Back to Journals » Vascular Health and Risk Management » Volume 19
Conclusions from an Observational Study of Patients with Vascular Diseases Using the FMSF Technique
Authors Mikosiński J, Mikosiński P, Kwapisz A, Katarzynska J , Gebicki J
Received 17 October 2023
Accepted for publication 17 November 2023
Published 22 November 2023 Volume 2023:19 Pages 755—764
DOI https://doi.org/10.2147/VHRM.S442344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Jacek Mikosiński,1 Paweł Mikosiński,1 Aleksandra Kwapisz,1 Joanna Katarzynska,2 Jerzy Gebicki2,3
1MIKOMED Medical Center, Lodz, Poland; 2Angionica Ltd, Lodz, Poland; 3Institute of Applied Radiation Chemistry, Lodz University of Technology, Lodz, Poland
Correspondence: Jacek Mikosiński, MIKOMED Medical Center, Traktorowa 13, Lodz, 94-238, Poland, Tel +48 459 595 021, Email [email protected] Jerzy Gebicki, Institute of Applied Radiation Chemistry, Lodz University of Technology, Lodz, 90-924, Poland, Tel +48 42 631 31 71, Email [email protected]; [email protected]
Purpose: There is great demand for a diagnostic tool for non-invasive assessment of vascular circulation and metabolic regulation. Assessing both these functions is crucial, as each can have a distinct response to hypoxia.
Patients and Methods: The Flow Mediated Skin Fluorescence (FMSF) technique appears uniquely suitable for analysis of vascular circulation and metabolic regulation. In this observational study, the FMSF technique was used to diagnose patients with various vascular diseases. The study group consisted of 482 patients (264 females and 218 males) between the ages of 40– 94 years with various vascular problems (arterial hypertension, cardiovascular disease, diabetes, hypercholesterolemia, and chronic venous disease).
Results: Three major FMSF parameters were used: Ischemic Response (IRmax), Hyperemic Response (HRmax), and Reactive Hyperemia Response (RHR). All three parameters were found to decrease with age with a distinguishable kinetics. The IRmax parameter was used for characterization of metabolic reaction to transient hypoxia and HRmax was used for characterization of macrocirculatory function. Both were sex-dependent.
Conclusion: Females were metabolically less adaptive to transient hypoxia than males. However, macrocirculatory function was better in females than among males. Microcirculatory function decreases gradually with age, while macrocirculatory function decreases much more slowly with age, with a tendency to stabilize after 70 years of age.
Keywords: vascular circulation, metabolic regulation, NADH fluorescence, FMSF technique
Introduction
Flow Mediated Skin Fluorescence (FMSF) is a new non-invasive technique for assessing vascular circulation and metabolic regulation.1–5 It measures stimulation of the vascular circulation in response to post-occlusive reactive hyperemia (PORH), based on dynamical changes in nicotinamide adenine dinucleotide (NADH) fluorescence from skin tissue. AngioExpert, produced by Angionica Ltd., is a diagnostic tool based on the FMSF technique. It has been registered in the European Union as a medical device (class IIa).
An AngioExpert unit has been installed in the MIKOMED Medical Center (Lodz, Poland). The device has been used frequently for analysis of patients with various vascular problems (arterial hypertension, cardiovascular disease, diabetes, hypercholesterolemia, and chronic venous disease). Over the course of a year, close to 500 patients were tested. As a sizeable group of patients had been examined, it was decided to summarize this observational study and draw general conclusions related to the use of the FMSF technique for the diagnosis of various vascular problems. Particular attention was given to the utility of specific FMSF parameters for monitoring vascular problems related specifically to vascular circulation and metabolic regulation. The effects of sex and age were also considered.
Based on the observational study, this paper will demonstrate the diagnostic utility of the key FMSF parameters for non-invasive assessment of vascular circulation and metabolic regulation. A clear distinction between circulatory function and metabolic regulation was observed with respect to sex and age.
Materials and Method
Study Population and Clinical Characteristics
The observational study of standard non-interventional nature included 482 patients who had been treated at the MIKOMED Medical Center over the previous 12 months. According to the Polish regulations such studies do not require a permission of bioethical commission. All formal regulations concerning the experimental data collection have been fully respected and the patients have been informed that the conducted measurements can be used for publication purposes with a full confidentiality and anonymity. The characteristics of the study group are displayed in Table 1. This study focuses only on the effects of sex and age on vascular circulation. An analysis of particular vascular problems will be presented in a separate publication. For example, very interesting observations were made for diabetic patients with microcirculatory complications and for patients with diabetic foot ulcers and venous leg ulcers, with clear differences appearing in macrocirculatory function.
 |
Table 1 Characteristics of the Study Group |
Brief Description of the FMSF Technique and the Measurement Protocol
Measurements were performed using AngioExpert, a device produced by Angionica Ltd. The AngioExpert device uses the Flow Mediated Skin Fluorescence (FMSF) technique, which measures changes in the intensity of nicotinamide adenine dinucleotide (NADH) fluorescence from the skin on the forearm as a response to blocking and releasing blood flow. The skin is the largest organ of the human body, and is characterized by a specific metabolism. The epidermal layer of skin is not directly vascularized, and oxygen and nutrients are transported from the dermis by diffusion. Therefore, epidermal cell metabolism can be considered a unique and sensitive marker of early dysfunction in vascular circulation and metabolic regulation.
The measurement protocol in the present study was as applied previously.2–4 For analysis of the results, three major FMSF parameters were used: Ischemic Response (IRmax), Hyperemic Response (HRmax), and Reactive Hyperemia Response (RHR). These parameters are defined in Figure 1A. It has been shown that these parameters differentiate statistically the patients with arterial hypertension or diabetes from the healthy group.
The FMSF technique utilizes a metabolic sensor for assessment of vascular circulation based on measurement of the changes in the NADH – NAD+ equilibrium in epidermal keratinocytes mediated by oxygen delivered by the blood flow. The RHR parameter characterizes the overall metabolic sensitivity of epidermal keratinocytes to oxygen delivered to the epidermis via blood circulation. RHR is a unique parameter based on a combination of the ischemic and hyperemic parts of the measured FMSF trace, as shown in Figure 1A. The RHR parameter characterizes both vascular circulation and metabolic regulation. It decreases with age and in cases of evident vascular dysfunction. The RHR parameter positively correlates with the log(FM) (FlowMotion) and log(HS) (Hypoxia Sensitivity) microcirculatory parameters (Figure 1S and Figure 2S). RHR values are particularly high in physically active young individuals (RHR > 40%) and low in patients with serious vascular dysfunction (RHR < 20%).4 The RHR parameter can be used to characterize a broad segment of the population and can be regarded as a vitality measure.
The IRmax parameter measures the response to brachial artery occlusion resulting in the complete blockage of oxygen delivery to the epidermis. There is no blood flow during the ischemic phase of the FMSF measurement and microcirculatory oscillations are not present on the ischemic curve. The IRmax parameter calculated from the ischemic response should be treated as a metabolic parameter characterizing the changes in NADH – NAD+ equilibrium in keratinocytes due to transient ischemia. The gradual shift of the NADH – NAD+ equilibrium towards reduction, seen as an increase in NADH fluorescence (ischemic response), depends on the residual bioavailability of oxygen and glucose in epidermal keratinocytes after brachial artery occlusion. In principle, such residual bioavailability of oxygen and glucose may reflect skin microcirculatory function, among other variables related to mitochondrial function of epidermal cells. In cases of profound microcirculatory dysfunction, the transport of oxygen and glucose to epidermal keratinocytes may be poor and brachial artery occlusion may result in complete blockage of metabolic processes in the epidermis, seen as a decrease in NADH fluorescence. Such decreases may be due to the transformation of pyruvate to lactate, with use of NADH as a cofactor and subsequent accumulation of NAD+ as it is not effectively consumed in glycolysis (due to low glucose bioavailability).6,7 An FMSF trace with a negative ischemic response (IRmax < 0) is illustrated in Figure 1B. The negative ischemic response parameter is not observed in healthy individuals, but it is seen quite frequently in patients with serious microcirculation dysfunction (see Results).
The HRmax parameter measures the response to releasing the pressure from the occlusion cuff, which results in immediate hyperemia (hyperemic response). This response is related to the production of nitric oxide (NO) in the vasculature due to reactive hyperemia, and predominantly reflects macrocirculatory function.4
As the results of this study show, the three parameters RHR, IRmax, and HRmax, are mutually correlated, but they differentially decrease with age and are also sex dependent.
Statistical Analysis
The collected data were analyzed using the dedicated software installed on the AngioExpert device. The normality of the data distribution was tested with the Shapiro–Wilk test. The Levene’s test was used to assess the homogeneity of variance. A Student’s t-test or Mann–Whitney U-test was used (depending on the distribution) to compare differences between two independent groups. A one-way ANOVA with a post-hoc Scheffe test was used to compare groups by age. Correlations were analyzed with the Pearson or Spearman correlation coefficients, according to the test assumptions. All probability values were two-tailed and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed with OriginPro 2023 software.
Results
The NADH/NAD+ redox balance cannot be assessed reliably for individual patients based on the level of NADH fluorescence emitted from skin, due to the effects of skin pigmentation. It is well known that the concentration of melanin in the skin can seriously affect the measured fluorescence intensity.8,9 However, comparing groups with similar skin pigmentation is more feasible, particularly if the groups are very different in terms of NADH – NAD+ redox imbalance. We observed higher FLbase values of for patients with lower IRmax values. However, we decided to omit discussion of this observation in the present publication, as all patients were characterized by quite similar NADH/NAD+ redox imbalance.
Figure 2 compares the key FMSF parameters (IRmax, HRmax, RHR) for female and male groups. As can be seen in Figure 2A, the IRmax parameter is significantly lower for the female group. Since the IRmax parameter reflects predominantly the metabolic status of the epidermis under hypoxia, this supports the notion that females are less adaptive to transient hypoxia than males. This observation is closely related to the status of microcirculation. The HRmax parameter is higher for the female group (Figure 2B), which suggests better macrocirculatory function. The RHR parameter is unable to distinguish female from male groups.
Table 2 shows the characteristics of the subgroups by age and the measured FMSF parameters. Figure 3 illustrates the changes in IRmax and HRmax parameters with age. The decrease in IRmax with age shown in segment A is much more pronounced than the decrease in HRmax shown in segment 3B. In fact, the HRmax values for the 70–79 y. age group are similar to those for the 80–94 y. age group. This suggests that the function of metabolic regulation gradually deteriorates with age, as seen in segment 3A, in contrast to the function of macrocirculation which stabilizes at ages over 70 years (segment 3B).
 |
Table 2 Characteristics of the Groups by Age with Values of the Main FMFS Parameters |
Novel and surprising results were obtained concerning the IRmax parameter. As shown in Table 3, 162 of the total 482 patients (34%) had a negative ischemic response (IRmax < 0). This means that the skin metabolic regulation was seriously impaired, with the immediate inhibition of metabolic processes in epidermal cells after blocking blood flow in the brachial artery. If we compare the relative proportions of females to males in the groups with IRmax > 0 and IRmax < 0, we find that there is a larger proportion of females in the group with a negative ischemic response (Table 3). This intriguing observation requires special attention.
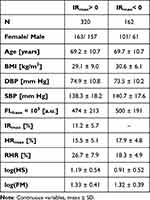 |
Table 3 Characteristics of the Groups by Ischemic Response |
Figure 4 compares two parameters, log(HS) and HRmax, for patients with IRmax > 0 and IRmax < 0.The Hypoxia Sensitivity (HS) parameter, expressed as a logarithm, represents the reaction of the microcirculation to hypoxia by stimulating myogenic microcirculatory oscillations.3 The significantly lower log(HS) parameter in the group with IRmax < 0 supports use of the IRmax parameter as a measure of skin metabolic reaction to hypoxia. The IRmax parameter correlates with the log(HS) parameter (Figure 3S). The increase in the HRmax parameter in the group with IRmax < 0 (segment 4B) suggests the operation of a compensatory mechanisms whereby the macrocirculation compensates for seriously dysfunctional metabolic regulation.
Discussion
There is an extensive literature on sex and age differences in endothelial function.10–13 Various aspects of epidemiology, pathophysiology, and outcomes have been discussed.14–16 Some differences related to sex and age effects on vascular circulation have been analyzed in terms of differences in mitochondrial function.17,18 However, there is still quite limited knowledge specifically concerning sex and age effects on vascular and metabolic functional differences associated with particular vascular diseases. The present article addresses this lack.
Our observational study clearly demonstrates the diagnostic potential of the FMSF technique for the non-invasive assessment of vascular function and metabolic regulation. The effects of sex and age differences on vascular circulation, monitored by the FMSF technique, are clearly visible. The IRmax parameter, characterizing predominantly the metabolic adaptation of skin to hypoxia, provides particularly important information. The cutaneous vascular function is also considered to be an indicator of general vascular function.19,20
As can be seen clearly in Figure 2, the ability of metabolic function to adapt to hypoxia is weaker in females than in males. On the other hand, the macrocirculation in females seems to be more functional. There are many possible explanations for such differences, including mechanisms related to the function of sex hormones.21 The most striking observation is linked to the negative ischemic response (IRmax < 0). About a third of the studied group had IRmax < 0. The majority in this segment were females. Some of the patients involved in this observational study had substantial dysfunction of vascular circulation and were in old age.
Very similar observations have been reported recently, indicating a negative response to hypoxia (an atypical skin fluorescence response) in hypertensive patients.22 Atypical ischemic patterns were observed in 32.3% of patients, with a larger proportion of females than males. The patients with an atypical FMSF signal had less favorable blood pressure profiles. Although the present observational study focused on patients in different age ranges, in both cases the observed negative ischemic responses identified the more severe clinical cases, regardless the vascular problem analyzed.
Important conclusions can be drawn from the data presented in Figure 3. The metabolic function related to the reaction to hypoxia represented by the IRmax parameter appears to gradually decrease with age. Macrocirculatory function decreases much more slowly, with a tendency to stabilize in the 80–94 y. age group. Super seniors, those aged 90 years and above, showed mean values of IRmax = 5.4% and HRmax = 14.8% (Table 1S). Such values for these parameters indicate that a satisfactory function of macrocirculation is very important, particularly given that metabolic regulation in this group is highly dysfunctional. For elderly people, a value for the HRmax parameter of about 15% can be considered a long life-expectancy value.
The results generated in this observational study are very interesting, and establish the FMSF technique as an important tool for monitoring the status of both vascular circulation and metabolic regulation. Expanding the study to involve a large segment of patients with dysfunctional vascular circulation seems justified. Future work should also concentrate on the analysis of particular diseases associated with impaired vascular function.
Conclusion
The following conclusions can be drawn from this study:
- The FMSF technique appears uniquely suitable for the analysis of vascular circulation and metabolic regulation.
- Females were metabolically less adaptive to transient hypoxia than males. However, macrocirculatory function was better in females than among males.
- Microcirculatory function gradually decreases with age, while macrocirculatory function decreases much more slowly with age, with a tendency to stabilize at ages over 70 years.
Disclosure
JK is employed by Angionica Ltd. JG is inventor of patents protecting the use of FMSF technology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Katarzynska J, Lipinski Z, Cholewinski T, et al. Non-invasive evaluation of microcirculation and metabolic regulation using flow mediated skin fluorescence (FMSF): technical aspects and methodology. Rev Sci Instrum. 2019;90(10):104104. doi:10.1063/1.5092218
2. Gebicki J, Katarzynska J, Cholewinski T, Sieron L, Marcinek A. Flowmotion Monitored by Flow Mediated Skin Fluorescence (FMSF): a tool for characterization of microcirculatory status. Front Physiol. 2020;11:702. doi:10.3389/fphys.2020.00702
3. Gebicki J, Marcinek A, Zielinski J. Assessment of microcirculatory status based on stimulation of myogenic oscillations by transient ischemia: from health to disease. Vasc Health Risk Manag. 2021;17:33–36. doi:10.2147/VHRM.S292087
4. Katarzynska J, Zielinski J, Marcinek A, Gebicki J. New approach to non-invasive assessment of vascular circulation based on the response to transient ischemia. Vasc Health Risk Manag. 2022;18:113–116. doi:10.2147/VHRM.S358983
5. Marcinek A, Katarzynska J, Sieron L, et al. Non-invasive assessment of vascular circulation based on Flow Mediated Skin Fluorescence (FMSF). Biology. 2023;12(3):385. doi:10.3390/biology12030385
6. Balu M, Mazhar A, Hayakawa CK, et al. In vivo multiphoton fluorescence reveals depth-dependent keratinocyte metabolism in human skin. Biophys J. 2013;104(1):258–267. doi:10.1016/j.bpj.2012.11.3809
7. Croce AC, Bottirioli G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur J Histochem. 2014;58(4):2461. doi:10.4081/ejh.2014.2461
8. Dremin VV, Dunaev AV. How the melanin concentration in the skin affects the fluorescence-spectroscopy signal formation. J Opt Technol. 2016;83(1):43–48. doi:10.1364/JOT.83.000043
9. Heikal AA. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark Med. 2010;4(2):241–263. doi:10.2217/bmm.10.1
10. Skaung E-A, Aspenes ST, Oldervoll L, et al. Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prevet Cardiol. 2012;20(4):531–540. doi:10.1177/2047487312444234
11. Chen H. Mechanism of gender-related differences in vascular function. Cardiovasc Pharm Open Access. 2018;7(04):246. doi:10.4172/2329-6607.1000246
12. Peters SAE, Woodward M. Sex differences in the burden and complications of diabetes. Curr Diab Rep. 2018;18:33. doi:10.1007/s11892-018-1005-5
13. Ji H, Kwan AC, Chen MT, et al. Sex difference in myocardial and vascular aging. Circul Res. 2022;130(4):566–577. doi:10.1161/CIRCRESAHA.121.319902
14. Seeland U, Nemcsik J, Lonnebakken MT, et al. Sex and gender aspects in vascular ageing - focus on epidemiology, pathophysiology, and outcomes. Heart Lung Circul. 2021;30(11):1637–1646. doi:10.1016/j.hlc.2021.07.006
15. Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775385 individuals and 12539 strokes. Lancet. 2014;383(9933):1973–1980. doi:10.1016/S0140-6736(14)60040-4
16. Chatterjee S, Peters SAE, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabet Care. 2016;39(2):300–307. doi:10.2337/dc15-1588
17. Ventura-Clapier R, Moulin M, Piquereau J, et al. Mitochondria: a central target for sex differences in pathologies. Clin Sci. 2017;131(9):803–822. doi:10.1042/CS20160485
18. Cardinale DA, Larsen FJ, Schiffer TA, et al. Superior intrinsic mitochondrial respiration in women than in men. Front Physiol. 2018;9:1133. doi:10.3389/fphys.2018.01133
19. Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105(1):370–372. doi:10.1152/japplphysiol.00858.2007
20. Hellmann M, Roustit M, Cracowski JL. Skin microvascular function as a biomarker in cardiovascular diseases? Pharmacol Rep. 2015;67(4):803–810. doi:10.1016/j.pharep.2015.05.008
21. Pabbidi MR, Kuppusamy M, Didon S, et al. Sex differences in the vascular function and related mechanisms: role of 17β-estradiol. Am J Physiol Heart Circ Physiol. 2018;315(6):H1499–H1518. doi:10.1152/ajpheart.00194.2018
22. Pawlak-Chomicka R, Urski P, Krauze T, et al. Arterial blood pressure features of hypertensive patients with typical and atypical 460 nm skin fluorescence response to transient ischaemia. J Clin Med. 2023;12(18):5886. doi:10.3390/jcm12185886
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




