Back to Journals » Journal of Pain Research » Volume 16
Compression Degree of Trigeminal Nerve and Type of Conflicting Vessels Determine Short- and Long-Term Complete Pain Relief in Adult Patients with Primary Trigeminal Neuralgia After Microvascular Decompression: A Three-Year Retrospective Study of 200 Adult Patients with Primary Trigeminal Neuralgia
Authors Zhao ZF, Liu BB, Zhang JN, Shi N, Zhang P, Yang GZ, Cao YF, Tian LH, Qiu CC, Gao Y
Received 4 September 2023
Accepted for publication 22 November 2023
Published 7 December 2023 Volume 2023:16 Pages 4191—4207
DOI https://doi.org/10.2147/JPR.S429713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rushna Ali
Zhe-Feng Zhao,1,* Bing-Bing Liu,1,* Jian-Nan Zhang,2,* Ni Shi,3,* Ping Zhang,1,* Gang-Zhi Yang,4 Yu-Fu Cao,4 Long-He Tian,4 Cheng-Cai Qiu,5 Yang Gao6,7
1Department of Neurosurgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China; 2Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China; 3Department of Critical Care Medicine, The Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China; 4Department of Neurosurgery, People’s Hospital of Hegang City, Hegang, Heilongjiang, People’s Republic of China; 5Department of Neurosurgery, Traditional Chinese Medicine Hospital of Jixi City, Jixi, Heilongjiang, People’s Republic of China; 6Department of Critical Care Medicine, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China; 7Institute of Critical Care Medicine, The Sino Russian Medical Research Center of Harbin Medical University, Harbin, Heilongjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhe-Feng Zhao, Department of Neurosurgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China, Email [email protected] Yang Gao, Department of Critical Care Medicine, The Sixth Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China, Email [email protected]
Objective: In this study, we aimed to explore the demographic and clinical factors that could determine short- and long-term complete pain relief (CPR) in adult patients with primary trigeminal neuralgia (PTN) after microvascular decompression (MVD) to guide clinical practice.
Methods: This single-center retrospective study included adult patients with PTN who underwent MVD as their initial neurosurgical procedure in the Department of Neurosurgery at the Second Affiliated Hospital of Harbin Medical University from January 2017 to December 2019 and completed a 3-year post-surgery follow-up. Demographic and clinical information was obtained from medical records. Pain relief of adult patients with PTN at various time points after sufficient decompression of trigeminal nerve (TN) during MVD was determined and classified by the patient’s subjective response and medications use. Pain relief of local patients was evaluated by outpatient follow-up at various time points, whereas that of local cases who could not return to outpatient or non-local cases was assessed through telephone or WeChat.
Results: In univariate analysis, compression degree of TN and type of conflicting vessels constantly showed significant differences between the two groups at 3 months, 6 months, 1 year, 2 years, and 3 years after MVD. Compression degree of TN and type of conflicting vessels at various time points after MVD were always the related factors to CPR in logistic regression analysis, with the former having the greatest impact. The areas under the receiver operating characteristic (ROC) curve of CPR at various time points after MVD were 0.937, 0.874, 0.879, 0.864, and 0.869, respectively.
Conclusion: In summary, compression degree of TN and type of conflicting vessels can determine short- and long-term CPR in adult patients with PTN after MVD.
Keywords: compression degree of trigeminal nerve, type of conflicting vessels, microvascular decompression, sufficient decompression of trigeminal nerve, complete pain relief, primary trigeminal neuralgia
Introduction
The main clinical manifestation of primary trigeminal neuralgia (PTN) is touch-evoked sudden unilateral transient burning or shock-like paroxysmal or continuous onset of stabbing pain in one or more divisions of the trigeminal nerve (TN), which seriously lowers patient’s quality-of-life (QOL).1 Long-term suffering from this refractory excruciating neuralgia may result in sleep disturbance, interference in activities of daily living, anxiety, depression, and even suicide in extreme cases.2,3 Multiple known genes and the subsequent ion channels and other downstream products they produce may play a role in the genetic predisposition to PTN.4 In addition to other unknown etiologies, pathological demyelination along the trigeminal afferent pathway triggered by neurovascular conflict (NVC) with physical compression and morphological changes of TN is the predominant pathogenesis and pathophysiological mechanism of PTN, and subsequent activated neuroimmune cells, released inflammatory cytokines, significant molecular changes, channelopathies, and electrophysiological abnormalities pave the way for a generation of ectopic impulses and ephaptic crosstalk.1,5–9 In adult patients with PTN, three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA) prior to surgery has demonstrated a better capacity to identify the presence of NVC with nearby specific offending vessels, and thus neuroimaging using magnetic resonance imaging (MRI) with high resolution can be available as a reliable objective imaging predictor of therapeutic responsiveness and outcome to neurosurgical interventions.10,11
The initial standard management of adult patients with PTN is conventional pharmacotherapy with different action mechanisms, either monotherapy or combination therapy. However, up to half of adult patients with PTN require neurosurgical procedures when it is not sufficiently controlled or poorly tolerated with pharmacotherapies.12 Microvascular decompression (MVD), one of many different neurosurgical procedures, is currently recommended as the first-line neurosurgical option and gold standard for PTN because it is an efficient and secure neurosurgical intervention to directly alleviate or eliminate NVC.13–16 Till now, few clinical trials have focused on potential candidates suitable for MVD and short- and long-term complete pain relief (CPR) after sufficient decompression of TN during MVD in adult patients with PTN. To address the above issue, we aimed to explore the demographic and clinical factors that could determine short- and long-term CPR after sufficient decompression of TN during MVD in adult patients with PTN, which will contribute to better characterizing potential candidates suitable for MVD and thus optimizing the planning and implementation of MVD.
Methods
Study Design
This single-center retrospective study included adult patients with PTN who underwent MVD as their initial neurosurgical procedure in the Department of Neurosurgery at the Second Affiliated Hospital of Harbin Medical University from January 2017 to December 2019 and completed a 3-year post-surgery follow-up. Demographic data, including gender and age, as well as clinical data, involving affected side (left or right) of PTN, duration of PTN and medications use before MVD, compression degree (mild or severe) and location (nerve root or others) of TN, and type of conflicting vessels (artery or vein) during MVD were obtained and collated from medical records. After sufficient decompression of TN during MVD, pain relief of local patients was evaluated by outpatient follow-up at various time points, whereas that of local cases who were unable to return for outpatient follow-up or non-local cases was assessed through telephone or WeChat. This study protocol was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (IRB number: KY-2022-308). In this study, participants’ identities and other relevant data were guaranteed to remain anonymous and confidential. The requirement for written informed consent was waived according to the retrospective nature of this study, institutional requirements and national law.
Study Population
In this study, the inclusion criteria contained age ≥18 years old; admission to the Department of Neurosurgery at the Second Affiliated Hospital of Harbin Medical University from January 2017 to December 2019; definitive diagnosis of PTN; explicit NVC through available preoperative 3D-TOF-MRA; implementation of standard MVD as initial neurosurgical procedure; completion of 3-years post-surgery follow-up. Patients who met the following criteria were excluded, including cardiopulmonary dysfunction intolerance to general anesthesia (GA), absence of NVC during MVD, chronic organ failures such as chronic renal failure (CKD) or cirrhosis, simultaneous arterial and venous compression on TN, or incomplete medical records (Figure 1).
Diagnosis of PTN
PTN was discriminated against from all other types of orofacial pain by the patient’s medical history, typical clinical manifestations, and physical examination.17
Implementation of MVD
MVD was implemented in a standard manner as the initial neurosurgical procedure by the same team of skilled neurosurgeons in the Department of Neurosurgery at the Second Affiliated Hospital of Harbin Medical University from January 2017 to December 2019, in which Teflon grafts were used to alter the path of conflicting vessels away from TN rather than directly contacting the compression site of TN.17
Determination and Classification of Compression Degree of TN
During MVD, compression degree of TN was determined and classified, in which mild compression was defined as TN in contact with a nearby specific offending vessel without local deformation, whereas severe compression not only came into contact with a nearby specific offending vessel but also exhibited significant deformation.
Determination and Classification of Pain Relief
Pain relief of adult patients with PTN at 3 months, 6 months, 1 year, 2 years, and 3 years after sufficient decompression of TN during MVD was determined and classified by the patient’s subjective response and medications use as follows: CPR (no pain without medications use); nearly CPR (no pain with small doses of medications use, or 90%–99% pain relief with or without medications use); partial pain relief (50%-89% pain relief with or without medications use); poor pain relief (<50% pain relief).18 In this study, adult patients with PTN after sufficient decompression of TN during MVD were divided into two subgroups for statistical analysis: the CPR group and the other group, with the latter consisting of those with nearly CPR, partial and poor pain relief.
Data Collection
Demographic information, involving gender and age, as well as clinical information, including affected side of PTN, duration of PTN and medications use before MVD, compression degree and location of TN, and type of conflicting vessels during MVD were obtained and collated from medical records during hospitalization, whereas pain relief of adult patients with PTN at 3 months, 6 months, 1 year, 2 years, and 3 years after sufficient decompression of TN during MVD was followed up and recorded by dedicated personnel in our research team. Other than what was required for this study, none of the other members in our research team had access to the personal data of the enrolled patients.
Statistical Analyses
SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. Quantitative data without normal distribution were presented as interquartile ranges (IQRs), and Mann–Whitney U-test was adopted for inter-group comparisons. In univariate analysis, Chi-square test was employed for inter-group comparisons of qualitative data. The significant variables in univariate analysis were included in multivariate analysis, and then logistic regression analysis was performed. The receiver operating characteristic (ROC) curve of CPR was analyzed, and then the area under the ROC curve, and the sensitivity and specificity of the ROC curve were calculated, respectively. A p-value <0.05 were considered to indicate statistical significance.
Results
Demographic and Clinical Data of Adult Patients with PTN
A total of 200 adult patients with PTN who underwent MVD as their initial neurosurgical procedure in the Department of Neurosurgery at the Second Affiliated Hospital of Harbin Medical University from January 2017 to December 2019 and completed a 3-year post-surgery follow-up were enrolled in this study. Median age, and duration of PTN and medications use before MVD in this study population were 61 years, 36 months, and 24 months, respectively. Adult patients with PTN usually received oral medications, such as carbamazepine, oxcarbazepine, pregabalin, and gabapentin to alleviate pain and/or relieve symptoms before MVD, and the dosage varied from person to person. The remaining demographic and clinical information were displayed in Table 1. CPR rates of adult patients with PTN at 3 months, 6 months, 1 year, 2 years, and 3 years after sufficient decompression of TN during MVD were 76.0%, 71%, 69%, 68%, and 67.5%, respectively (Figure 2).
 |
Table 1 The Remaining Demographic and Clinical Data of Adult Patients with PTN |
CPR of Adult Patients with PTN at 3 Months After MVD
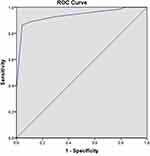
In univariate analysis, gender, compression degree of TN, and type of conflicting vessels showed significant differences between the two groups (p = 0.009, p = 0.000, p = 0.000, respectively) (Table 2). In logistic regression analysis, the related factors to CPR included gender, compression degree of TN, and type of conflicting vessels. CPR rates of male, arterial and severe compression were 3.77, 8.329 and 84.382 times as much as those of female, venous and mild compression, respectively, indicating that compression degree of TN had the greatest impact (Table 3). The area under the ROC curve and the sensitivity and specificity of the ROC curve were 0.937, 86.2%, and 4.2%, respectively (Figure 3 and Table 4).
 |
Table 2 Univariate Analysis of CPR of Adult Patients with PTN at 3 Months After MVD |
 |
Table 3 Logistic Regression Analysis of CPR of Adult Patients with PTN at 3 Months After MVD |
 |
Table 4 Area Under the ROC Curve at 3 Months After MVD |
CPR of Adult Patients with PTN at 6 Months After MVD
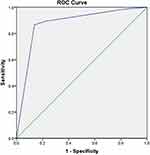
In univariate analysis, compression degree of TN and type of conflicting vessels showed significant differences between the two groups (p = 0.000, p = 0.000, respectively) (Table 5). In logistic regression analysis, the related factors to CPR included compression degree of TN and type of conflicting vessels. CPR rates of arterial and severe compression were 8.509 and 30.035 times greater than those of venous and mild compression, indicating that compression degree of TN had the largest effect (Table 6). The area under the ROC curve and the sensitivity and specificity of the ROC curve were 0.874, 86.6%, and 13.8%, respectively (Figure 4 and Table 7).
 |
Table 5 Univariate Analysis of CPR of Adult Patients with PTN at 6 Months After MVD |
 |
Table 6 Logistic Regression Analysis of CPR of Adult Patients with PTN at 6 Months After MVD |
 |
Table 7 Area Under the ROC Curve at 6 Months After MVD |
CPR of Adult Patients with PTN at 1 Year After MVD
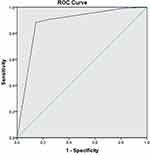
In univariate analysis, compression degree of TN and type of conflicting vessels showed significant differences between the two groups (p = 0.000, p = 0.000, respectively) (Table 8). In logistic regression analysis, the related factors to CPR included compression degree of TN and type of conflicting vessels. CPR rates of arterial and severe compression were 17.361 and 34.344 times greater than those of venous and mild compression, indicating that compression degree of TN had the largest effect (Table 9). The area under the ROC curve and the sensitivity and specificity of the ROC curve were 0.879, 88.4%, and 14.5%, respectively (Figure 5 and Table 10).
 |
Table 8 Univariate Analysis of CPR of Adult Patients with PTN at 1 Year After MVD |
 |
Table 9 Logistic Regression Analysis of CPR of Adult Patients with PTN at 1 Year After MVD |
 |
Table 10 Area Under the ROC Curve at 1 Year After MVD |
CPR of Adult Patients with PTN at 2 Years After MVD
In univariate analysis, compression degree of TN and type of conflicting vessels showed significant differences between the two groups (p = 0.000, p = 0.000, respectively) (Table 11). In logistic regression analysis, the related factors to CPR included compression degree of TN and type of conflicting vessels. CPR rates of arterial and severe compression were 14.528 and 28.286 times higher than those of venous and mild compression, indicating that compression degree of TN had the largest effect (Table 12). The area under the ROC curve and the sensitivity and specificity of the ROC curve were 0.864, 88.2%, and 17.2%, respectively (Figure 6 and Table 13).
 |
Table 11 Univariate Analysis of CPR of Adult Patients with PTN at 2 Years After MVD |
 |
Table 12 Logistic Regression Analysis of CPR of Adult Patients with PTN at 2 Years After MVD |
 |
Table 13 Area Under the ROC Curve at 2 Years After MVD |
CPR of Adult Patients with PTN at 3 Years After MVD
In univariate analysis, compression degree of TN and type of conflicting vessels showed significant differences between the two groups (p = 0.000, p = 0.000, respectively) (Table 14). In logistic regression analysis, the related factors to CPR included compression degree of TN and type of conflicting vessels. CPR rates of arterial and severe compression were 14.327 and 31.408 times greater than those of venous and mild compression, indicating that compression degree of TN showed the greatest effect (Table 15). The area under the ROC curve and the sensitivity and specificity of the ROC curve were 0.869, 88.9%, and 16.9%, respectively (Figure 7 and Table 16).
 |
Table 14 Univariate Analysis of CPR of Adult Patients with PTN at 3 Years After MVD |
 |
Table 15 Logistic Regression Analysis of CPR of Adult Patients with PTN at 3 Years After MVD |
 |
Table 16 Area Under the ROC Curve at 3 Years After MVD |
Discussion
PTN is a common and severe intermittently recurring orofacial lancinating pain syndrome confined to the somatosensory distribution of TN that can cause significant chronic debilitating neuropathic pain and great discomfort to the patient, with clear-cut pain-free intervals and a tendency to occur in the elderly.19 The clinical practice needs to be urgently improved through prompt recognition of the clinical picture, accurate evaluation of the neurosensory system, and appropriate adherence to the specific guidelines despite the common clinical features and available international diagnostic criteria of PTN. Misdiagnosis, delayed diagnosis, and even mistreatment are still not negligible in clinical practice.20,21 Vascular compression-induced NVC in TN root entry zone (REZ), whether arterial or venous compression, can cause demyelination of trigeminal sensory fibers and atrophic changes of TN, which is the primary hypothesis for the underlying neural mechanism and thus plays an important causative role in PTN.14,22 NVC may be more likely to develop and progress if the posterior cranial fossa (PCF) is crowded or a sharp trigeminal-pontine angle is present, both of which are risk factors for PTN.23,24 Current gold standard of conventional pharmacotherapy for PTN is sodium channel blockers, including carbamazepine and oxcarbazepine, with the need for titration and poor tolerability, such as the occurrence of drowsiness, diplopia and ataxia, although they are effective in pain control.25 If adult patients with PTN are unable to respond to conventional pharmacotherapy or have a low tolerance for it, neurosurgical interventions should be considered.26
Compared with other neurosurgical interventions, standard MVD has the highest pain relief, success rate and patient satisfaction as well as the lowest incidence of complications and recurrence rate, and therefore is generally well-accepted as the preferred choice for adult patients with PTN in clinical practice.27–30 Sufficient decompression of TN and thorough exploration of both sensory and motor roots during MVD is a prerequisite to obtaining optimal pain relief in adult patients with PTN.31 The main purpose of this study is derived from this clinical fact that it is still unclear why a sizable portion of adult PTN patients with explicit NVC are unable to obtain satisfactory short- and long-term CPR even after sufficient decompression of TN during MVD. According to reports, MVD has a recurrence rate of about 10%, which is significantly lower than that of traditional pharmacotherapy and other neurosurgical interventions, with non-arterial compression being one of the contributing factors.32 Improvements in the advanced imaging technologies and neurosurgical techniques of MVD or combination with other neurosurgical interventions, such as partial sensory rhizotomy (PSR), may be fruitful in attempting to lower the recurrence rate.32 Even for persistent or recurrent PTN, repeated MVD (Re-MVD) and PCF exploration remain feasible and reliable options for salvage therapy.33–36
This study population was characterized by a predominance of females and a preference for right-sided, arterial, and nerve root compression. CPR rate in adult patients with PTN gradually decreased from 76% at 3 months to 67.5% at 3 years after sufficient decompression of TN during MVD, which was consistently within the clinically acceptable range. In general, our results are consistent with the fact that MVD is an effective therapeutic option independent of patient’s age.37 In addition, our results showed that compression degree of TN constantly had the greatest impact on CPR in adult patients with PTN at different time points after sufficient decompression of TN during MVD, which was under previous results.38–40 From our results, although the specificity and negative predictive value of the ROC curve at various time points after MVD were constrained in this case series, adult PTN patients with severe and arterial compression on TN had better short- and long-term CPR after sufficient decompression of TN during MVD as their initial neurosurgical procedure. Therefore, preoperative neuroimaging as a prognostic marker of optimal management strategy, especially 3D-TOF-MRA with distinct signal, contributes to determining the optimal candidates suitable for MVD, guiding the appropriate treatment plan, and thus obtaining the best curative effect by demonstrating explicit NVC with nearby specific offending vessels and its severity.
Limitations
There are several limitations in the present study. First of all, the nature of a single-center small-sample retrospective study constrained the reliability and generalizability of our conclusion. Second, the conclusion of our study could not be directly generalized to other patient populations because it was only applicable to adult PTN patients with explicit NVC prior to surgery who underwent MVD as their initial neurosurgical procedure. Third, the comparable demographic and clinical data were limited in this study. Finally, the post-surgery follow-up was discontinued at 3 years without further follow-up information available.
Conclusion
In conclusion, compression degree of TN and type of conflicting vessels can determine short- and long-term CPR in adult patients with PTN after sufficient decompression of TN during MVD, which will contribute to better characterizing potential candidates suitable for MVD and thus optimizing the planning and implementation of MVD. Future multi-center prospective clinical trials with a sizable sample size are required to further validate our findings.
Data Sharing Statement
The data are available from the corresponding author on reasonable request.
Ethics statement
The requirement for written informed consent was waived according to the retrospective nature of the study, institutional requirements and national law. All operative procedure had undergone rigorous ethical review for keeping the safety of medical care and individual privacy. All patients’ data were reserved confidentially under the inspection of the ethical committee of the Second Affiliated Hospital of Harbin Medical University even if without written informed consent. We will always comply with relevant laws, regulations, and international ethical guidelines, particularly the “Declaration of Helsinki”.
Acknowledgments
The authors are grateful to everyone who offered their time, insight, and assistance to this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37(7):648–657. doi:10.1177/0333102416687280
2. Ma Y, Hsu G, Zhang F. The applicability and efficacy of magnetic resonance-guided high intensity focused ultrasound system in the treatment of primary trigeminal neuralgia. Med Hypotheses. 2020;139:109688. doi:10.1016/j.mehy.2020.109688
3. Godazandeh K, Martinez Sosa S, Wu J, Zakrzewska JM. Trigeminal neuralgia: comparison of characteristics and impact in patients with or without multiple sclerosis. Mult Scler Relat Disord. 2019;34:41–46. doi:10.1016/j.msard.2019.06.015
4. Smith CA, Paskhover B, Mammis A. Molecular mechanisms of trigeminal neuralgia: a systematic review. Clin Neurol Neurosurg. 2021;200:106397. doi:10.1016/j.clineuro.2020.106397
5. Chen Q, Yi DI, Perez JNJ, et al. The molecular basis and pathophysiology of trigeminal neuralgia. Int J Mol Sci. 2022;23(7):3604. doi:10.3390/ijms23073604
6. Chen GQ, Wang XS, Wang L, Zheng JP. Arterial compression of nerve is the primary cause of trigeminal neuralgia. Neurol Sci. 2014;35(1):61–66. doi:10.1007/s10072-013-1518-2
7. Lin J, Zhang Y, Li W, Yan J, Ke Y. Flatness of the Meckel cave may cause primary trigeminal neuralgia: a radiomics-based study. J Headache Pain. 2021;22(1):104. doi:10.1186/s10194-021-01317-4
8. Lin J, Zhou L, Luo Z, et al. Flow cytometry analysis of immune and glial cells in a trigeminal neuralgia rat model. Sci Rep. 2021;11(1):23569. doi:10.1038/s41598-021-02911-x
9. Liu MX, Zhong J, Xia L, Dou NN, Li ST. A correlative analysis between inflammatory cytokines and trigeminal neuralgia or hemifacial spasm. Neurol Res. 2019;41(4):335–340. doi:10.1080/01616412.2018.1564188
10. Hu M, Zhou W, Shen W, Zhang H, Shen J. A combination of 3D TOF MRA and FIESTA predicts surgery-needed primary trigeminal neuralgia and specific offending vessels. J Integr Neurosci. 2022;21(6):169. doi:10.31083/j.jin2106169
11. Wang Z, Zhao Z, Song Z, Wang Y, Zhao Z. The application of magnetic resonance imaging (MRI) for the prediction of surgical outcomes in trigeminal neuralgia. Postgrad Med. 2022;134(5):480–486. doi:10.1080/00325481.2022.2067612
12. Sterman-Neto H, Fukuda CY, Duarte KP, et al. Balloon compression vs radiofrequency for primary trigeminal neuralgia: a randomized, controlled trial. Pain. 2021;162(3):919–929. doi:10.1097/j.pain.0000000000002070
13. Bendtsen L, Zakrzewska JM, Abbott J, et al. European Academy of Neurology guideline on trigeminal neuralgia. Eur J Neurol. 2019;26(6):831–849. doi:10.1111/ene.13950
14. Zhao H, Wang XH, Zhang Y, et al. Management of primary bilateral trigeminal neuralgia with microvascular decompression: 13-case series. World Neurosurg. 2018;109:e724–e730. doi:10.1016/j.wneu.2017.10.072
15. Wang Z, Wang Z, Li K, Su X, Du C, Tian Y. Radiofrequency thermocoagulation for the treatment of trigeminal neuralgia. Exp Ther Med. 2022;23(1):17. doi:10.3892/etm.2021.10939
16. Kanekar S, Saif M, Kanekar S. Imaging of cranial neuralgias. Neurol Clin. 2022;40(3):591–607. doi:10.1016/j.ncl.2022.02.008
17. Functional Neurosurgery Group of Chinese Society of Neurosurgery. Functional neurosurgery expert committee of Chinese medical doctors association, etc. Chinese expert consensus on the diagnosis and treatment of trigeminal neuralgia. Chin J Surg. 2015;53(9):657–664. doi:10.3760/cma.j.issn.0529-5815.2015.09.005
18. Zhang P, Brisman R, Choi J, Li X. Where to locate the isocenter? The treatment strategy for repeat trigeminal neuralgia radiosurgery. Int J Radiat Oncol Biol Phys. 2005;62(1):38–43. doi:10.1016/j.ijrobp.2004.09.005
19. Theodros D, Rory Goodwin C, Bender MT, et al. Efficacy of primary microvascular decompression versus subsequent microvascular decompression for trigeminal neuralgia. J Neurosurg. 2017;126(5):1691–1697. doi:10.3171/2016.5.JNS151692
20. Antonaci F, Arceri S, Rakusa M, et al. Pitfals in recognition and management of trigeminal neuralgia. J Headache Pain. 2020;21(1):82. doi:10.1186/s10194-020-01149-8
21. Khan ZA. Revisiting the corneal and blink reflexes for primary and secondary trigeminal facial pain differentiation. Pain Res Manag. 2021;2021:6664736. doi:10.1155/2021/6664736
22. Cheng J, Meng J, Liu W, Zhang H, Hui X, Lei D. Nerve atrophy in trigeminal neuralgia due to neurovascular compression and its association with surgical outcomes after microvascular decompression. Acta Neurochir. 2017;159(9):1699–1705. doi:10.1007/s00701-017-3250-9
23. Cheng J, Meng J, Liu W, Zhang H, Hui X, Lei D. Primary trigeminal neuralgia is associated with posterior fossa crowdedness: a prospective case-control study. J Clin Neurosci. 2018;47:89–92. doi:10.1016/j.jocn.2017.10.032
24. Pang H, Sun H, Fan G. Correlations between the trigeminal nerve microstructural changes and the trigeminal-pontine angle features. Acta Neurochir. 2019;161(12):2505–2511. doi:10.1007/s00701-019-04099-6
25. Zakrzewska JM, Palmer J, Morisset V, et al. Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: a double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol. 2017;16(4):291–300. doi:10.1016/S1474-4422(17)30005-4
26. Ziegeler C, Beikler T, Gosau M, May A. Idiopathic facial pain syndromes–an overview and clinical implications. Dtsch Arztebl Int. 2021;118(6):81–87. doi:10.3238/arztebl.m2021.0006
27. Alshukry A, Salburgo F, Jaloux L, Lavieille JP, Montava M. Trigeminal neuralgia (TN): a descriptive literature analysis on the diagnosis and management modalities. J Stomatol Oral Maxillofac Surg. 2017;118(4):251–254. doi:10.1016/j.jormas.2017.06.010
28. Sharma R, Phalak M, Katiyar V, Borkar S, Kale SS, Mahapatra AK. Microvascular decompression versus stereotactic radiosurgery as primary treatment modality for trigeminal neuralgia: a systematic review and meta-analysis of prospective comparative trials. Neurol India. 2018;66(3):688–694. doi:10.4103/0028-3886.232342
29. Dai ZF, Huang QL, Liu HP, Zhang W. Efficacy of stereotactic gamma knife surgery and microvascular decompression in the treatment of primary trigeminal neuralgia: a retrospective study of 220 cases from a single center. J Pain Res. 2016;9:535–542. doi:10.2147/JPR.S110161
30. Diana C, Kumar RD, Bodh R, Kumari S. Does the surgical intervention for trigeminal neuralgia refractory to pharmacotherapy improve quality-of-life? - a systematic review. J Oral Maxillofac Surg. 2021;79(11):2227–2239. doi:10.1016/j.joms.2021.03.003
31. Huang Z, Pu B, Li F, et al. Analysis of failed microvascular decompression in patients with trigeminal neuralgia. J Neurol Surg B Skull Base. 2020;81(5):567–571. doi:10.1055/s-0039-1692683
32. Chen F, Niu Y, Meng F, et al. Recurrence rates after microvascular decompression in patients with primary trigeminal neuralgia and its influencing factors: a systematic review and meta-analysis based on 8172 surgery patients. Front Neurol. 2021;12:738032. doi:10.3389/fneur.2021.738032
33. Hussain MA, Konteas A, Sunderland G, et al. Re-Exploration of microvascular decompression in recurrent trigeminal neuralgia and intraoperative management options. World Neurosurg. 2018;117:e67–e74. doi:10.1016/j.wneu.2018.05.147
34. Patra DP, Savardekar AR, Dossani RH, Narayan V, Mohammed N, Nanda A. Repeat Gamma Knife radiosurgery versus microvascular decompression following failure of GKRS in trigeminal neuralgia: a systematic review and meta-analysis. J Neurosurg. 2018;1–10. doi:10.3171/2018.5.JNS18583
35. Chen JN, Yu WH, Du HG, Jiang L, Dong XQ, Cao J. Prospective comparison of redo microvascular decompression and percutaneous balloon compression as primary surgery for recurrent trigeminal neuralgia. J Korean Neurosurg Soc. 2018;61(6):747–752. doi:10.3340/jkns.2017.0196
36. Jiao L, Ye H, Lv J, et al. A systematic review of repeat microvascular decompression for recurrent or persistent trigeminal neuralgia. World Neurosurg. 2022;158:226–233. doi:10.1016/j.wneu.2021.11.129
37. Keric N, Kalasauskas D, Kreth SL, et al. An age-dependent outcome analysis of microvascular decompression and percutaneous thermocoagulation in trigeminal neuralgia. BMC Neurol. 2021;21(1):182. doi:10.1186/s12883-021-02197-6
38. Sindou M, Leston J, Decullier E, Chapuis F. Microvascular decompression for primary trigeminal neuralgia: long-term effectiveness and prognostic factors in a series of 362 consecutive patients with clear-cut neurovascular conflicts who underwent pure decompression. J Neurosurg. 2007;107(6):1144–1153. doi:10.3171/JNS-07/12/1144
39. Fukuoka T, Nishimura Y, Hara M, et al. Flat posterior cranial fossa affects outcomes of microvascular decompression for trigeminal neuralgia. World Neurosurg. 2018;111:e519–e526. doi:10.1016/j.wneu.2017.12.114
40. Ishaque AH, Xie H, Danyluk H, et al. Comparison of prognostic scoring systems to predict durable pain relief after microvascular decompression for trigeminal neuralgia. World Neurosurg. 2022;157:e432–e440. doi:10.1016/j.wneu.2021.10.111
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.