Back to Journals » International Journal of General Medicine » Volume 16
Computed Tomography Manifestations in Patients with Rifampin Primary Drug-Resistant Tuberculosis in an Infectious Disease Hospital in the Yi Autonomous Prefecture, China
Authors Wang T, Yang Q, Gao Y, Zhang R, Zhou C, Kong W, Zhang G, Chen X, Pu H, Shang L
Received 4 July 2023
Accepted for publication 31 October 2023
Published 6 November 2023 Volume 2023:16 Pages 5109—5118
DOI https://doi.org/10.2147/IJGM.S428962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Tao Wang,1,2,* Qianwen Yang,3,* Yan Gao,2 Rongping Zhang,2 Chaoxin Zhou,2 Weifang Kong,1 Guojin Zhang,1 Xinyue Chen,4 Hong Pu,1 Lan Shang1
1Department of Radiology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 2Department of Radiology, The First People’s Hospital of Liangshan Yi Autonomous Prefecture, Xichang, Sichuan, People’s Republic of China; 3School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 4CT Collaboration, Siemens-Healthineers, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Pu; Lan Shang, Department of Radiology, The First Department of Radiology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, No. 32, West Section 2, 1st Ring Road, Chengdu, Sichuan Province, 60072, People’s Republic of China, Tel +86 13882267217, Fax +86 87294280, Email [email protected]; [email protected]
Purpose: This study aimed to investigate clinical features and computed tomography (CT) manifestations of rifampicin primary drug-resistant pulmonary tuberculosis in Liangshan Yi Autonomous Prefecture.
Patients and Methods: A total of 100 inpatients with confirmed primary rifampicin-resistant pulmonary tuberculosis were recruited from January 2020 to December 2022 at an infectious disease hospital located in the Liangshan Yi Autonomous Prefecture. Additionally, 100 inpatients with confirmed drug-susceptible pulmonary tuberculosis during the same period were matched to the rifampicin-resistant group based on gender, age, and ethnicity. The clinical characteristics of the two groups were recorded separately. Furthermore, the CT manifestations in these patients were independently analyzed by three radiologists.
Results: The results showed that comorbid diabetes mellitus was more prevalent in the drug-resistant tuberculosis (DR-TB) group than in the drug-susceptible tuberculosis (DS-TB) group (9% vs 0%, p=0.0032). In terms of imaging presentation, DR-TB patients exhibited a higher frequency of calcifications (55% vs 35.00%, p=0.0068), greater median number of cavities (5 vs 2, p=0.0027), and larger maximum cavity diameter (52.08± 25.55 mm vs 42.72± 17.48 mm, p=0.0097). Additionally, bilateral involvement was more common in DR-TB patients at the site of the lesion (89% vs 76%, p=0.0246), with a higher prevalence in the right middle (82% vs 68%, p=0.0332), right lower (82% vs 68%, p=0.0332), left upper (91% vs 77%, p=0.0113), and left lower lobes (92% vs 66%, p< 0.0001). Conversely, the involvement of only one lobe was less frequent in patients with DR-TB than in those with DS-TB (4% vs 13%, p=0.0398), whereas the involvement of all five lobes was more common (68% vs 51%, p=0.0209).
Conclusion: Patients with DR-TB exhibit a higher prevalence of severe imaging manifestations, highlighting the importance of CT in the early detection and diagnosis of DR-TB.
Keywords: drug-resistant tuberculosis, drug-susceptible tuberculosis, tuberculosis, comorbid diabetes mellitus
Introduction
Drug-resistant tuberculosis (DR-TB) poses a significant public health challenge owing to its high incidence, high relapse rate, high mortality, prolonged treatment duration, and substantial medical costs. It is recognized as a major public health problem associated with tuberculosis (TB).1,2 The primary causes of the emergence of DR-TB are non-standard anti-TB treatment practices and the transmission of drug-resistant strains.3 A World Health Organization (WHO) modeling study has also established that poverty is a risk factor for TB incidence, with the potential to reduce the global TB incidence by 4.95% through the elimination of extreme poverty.4
The Liangshan Prefecture, located in Sichuan Province, China, is home to the largest Yi community and is among the least economically developed regions in the country. It suffers from poor infrastructure and weak public health. The epidemiological situation of DR-TB within the Yi settlement in Liangshan is severe, with a TB incidence rate of 10.34%, which is significantly higher than the national average of 4.23%.5,6 Additionally, the treatment cost for DR-TB is substantial. Economic status, education, medical conditions, and disease management often contribute to a delayed diagnosis and irregular treatment in high TB burden areas, further burdening the affected families.
While sputum culture and drug sensitivity testing remain the gold standard for detecting DR-TB, the time required to obtain results, typically 4–8 weeks, limits their applicability in high TB burden areas, leading to delayed diagnosis, empirical use of second-line drugs, and poor treatment efficacy due to drug resistance.
Early detection and treatment are crucial for successful outcomes and for reducing mortality in DR-TB cases. Chest CT plays a vital role in the early diagnosis and prognostic assessment of DR-TB. At present, we have only found one study describing the CT manifestations in patients with primary DR-TB.7 This study specifically focused on clinical and CT manifestations in patients hospitalized for primary rifampicin-resistant pulmonary tuberculosis in the Liangshan region of China. Unlike previous studies that included populations with a combination of secondary and primary drug resistance, in which primary drug resistance accounted for a range of percentages,8,9 our study exclusively included patients with primary drug-resistant tuberculosis who had not yet received anti-TB therapy. These precise inclusion criteria allowed a more targeted and relevant study population, thereby enhancing the significance of the findings. These findings will contribute to the timely detection and treatment of DR-TB in this high TB burden population.
Patients and Methods
Patients
This retrospective cross-sectional study involved the collection of data from 2400 adult patients hospitalized for pulmonary tuberculosis at the largest infectious disease hospital in Liangshan Yi Autonomous Prefecture from January 2020 to December 2022. The study population was selected based on the following inclusion criteria:
- Patients with confirmed tuberculosis using bacteriological methods such as sputum smear, culture, or polymerase chain reaction (PCR) of the respiratory specimens (sputum, bronchoalveolar lavage, and open or percutaneous lung tissue biopsy).
- Pretreatment examination conducted using chest CT.
Exclusion criteria were applied to eliminate patients with confounding factors, such as:
- Coexisting conditions, including human immunodeficiency virus (HIV) infection, immunosuppressive therapy, underlying malignancy, and other lung diseases (such as lung cancer, pneumoconiosis, and other lung infections).
- Cases with inadequate CT image quality that did not meet the diagnostic criteria or incomplete case information.
- Patients who had received treatment with tuberculosis drugs previously.
From the eligible patient pool, two groups were formed for the study:
- Rifampicin-resistant TB group: The gold standard for confirming DR-TB is sputum culture and drug sensitivity testing of Mycobacterium tuberculosis; however, this method has limitations in terms of long detection periods and limited accessibility in areas with limited resources.10 Therefore, rifampicin resistance was detected using GeneXpert MTB/RIF assay, a novel, fast-detection nucleic acid amplification test with an established role for rapid diagnosis of Mycobacterium tuberculosis and detection of rifampicin resistance.11 A total of 100 patients with DR-TB were included in this group.
- Drug-susceptible TB (DS-TB) group: To compare the characteristics, 100 patients with active DS-TB were randomly selected, matching the age within ±3 years, gender, and ethnicity of the rifampicin-resistant TB group at the time of their first hospital admission.
Ethical Statement
The data for this study were collected from patient hospital records, ensuring the anonymization of personal information. The study protocol, including the collection of clinical data and specimens, was approved by the Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital (protocol 20220-254). Considering the retrospective nature of the study and the use of anonymized data from existing records, the institutional review board approved the waiver of patient informed consent. This study is in line with the fundamental principles of the Declaration of Helsinki to promote and ensure respect for human subjects and to protect their health and rights.
Clinical Data Collection
The clinical data collected for all patients included age, sex, ethnicity, smoking history, presence of diabetes mellitus, extrapulmonary tuberculosis, and clinical symptoms. The clinical symptoms assessed included dry or productive cough, fever, chest pain, and other symptoms such as weight loss, hemoptysis, and headaches.
CT Scan and Image Evaluation
All enrolled patients underwent a chest CT before TB diagnosis and treatment. The CT scans were independently reviewed by three experienced radiologists, including two attending radiologists with 8 and 10 years of experience in diagnostic chest radiology and one chief radiologist with 20 years of experience. In cases of disagreement, a consensus was reached through consultation and discussion among the three of them.
Chest CT was performed using various multi-detector row CT scanners, including a 16-row CT scanner (Siemens Somatom Sensation 16), a 128-layer spiral CT (GE Optima CT 660), and a dual-source spiral CT (Somatom Definition Flash Drive). The images were extracted using a slice thickness of 8–10 mm from the raw data, and all the images were displayed at standard window settings that allowed viewing the lung parenchyma (window level, −500–600 HU; window width, 1500–1800 HU) and mediastinum (window level, 40–50 HU; window width, 250–350 HU).3 The analysis of the CT scans included the following parameters (Table 1).
 |
Table 1 The Parameters and Details of CT Scans |
Statistical Analysis
For the analysis of continuous variables with a normal distribution, the unpaired t-test was used, whereas the Wilcoxon signed-rank test was used to analyze variables that did not show a normal distribution. Fisher’s exact test was used to compare categorical variables between the two groups, and statistical analyses were performed using the GraphPad Prism 8 software.
Results
Baseline Characteristics
A total of 200 patients with pulmonary tuberculosis were included in this study, with 100 in the DR-TB group and 100 in the DS-TB group. The specific procedure and patient selection process are illustrated in Figure 1.
The clinical characteristics of the two groups are summarized in Table 2. The two groups were matched for age (±3 years), sex, and ethnicity, and there were no statistically significant differences in the history of alcohol consumption or extrapulmonary TB between the groups. The presence of comorbid diabetes mellitus was more common in the DR-TB group than in the DS-TB group (P=0.0032). Regarding clinical symptoms, febrile symptoms were more frequently reported in the DS-TB group (P<0.0001), whereas no significant differences were observed in the other symptoms between the two groups.
 |
Table 2 Clinical Characteristics in PTB Patients with or Without DR-TB |
Characteristics of CT Findings
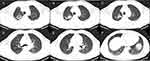
Compared to the patients with drug-susceptible tuberculosis (DS-TB), DR-TB patients exhibited several notable differences in imaging findings (Figures 2 and 3). DR-TB patients had a higher prevalence of calcifications (55% vs 35.00%, P=0.0068), greater number of cavities (P=0.0027), and larger maximum cavity diameters (52.08±25.55 mm vs 42.72±17.48 mm, P=0.0097). However, no significant differences in other radiological signs were observed between the two groups.
Regarding the distribution of lesions, DR-TB patients had a higher frequency of bilateral involvement (P=0.0246) and a higher occurrence of lesions in specific lung lobes, such as the right middle lobe (P=0.0332), right lower lobe (P=0.0332), left upper lobe (P=0.0113), and left lower lobe (P<0.0001). In contrast, involvement of only one lobe was less common in DR-TB patients (P=0.0398), whereas involvement of all five lobes more prevalent (P=0.0209) among them. Further details regarding the CT characteristics of the two patient groups are shown in Table 3, and the distribution of the lesion sites is provided in Table 4.
 |
Table 3 Analysis of Chest CT Images in Patients with PTB in the DR-TB and DS-TB Groups |
 |
Table 4 Analysis of Diseased Region in Patients with PTB in DR-TB and DS-TB Group |
Discussion
Liangshan Yi Autonomous Prefecture is located in the Southwestern Sichuan Province, China, and characterized by mountainous and rural environments. It serves as the largest concentration of the Yi ethnic group in China and is home to the most populous and geographically widespread branch of the Yi ethnic group. The health literacy level of the residents in this region remains relatively low, with only 11.62% reported in recent data, which is lower than the overall health literacy level of the Sichuan Province in 2019 (19.40%) and rural residents in China in 2017 (14.5%).12 Some Yi autonomous counties have even lower health literacy levels, estimated at approximately 1%.13 The fragile economy, limited healthcare resources, challenging transportation conditions, and low educational attainment collectively contribute to the region’s vulnerability to serious DR-TB epidemics. In the current study, the Yi ethnic group accounted for a significant proportion, comprising 75.68% (n=56) of DR-TB patients, which is notably higher than that of the Han population in the region.
In our study, the majority of DR TB patients were young people between the ages of 18 and 44 years, comprising approximately 77.03% of the cases. This finding suggests that there may be a correlation between the high incidence of DR-TB in this age group and certain factors, such as extensive social interactions, increased exposure to the outside world, stressful lifestyles, and a lack of attention to personal health.3,14 These factors can contribute to a delayed diagnosis, poor treatment adherence, and inadequate access to timely healthcare among young individuals.
It was found that the DR-TB group had a significantly higher prevalence of coexisting diabetes than in the DS-TB group. This observation led to the hypothesis that diabetes could be a risk factor for the development of drug resistance in TB patients.15,16 Supporting this notion, a systematic retrospective analysis concluded that HbA1c classification (≥7%) was an independent risk factor for isoniazid and multidrug resistance in TB patients with type 2 diabetes.17
In our study, we observed significant differences in the distribution of lung lesions and the number of lobes involved between DS-TB and DR-TB patients, based on chest CT findings. The percentage of patients with single-lobe involvement was higher in the DS-TB group (13%) than in the DR-TB group (4%) (P=0.0398). Conversely, the involvement of all five lung lobes was more common in the DR-TB group (68%) than in the DS-TB group (51%) (P=0.0209), which was consistent with the findings of previous studies.3,18,19 Except for the upper lobe of the right lung, where no significant differences were observed between the two groups, there were significant differences in the involvement of the middle and lower lobes of the right lung, as well as the upper, middle, and lower lobes of the left lung. Particularly, the involvement of the lower lobe of the left lung was significantly higher in the DR-TB group (92%) than in DS-TB group (P<0.0001), suggesting that the lower lobe of the left lung may be a distinguishing manifestation of DR-TB from DS-TB, although further investigation is required to understand the underlying reasons.
Tree buds and lobular central nodules are common CT findings in pulmonary TB,20 with the tree bud sign being the most frequent and the earliest feature associated with the bronchial dissemination of TB. This corresponds to the presence of peribronchial granulomas following intrabronchial caseous necrosis observed in pathological specimens. Our study shows a higher incidence of this lesion in DR-TB than in DS-TB, which is consistent with the findings of some previous studies.19,21
Our study demonstrated a higher number of cavities (P=0.0027) and larger cavity diameters (P=0.0097) in the lungs of DR-TB patients. Multiple cavities, especially when the number is ≥3 and the diameter is ≥30 mm, can be considered a highly specific sign of DR-TB on imaging.22 This may be attributed to the enhanced extracellular matrix degradation ability of DR-TB, which promotes the action of matrix metalloproteinases (MMPs), resulting in increased and enlarged cavities.3 Although our study did not find a statistical difference in cavity wall thickness between the two groups, some studies have suggested that cavity walls may be thicker in extensively drug-resistant TB compared to DR-TB.23,24 Cavitation can serve as a crucial marker for the development of drug resistance, particularly in the presence of multiple and thick-walled cavities.
Previous studies have reported varying results regarding the presence of calcified foci, with some suggesting a higher incidence of calcification in DR-TB patients,3 whereas others have indicated no significant difference in the incidence of calcification between DR-TB and DS-TB patients.18 In our study, we observed a higher incidence of calcification in the DR TB group (P=0.0068). Intrapulmonary calcified foci are generally associated with a longer duration of TB, and since DR-TB often has a more prolonged disease duration, the likelihood of observing calcified foci is higher in patients with DR-TB.
The present study has some limitations. First, although our cross-sectional study suggests a role for CT in aiding the differentiation of patients with drug-resistant tuberculosis (DR-TB) and drug-susceptible tuberculosis (DS-TB), we did not conduct a more in-depth quantitative assessment of this relationship using mathematical modeling. Second, we did not compare and analyze the clinical features and imaging manifestations of different drug-resistant types separately in our study. This limitation prevented us from capturing the potential differences in imaging findings among patients with different types of DR-TB. Further studies specifically addressing this aspect are warranted to gain a more comprehensive understanding of the imaging manifestations associated with different types of DR-TB. In addition, it is important to note that our study was conducted at a single center with a relatively small sample size. This limited scope may restrict the generalizability of our findings to a larger population. Finally, this was an observational study, and potential selection bias could not be avoided in the study cohort.
Conclusion
The present study found that adverse imaging manifestations were more common in patients with DR-TB than in those with DS-TB and showed significantly different imaging manifestations from DS-TB.
Abbreviations
WHO, World Health Organization; CT, computed tomography; PCR, polymerase chain reaction; MMPs, matrix metalloproteinases; DR-TB, drug-resistant tuberculosis; DS-TB, drug-susceptible tuberculosis; infectious disease; HIV, human immunodeficiency virus.
Data Sharing Statement
The datasets generated during and analyzed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The data for this study were collected from patients’ hospital records, ensuring the anonymization of personal information. The study protocol, including the collection of clinical data and specimens, was approved by the Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital (protocol 20220-254). Considering the retrospective nature of the study and the use of anonymized data from existing records, the institutional review board approved the waiver of patient’s informed consent.
Acknowledgments
We would like to thank the infectious disease ward, radiology department, and laboratory department of the First People’s Hospital of Liangshan Prefecture for providing relevant data.
Funding
This study was supported by the Liangshan Science and Technology Bureau (Grant No. 21ZDY0058; No. 21ZDY0069), the Sichuan Provincial Cadre Health Research Project (Grant No. 2021−230; No. 2022 −208), the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital Research Fund (Grant No. 2022QN25), and Sichuan Science and Technology Program(Grant No.2022YFS0075).
Disclosure
All authors declare no conflicts of interest for this work.
References
1. Migliori GB, Tiberi S, Zumla A, et al. MDR/XDR-TB management of patients and contacts: challenges facing the new decade. The 2020 clinical update by the Global Tuberculosis Network. Int J Infect Dis. 2020;92S:S15–S25. doi:10.1016/j.ijid.2020.01.042
2. Li Q, Lu M, Hsieh E, et al. Time to sputum culture conversion and its predictors among patients with multidrug-resistant tuberculosis in Hangzhou, China: a retrospective cohort study. Medicine. 2020;99(50):e23649. doi:10.1097/MD.0000000000023649
3. Cheng N, Wu S, Luo X, et al. A comparative study of chest computed tomography findings: 1030 cases of drug-sensitive tuberculosis versus 516 cases of drug-resistant tuberculosis. Infect Drug Resist. 2021;14:1115–1128. doi:10.2147/IDR.S300754
4. Carter DJ, Glaziou P, Lonnroth K, et al. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1. Lancet Glob Health. 2018;6(5):e514–e522. doi:10.1016/S2214-109X(18)30195-5
5. Gao W, Chen X, Yao L, et al. Drug Resistance of Mycobacterium tuberculosis Based on Whole-Genome Sequencing in the Yi Ethnic Group, Sichuan Province, China. J Immunol Res. 2023;2023:4431209. doi:10.1155/2023/4431209
6. World Health Organization: Global tuberculosis report 2022. Available from: https://www.who.int/publications/m/item/global-tuberculosis-report-2022-factsheet.
7. Li D, He W, Chen B, Lv P. Primary multidrug-resistant tuberculosis versus drug-sensitive tuberculosis in non-HIV-infected patients: comparisons of CT findings. PLoS One. 2017;12(6):e176354.
8. Chuchottaworn C, Thanachartwet V, Sangsayunh P, et al. Risk factors for multidrug-resistant tuberculosis among patients with pulmonary tuberculosis at the Central Chest Institute of Thailand. PLoS One. 2015;10(10):e139986. doi:10.1371/journal.pone.0139986
9. Bhering M, Kritski A. Primary and acquired multidrug-resistant tuberculosis: predictive factors for unfavorable treatment outcomes in Rio de Janeiro, 2000–2016. Revista Panamericana de Salud Pública. 2020;44(1):178. doi:10.26633/RPSP.2020.178
10. Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185–192. doi:10.2147/IDR.S135935
11. Sasikumar C, Utpat K, Desai U, Joshi J. The role of genexpert in the diagnosis of Mycobacterium tuberculosis. Adv Respir med. 2020;88(3):183–188. doi:10.5603/ARM.2020.0102
12. Hu L, Zhu W, Yu J, et al. Family-based improvement for health literacy among the Yi nationality (FAMILY) inLiangshan: protocol of an open cohort stepped wedge cluster randomized controlled trial. BMC Public Health. 2022;22(1):1543. doi:10.1186/s12889-022-13782-w
13. Wang H, Liao R, Chen X, et al. How to improve the COVID-19 health education strategy in impoverished regions: a pilot study. Infect Dis Poverty. 2022;11(1). doi:10.1186/s40249-022-00963-3
14. Liu X, Zhao W, Hu F, et al. Comorbid anxiety and depression, depression, and anxiety in comparison in multi-ethnic community of west China: prevalence, metabolic profile, and related factors. J Affect Disord. 2022;298(Pt A):381–387. doi:10.1016/j.jad.2021.10.083
15. Yang Q, Zhang R, Gao Y, et al. Computed tomography findings in patients with pulmonary tuberculosis and diabetes at an infectious disease hospital in China: a retrospective cross-sectional study. BMC Infect Dis. 2023;23(1):436. doi:10.1186/s12879-023-08386-7
16. Xia LL, Li SF, Shao K, Zhang X, Huang S. The correlation between CT features and glycosylated hemoglobin level in patients with T2DM complicated with primary pulmonary tuberculosis. Infect Drug Resist. 2018;11:187–193. doi:10.2147/IDR.S146741
17. Lyu M, Wang D, Zhao J, et al. A novel risk factor for predicting anti-tuberculosis drug resistance in patients with tuberculosis complicated with type 2 diabetes mellitus. Int J Infect Dis. 2020;97:69–77. doi:10.1016/j.ijid.2020.05.080
18. Shin HS, Choi DS, Na JB, et al. Low pectoralis muscle index, cavitary nodule or mass and segmental to lobar consolidation as predictors of primary multidrug-resistant tuberculosis: a comparison with primary drug sensitive tuberculosis. PLoS One. 2020;15(10):e239431. doi:10.1371/journal.pone.0239431
19. Xu L, Xu S. CT imaging characteristics of nontuberculous mycobacteria lung disease, active tuberculosis and multi-drug resistant tuberculosis. Sarcoidosis Vasc Diffuse Lung Dis. 2022;39(2):e2022008. doi:10.36141/svdld.v39i2.11829
20. Lawal IO, Mokoala KMG, Mathebula M, et al. Correlation between CT features of active tuberculosis and residual metabolic activity on end-of-treatment FDG PET/CT in patients treated for pulmonary tuberculosis. Front Med. 2022;9:791653. doi:10.3389/fmed.2022.791653
21. Cha J, Lee HY, Lee KS, et al. Radiological findings of extensively drug-resistant pulmonary tuberculosis in non-AIDS adults: comparisons with findings of multidrug-resistant and drug-sensitive tuberculosis. Korean J Radiol. 2009;10(3):207–216. doi:10.3348/kjr.2009.10.3.207
22. Wáng YXJ, Chung MJ, Skrahin A, Rosenthal A, Gabrielian A, Tartakovsky M. Radiological signs associated with pulmonary multi-drug resistant tuberculosis: an analysis of published evidences. Quant Imag Med Surg. 2018;8(2):161–173. doi:10.21037/qims.2018.03.06
23. Shin SS, Keshavjee S, Gelmanova IY, et al. Development of extensively drug-resistant tuberculosis during Multidrug-resistant tuberculosis treatment. Am J Resp Crit Care. 2010;182(3):426–432. doi:10.1164/rccm.200911-1768OC
24. Song QS, Zheng CJ, Wang KP, et al. Differences in pulmonary nodular consolidation and pulmonary cavity among drug-sensitive, rifampicin-resistant and multi-drug resistant tuberculosis patients: a computerized tomography study with history length matched cases. J Thorac Dis. 2022;14(7):2522. doi:10.21037/jtd-22-145
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.