Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Conditions and Factors That Raise the Risk of Developing Skin Lesions After Shingles
Authors Xue Q, Ji J, Fan WG, Pan JP, Wei M, Ding H, Zhao J
Received 5 July 2023
Accepted for publication 25 September 2023
Published 13 October 2023 Volume 2023:16 Pages 2869—2878
DOI https://doi.org/10.2147/CCID.S429143
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Qiao Xue,1 Jie Ji,2 Wen-Ge Fan,1 Jian-Peng Pan,3 Mei Wei,1 Hao Ding,1 Jun Zhao1
1Department of Dermatology, Changshu No. 1 People’s Hospital, Changshu Hospital Affiliated to Soochow University, Changshu, Jiangsu Province, 215500, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, 210000, People’s Republic of China; 3Department of Hand Surgery, Changshu No. 2 People’s Hospital, Changshu Hospital Affiliated to Yangzhou University, Changshu, Jiangsu Province, 215500, People’s Republic of China
Correspondence: Jun Zhao, Department of Dermatology, Changshu Hospital Affiliated to Soochow University, Changshu No. 1 People’s Hospital, No. 1 Shuyuan Street, Yushan Town, Changshu, Jiangsu Province, 215500, People’s Republic of China, Tel +86-15051774136, Email [email protected]
Objective: To understand the situation and risk factors of skin lesions following the eruption of shingles.
Methods: We selected 275 patients with shingles who had been diagnosed and treated in the Dermatology Department of Changshu No. 1 People’s Hospital between July 2017 and March 2022. Age, gender, skin lesion site, skin lesion type, prodromal pain, history of diabetes, history of hypertension, history of other immune diseases, as well as other pertinent clinical data, were collected. The severity and pain of patients with severe shingles were evaluated, and their fasting blood sugar and plasma albumin were measured for routine antiviral treatment. They were followed up 6 months—the types of skin lesions and pertinent clinical data were compared, and the risk factors for skin lesions were analyzed.
Results: There were no statistically significant differences in gender, age, or site among the different types of skin lesions (P > 0.05). The severity of skin lesions, acute pain, history of diabetes, history of scars, low immune function, combined with hypoproteinemia, squeezing and stripping behavior, and post-herpetic neuralgia (PHN) were significantly associated with skin lesions (P < 0.05). The results of multivariate analysis showed that: age ≥ 60 years old, severe skin injury combined with diabetes, low immune function, scar history, squeezing and stripping were independent risk factors for the development of skin lesions due to shingles.
Conclusion: There is no significant difference in age, gender, site, or other characteristics between the types of skin lesions due to shingles. The independent risk factors of skin lesions due to shingles are old age, severe rash, history of scars, diabetes, low immunity, squeezing, and peeling.
Keywords: risk factors, skin lesions due to shingles
Introduction
Varicella and shingles are both caused by the varicella-shingles virus (VZV).1 This virus predominantly infects infants, and scarring is common after infection. Studies suggest that the likelihood of scarring after varicella is approximately 18%, with no significant differences in gender.2 The clinical manifestations and histopathology of the primary skin lesions due to shingles are extremely similar to those of varicella.3 Elderly and malnourished patients infected with this virus and having skin lesions can have severe adverse reactions, such as necrosis and are more likely to have scarring, a condition known as gangrenous shingles.1 Previous studies have found that the virus infection and skin response delay between the time range is very wide, from a few days to a few years.4 The pathogenesis of these skin reactions is not fully clear. Earlier studies have proposed virus and immunological etiology, virus infection can result in high local skin immune response or immunosuppression, which leads to the formation of a granuloma or pigmentation.5 The occurrence of skin lesions, such as erythema, hypopigmentation, a sunken scar, and hypertrophic scar, after the peeling off of a shingles scab has been rarely reported. In this paper, we report the results of our treatment of patients with shingles to comprehend the condition of skin lesions due to shingles and investigate the relevant risk factors associated with its occurrence.
Clinical Data and Methods
Clinical Data
Case Selection
We selected 275 patients with shingles who were treated and diagnosed at the Dermatology Clinic of Changshu No. 1 People’s Hospital between July 2017 and March 2022. The study participants were selected based on the inclusion and exclusion criteria, and signed the informed consent form. This study was approved by the hospital’s ethics committee.
Inclusion Criteria
All patients met the diagnostic criteria for shingles described in the literature,1 all patients signed the informed consent form and agreed to the use of their photos for scientific research. All patients were ≥18 years old, patients ≥60 years old were included in the elderly group, and patients <60 years old were included in the non-elderly group.
Exclusion Criteria
- Patients unwilling to cooperate with respect to medical history collection, diagnosis, treatment, follow-up, and who lack the ability to take decisions on their own.
- Pregnant and lactating females.
Experimental Methods
All patients were treated at the dermatology clinic. Two dermatologists collected and assessed relevant clinical data: age, gender, skin lesion site, onset time, prodromal pain, history of diabetes and hypertension, systemic immune diseases, and scar history. To evaluate the severity of rash and acute pain at the time of treatment, valaciclovir [Trade Name: Valaciclovir Hydrochloride Tablets, LIVZON Pharmaceutical Factory of LIVZON Group, National [YZZ H10960079] 0.3 g was administered orally twice daily before meals for 10 consecutive days. Penciclovir ointment was applied topically [Trade Name: Penciclovir Cream, Chongqing HUAPONT PHARM., GYZZ H20000189] twice daily until all scabs fell off. Abdominal blood glucose and plasma albumin were measured at the time of the visit. After 6 months of treatment, follow-up visits were conducted through outpatient follow-up, phone calls, WeChat, and other methods to inquire about the peeling behavior after scabbing, the skin lesions at the affected area, and the condition of nerve pain. The classification of skin lesions (erythema pigmentation, depigmentation, sunken scar, hypertrophic scar, etc.) was confirmed during outpatient reexamination or via WeChat images. The differences in clinical data between individuals with and without skin lesions were compared, to identify the risk factors for the development of skin lesions.
Evaluation Methods
- The severity of rashes was categorized as mild to moderate, mild to bruising, moderate to classic, blisters, pustules, severe to bullous, blood blisters, and gangrene.
- Acute pain evaluation criteria: The Pain Visual Analog Scale (VAS) was utilized, with higher scores indicating more severe pain. A score of 0 indicates no pain; a score of 10 indicates severe pain tolerance; a score of 1–3 indicates mild pain; a score of 4–6 indicates moderate pain; and a score of 7–10 indicates severe pain.6
Statistical Methods
SPSS 26.0 statistical software was used for analysis. The counting data are represented by the number of patients (percentage), while the measurement data are represented by the mean ± standard deviation (X ± SD). The counting data were evaluated using the chi-squared test. A single factor screening (P < 0.05) was incorporated into the multivariate logistic regression analysis. Inspection level α = 0.05.
Results
General Information and Follow-Up Results Statistics
In all, 270 of the 275 patients with shingles completed follow-up, which included 129 males (57.66 ± 13.06 years old) and 141 females (57.84 ± 14.44 years old). The infection sites in 61 cases (22.59%) were in the trigeminal nerve area, 124 cases (45.93%) were in the intercostal nerve area, 52 cases (19.26%) were in the cervical brachial plexus area, and 33 cases (12.22%) were in the lumbosacral nerve area. Among them, 35 cases had diabetes (13.01%), 140 cases had hypertension (51.58%), 28 cases had low immunity (10.41%) (17 cases had a history of malignant tumor, 3 cases had systemic lupus erythematosus (SLE), 1 case had Sjogren’s syndrome, 2 cases had HIV infections, 5 cases had nephrotic syndrome), 22 cases had hypoproteinemia (plasma albumin <30 g/L) (8.15%), and 9 cases had a history of scarring (3.33%). Three months post-surgery, 53 cases (19.63%) developed post-herpetic neuralgia (PHN), 44 cases (16.29%) had skin lesions, and 41 cases (15.19%) had peeling behavior.
Conditions of Skin Lesions
There were 44 cases with skin lesions, including 24 males, with an average age of 61.28 ± 12.78 years and incidence rate of 18.60%, and 20 females, with an average age of 60.75 ± 7.99 years and incidence rate of 14.18%. The incidence of skin lesions in males was higher than that in females, but there was no statistically significant difference (P = 0.326). Among the 44 patients with skin lesions, 17 cases (39.53%) had related immune system diseases (2 cases had SLE, 2 cases had HIV infection, 3 cases had nephrotic syndrome, 10 cases had malignant tumors), 12 cases (27.27%) had a history of diabetes, 28 cases (63.63%) had hypertension, 7 cases (15.90%) had hypoproteinemia (serum albumin below 30 g/L), 6 cases (13.64%) had a history of scarring, 19 cases (43.18%) involved squeezing and picking behavior, and 20 cases (45.45%) had PHN. See Table 1.
 |
Table 1 Single Factor Analysis Results of Skin Lesions Due to Shingles |
Analysis of Skin Lesions in Different Areas
There were 44 cases with skin lesions, 7 cases of whom were infected in the trigeminal nerve area, 23 cases in the intercostal nerve area, 7 cases in the brachial plexus area, and 7 cases in the sacrococcygeal plexus area. There were 19 cases with erythema pigmentation, among whom, 3 cases were infected in the trigeminal nerve area (see Figure 1), 9 cases in the intercostal nerve area, 5 cases in the cervical brachial plexus area, and 2 cases in the sacrococcygeal plexus area. There were 11 cases with hypopigmentation (see Figure 2), among whom 2 cases were infected in the trigeminal nerve region, 6 cases in the intercostal nerve region, 1 case in the cervical brachial plexus region, and 2 cases in the sacrococcygeal plexus region. There were 4 cases were with hypertrophic scars (see Figure 3), among whom 2 cases had scarring in the intercostal nerve area, 1 case had scarring in the cervical brachial plexus region, and 1 case had scarring in the sacrum area. There were 4 cases were with sunken scar (see Figure 4), among whom 1 case was infected in the trigeminal nerve area, 2 cases were in the intercostal nerve area, and 1 case was in the sacrococcygeal plexus area. There were 5 cases with pigmentation with sunken scar, among whom there was 1 case with pigmentation in the trigeminal nerve plexus, 2 cases in the intercostal nerve area, 1 case in the cervical brachial plexus area, and 1 case with pigmentation combined with hyperplasia in the intercostal nerve area. The statistical results show that there were no statistically significant differences in the types of skin lesions among patients of different genders, sites, and ages (P > 0.05). See Table 2 for details.
 |
Table 2 Distribution of Shingles-Related Skin Lesions |
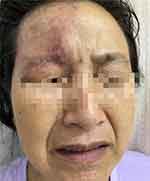 |
Figure 1 Erythema and pigmentation in the trigeminal nerve region. |
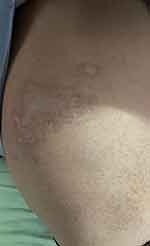 |
Figure 2 Depigmentation in the intercostal region. |
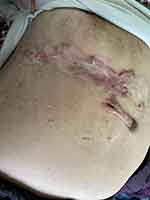 |
Figure 3 Hypertrophic Keloid in intercostal region. |
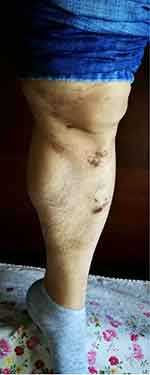 |
Figure 4 Pigmentation and depression in the sacroiliac and lower limbs. |
Univariate analysis of risk factors for skin lesions due to shingles revealed that patients with skin lesions had diabetes, low immunity, hypoproteinemia, history of scarring, and picking and peeling behavior, and that the proportion of patients with PHN was significantly higher among those with skin lesions over (P < 0.05). The age of onset, severity of skin lesions, and acute pain intensity of patients were significantly correlated with skin lesions (P < 0.05). However, the skin lesions were not correlated with the gender, site of onset, time of first diagnosis, forebody pain, or history of hypertension (P > 0.05). See Table 1 for details.
Multivariate analysis revealed that age ≥60 years old, severe skin lesions combined with diabetes, low immunity, history of scarring, and squeezing and stripping behavior, were significant independent risk factors for skin lesions after shingles (P < 0.05). See Table 3 for details.
 |
Table 3 Multifactor Logistic Regression Analysis of Skin Lesions Due to Shingles |
Discussion
It is known that shingles (herpes zoster) (HZ) is a segmental cluster of shingles formed in the ganglion area after the reactivation of the varicella-shingles virus. Patients with severe conditions may develop bullae, blood blisters, skin lesions necrosis, and scars after healing.1 Based on the six-month follow-up of 270 patients with HZ, we discovered that 44 patients had skin lesions after six months, including 20 males and 24 females, with no difference in gender (P < 0.05). Based on the results, 16.29% of all patients with HZ had skin lesions, which is comparable to the incidence rate reported in literature (11–20%).7 There were 25 cases (19+1+5) with skin lesions in patients with erythema pigmentation, which accounted for 54.50% of the total. There were 19 cases of simple erythema pigmentation, 5 cases of combined depressed scars, and 1 case of combined hypertrophic scars. VZV infection can cause both shingles and varicella. Pathological manifestations of ballooning degeneration are acute inflammatory changes.1 Post inflammatory hyperpigmentation (PIH) is a common consequence of acute or chronic skin inflammation. Previous studies have demonstrated that PIH is more likely to occur in Fitzpatrick III–VI skin types.8 In this study, we focused primarily on Asian populations with skin colors III and IV, which have a greater risk of developing pigmentation (54.50%). Among the patients with skin lesions, there were 5 who had hypertrophic scars, which were primarily located in the anterior chest (3 cases), posterior neck (1 case), and lumbosacral region (1 case), and were distributed in areas not dominated by the trigeminal nerve. This resembles the cicatricial healing site after skin injury caused by other factors in the past.9 This is considered to be primarily associated with the mechanical tension of skin lesions. Mechanical tension directly transmits to the nucleus via the integrin cytoskeleton LINC complex signal pathway, which affects chromatin. This activates the TGF-13/Smad pathway, influencing the expression of cell genes, promoting the transformation of skin fibroblasts into proliferative fibroblasts, and promoting the formation of hypertrophic scars.10 Our research suggests that patients who engage in squeezing and peeling behavior are more likely to have skin lesions. When squeezing and peeling the skin, the skin suffers secondary damage, cell proliferation is activated, and fibroblasts stimulate the formation of local inflammatory factors, inducing cells to synthesize a large quantity of osteogenic protein, resulting in the hypertrophic scars.11
The results of our study revealed that severe skin lesions are more likely to result in pigmentation and scars. In the early stages in severe cases, large blisters, blood blisters, gangrene, severe tissue damage to the dermis tissue, and severe degeneration of collagen fibers in the dermis hinder the recovery of the skin. More severe skin lesions correlate with a wider incidence area, which suggests a greater viral load,12 more severe inflammatory damage, and more severe lung and brain complications. In later stages, skin lesions are more common because they take longer to heal, and the body takes longer to recover. The presence of VZV virus particles in dermal dendritic cells of severe HZ skin lesions has been shown to significantly impact dermal dendritic cell healing, modifying their normal function and inducing scar formation.13 In our study, there were 17 patients with skin lesions who had immune dysfunction, 2 cases who were HIV positive, 2 cases who had SLE, 2 cases had nephrotic syndrome, and 10 cases had malignant tumors, accounting for 39.53% of the total. Skin lesions caused by shingles are more severe, take longer to heal, and have a lower recovery rate in patients with malignant tumors or autoimmune disorders, as has been confirmed by prior research.14,15 In addition to immune factors, hyperglycemia affects the healing of rashes in patients with HZ. Studies suggest that most patients with diabetes experience microvascular damage, and microcirculatory disorders expand capillaries. The oxygen radical caused by hypoxia in mitochondria rapidly transforms procollagen into collagen in the presence of proline and lysine. If IGF-I levels in patients with diabetes are high, collagen synthesis is stimulated. Skin damage is difficult to heal, and patients with diabetes develop scars.16 The results of this study revealed that the elderly are more prone to have scarring. In this study, 29 of the 44 patients were over 60 years old. The incidence rate of shingles in the elderly was approximately (3.6/1000), which is twice that of the young. The elderly had more severe skin lesions, and these lesions also healed and recovered more slowly. Long-term local inflammation increased the likelihood of scar formation.17 In addition, as age increases, the density of collagen in the dermis decreases by approximately 1% per year. The number of fibroblasts decreases, the elastic fragments of fibrin increase, the contraction force of wounds decreases, and the epidermal turnover time decreases accordingly. The decrease in the number of blood vessels in the dermis and the prevalence of numerous systemic diseases that occur in later years can decrease wound healing ability.18 In this study, we found that the skin lesions in patients with a history of scarring are more likely to leave scars or pigmentation. Hypertrophic scars have been linked to single-nucleotide polymorphism (SNP) by some Japanese researchers. Potential loci associated with hypertrophic scars have been identified on chromosomes 2q23, 7p11, and 10q23.31 in people of Japanese, African American, and Han Chinese ethnicities, but the dominant gene has not yet been identified.18,19
Like acne scars, the scars or pigmentation after shingles affect not only the appearance of patients, but also their quality of life and self-esteem. It is imperative to improve skin care for patients with severe skin lesions, control the inflammatory response as quickly as possible, and prevent infection. Patients with diabetes, immunodeficiency, and a history of scarring, should be administered antiviral treatment as soon as possible to control the development of local skin lesions. Patients with scabs in non-trigeminal areas should be cautioned against randomly peeling or squeezing them to avoid impeding the healing of skin lesions and causing severe scarring. There are still some limitations to this study. The cases in this study were all patients who received routine antiviral treatment at a local hospital or clinic. Thus, we could not predict the skin lesions of patients who have not received antiviral treatment for shingles. Due to the small sample size and the fact that all of the patients were from the local area, it is possible that our findings do not generalize to other regions or people of different skin tones. In future, we will increase the sample size and conduct a multicenter clinical study. Due to the limited study time, it was not possible to conduct a long-term follow-up on patients with skin lesions. Thus, longer study time is needed to track and follow-up on the prognosis of pigmentation abnormalities and hypertrophic scars to understand the condition of such patients.
Conclusion
The results of this study confirmed that age, severity of skin lesions, as well as the combination of diabetes, low immunity, history of scarring, squeezing and stripping behavior, and other factors affect the generation and regression of skin lesions after shingles, resulting in the formation of hypertrophic or concave scars in severe cases. With the widespread use of the shingles vaccine, the incidence of severe inflammatory skin lesions will be reduced to a certain extent, the duration of skin lesions after shingles have been shortened, and scarring and pigment abnormalities reduced. Furthermore, we did not identify any association between skin lesion morphology and external body structure. It is undeniable, however, that the surface structure of the body plays a role in scar formation. Furthermore, the sample size and scope of the current study need to be increased.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Changshu No.1 People’s Hospital (2017No.20). A written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from every individual whose data are included in this manuscript.
Funding
National Natural Youth Foundation (NO.81602396).
Disclosure
None of the authors have any financial disclosure or conflict of interest.
References
1. Zhao B. Chinese Clinical Dermatology. Jiangsu Science and Technology Press; 2010.
2. Leung AK, Kao CP, Sauve RS. Scarring resulting from chickenpox. Pediatr Dermatol. 2001;18(5):378–380. doi:10.1046/j.1525-1470.2001.01975.x
3. Nikkels AF, Delvenne P, Debrus S, et al. Distribution of varicella-zoster virus gpI and gpII and corresponding genome sequences in the skin. J Med Virol. 1995;46(2):91–96. doi:10.1002/jmv.1890460202
4. Wolf R, Brenner S, Ruocco V, Filioli FG. Isotopic response. Int J Dermatol. 1995;34(5):341–348. PMID: 7607796. doi:10.1111/j.1365-4362.1995.tb03616.x
5. Ruocco E, Baroni A, Cutrì FT, Filioli FG. Granuloma annulare in a site of healed herpes zoster: wolf’s isotopic response. J Eur Acad Dermatol Venereol. 2003;17(6):686–688. PMID: 14761138. doi:10.1046/j.1468-3083.2003.00730.x
6. Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. Entwicklung und Validierung des VAS-Wirbelsäulenscores [Development and validation of the Visual Analogue Scale (VAS) Spine Score]. Unfallchirurg. 2001;104(6):488–497. German. doi:10.1007/s001130170111
7. Bowsher D. The lifetime occurrence of Herpes zoster and prevalence of post-herpetic neuralgia: a retrospective survey in an elderly population. Eur J Pain. 1999;3(4):335–342. doi:10.1016/S1090-3801(99)90015-0
8. Kaufman BP, Aman T, Alexis AF. Postinflammatory hyperpigmentation: epidemiology, clinical presentation, pathogenesis and treatment. Am J Clin Dermatol. 2018;19(4):489–503. doi:10.1007/s40257-017-0333-6
9. Huang X, Shan SG, Wei HM, et al. Expression and significance of IGF-I and IGF-IR in different part of keloid. Chin New Clin Med. 2009;002(007):688–690.
10. Zhu MY, Kuang RX, Wang ZG, Li YS. Mechanism of hypertrophic scar formation induced by mechanical stress. Chin Med J. 2017;97(026):2072–2074.
11. Hinz B. The role of myofibroblasts in wound healing[J]. Curr Res Transl Med. 2016;171–177. doi:10.1016/j.retram.2016.09.003
12. Warren-Gash C, Forbes H, Maple P, Quinlivan M, Breuer J. Viral load and antibody boosting following herpes zoster diagnosis. J Clin Virol. 2018;103:12. doi:10.1016/j.jcv.2018.03.010
13. Persson A, Bergström T, Lindh M, Namvar L, Studahl M. Varicella-zoster virus CNS disease--viral load, clinical manifestations and sequels. J Clin Virol. 2009;46(3):249–253. doi:10.1016/j.jcv.2009.07.014
14. Li HY, Cai LM. Comparative observation of curative effect of herpes zoster complicated with malignant tumor or not. Chin Primary Med. 2011;18(014):1936–1937.
15. Zhang Q. Retrospective Analysis of Herpes Zoster in Patients with Systemic Lupus Erythematosus. Xinjiang Medical University; 2016.
16. Frykberg RG, Wang YZ. Pathogenesis of diabetic foot ulcer: factors hindering wound healing. Foreign Medicine (Endocrinology); 2004.
17. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. doi:10.3390/ijms18030606
18. Marneros AG, Norris JE, Watanabe S, Reichenberger E, Olsen BR. Genome scans provide evidence for keloid susceptibility loci on chromosomes 2q23 and 7p11. J Invest Dermatol. 2004;122(5):1126–1132. doi:10.1111/j.0022-202X.2004.22327.x
19. Chen Y, Gao JH, Yan X, Song M, Liu XJ. 中国汉一瘢痕疙瘩家系易感基因的定位研究 [Location of predisposing gene for one Han Chinese keloid pedigree]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2007;23(2):137–140. Chinese. Chinese.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
