. | ||
Podolsky DK. Inflammatory bowel disease (2). N Engl J Med. 1991; 325(14):1008–1016. |
Supplementary materials
  | Figure S1 Structures of small molecular colon-specific prodrugs. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.