Back to Journals » International Journal of General Medicine » Volume 16
Comprehensive Review of Ustekinumab Utilization in Inflammatory Bowel Diseases: Insights from the ClinicalTrials.gov Registry
Authors Alorfi NM , Alourfi MM, Bokhari GA, Alkhattabi A, Ibrahim NA , Alsabban AM, Almatrafi MJ, Zakri YA, Almahmoud AJ, Al-ghamdi KMA, Alsharif SN
Received 2 August 2023
Accepted for publication 14 September 2023
Published 20 September 2023 Volume 2023:16 Pages 4283—4294
DOI https://doi.org/10.2147/IJGM.S433636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nasser M Alorfi,1 Mansour M Alourfi,2,3 Ghfran Abdulrahman Bokhari,3 Abdullah Alkhattabi,3 Nihal Abdalla Ibrahim,4 Abdulmalik Mohammed Alsabban,5 Mohammad J Almatrafi,5 Yaser Abdulaziz Zakri,5 Abdullah Jasem Almahmoud,6 Khalid Mohammed A Al-ghamdi,6 Saeed Nasser Alsharif7
1Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia; 2Internal Medicine Department, King Faisal Medical City for Southern Region, Abha, Saudi Arabia; 3Department of Gastroenterology, East Jeddah Hospital, Jeddah, Saudi Arabia; 4College of Pharmacy, Ajman University, Ajman, United Arab Emirates; 5Gastroenterology Section, Department of Medicine, King Abdulaziz Medical City, Jeddah, Saudi Arabia; 6Gastroenterology Section, Internal Medicine Department, King Fahad Hospital, Jeddah, Saudi Arabia; 7Gastroenterology Department, Armed Force Hospital of Southern Region, Khamis Mushait, Saudi Arabia
Correspondence: Nasser M Alorfi, Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia, Email [email protected]
Background: Inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis, are chronic inflammatory conditions affecting the gastrointestinal tract. To achieve and sustain remission, effective treatment strategies are necessary. Ustekinumab, a biologic agent targeting interleukin-12 and interleukin-23, has emerged as a significant therapeutic option for moderate to severe IBD.
Aim: To gain insights into the utilization of Ustekinumab for IBD, we conducted a comprehensive review of the ClinicalTrials.gov registry.
Methods: A comprehensive search of the ClinicalTrials.gov was conducted to find all clinical trials involving the use of Ustekinumab in IBD patients. As of December 30th, 2022, 69 clinical trials were identified that included IBD and Ustekinumab. The study list was saved, and those clinical trials that fitted the definition of targeted therapy were included in the review.
Results: The results showed that Ustekinumab was associated with significant improvements in the clinical response and remission rates, in both Crohn’s disease and ulcerative colitis patients. Additionally, the safety profile of Ustekinumab was generally favourable, with low rates of adverse events reported. In terms of study design, most of the relevant studies found in the database were interventional studies. The investigation focused on completed studies and found that there were a limited number of clinical trials with interventional measures.
Conclusion: Ustekinumab appears to be a promising treatment option for patients with IBD, with the potential to provide significant clinical benefits and a favourable safety profile. Further research is warranted to confirm these findings and explore optimal dosing and treatment regimens.
Keywords: ustekinumab, pharmacology, biologics, IBD, clinical trials, gastroenterology
Introduction
Inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis, are long-term inflammatory conditions affecting the gastrointestinal tract that can cause significant morbidity and impact the quality of life of affected individuals. It can be divided into two subtypes, Crohn’s disease and ulcerative colitis (UC).1 The differences between these subtypes are the location of the pathology and the characteristics of the lesion. In ulcerative colitis, the inflammatory response is limited to the mucosal layer along the whole length of the colon. The lesions can be found with variable degrees of ulceration, oedema, and haemorrhage. While ulcerative colitis primarily impacts the colon and rectum, in chronic progressive ulcerative colitis, a substantial prevalence of fibrosis and thickening of the muscularis mucosae is frequently observed. These particular histological characteristics can carry clinical significance, potentially leading to motility abnormalities and heightened wall stiffness as consequential complications.2 Crohn’s disease can potentially affect any segment of the gastrointestinal tract, ranging from the mouth to the anus.3,4 The lesions in Crohn’s disease are separated by areas of normal, non-pathological bowel, known as “skip areas”.5,6 However, the inflammation associated with Crohn’s disease is not confined to the surface lining of the gut, and can penetrate deeper layers of the intestinal wall leading to the formation of fistulas or sinus tracts.7 The most commonly affected areas are the ileocecal area, terminal ileum, and the small bowel.8,9
The symptoms of IBD are not limited to gastrointestinal symptoms but are usually associated with extraintestinal manifestations related to intestinal disease activities.10–12 Moreover, abdominal pain, abdominal bloating, fever, weight loss, fatigue, persistent diarrhoea, and the presence of blood and mucus in the stool can also occur.13–16 In UC, diarrhoea is a dominant symptom, while postprandial abdominal pain is more common in Crohn’s disease.17,18 The cause of IBD is unknown but the suspected causes are the genetics and abnormalities of the immune system, with dysregulation or excessive T helper cells (TH)1 in Crohn’s disease or (TH)2 in ulcerative colitis. However, microbial factors, food and the environment might also be causes of the disease.19,20 Thus, while IBD can be found in both children and adults, it is frequently diagnosed in the age range 15–35.21 The prevalence of IBD varies significantly by region and is subject to change over time. In many Western countries, including the United States and Europe, the estimated prevalence of IBD is approximately 0.3% to 0.5% of the population. However, this figure can vary, and the prevalence may be higher in some regions or subpopulations.
The objectives of IBD treatment are to relieve the symptoms and prevent disease flare-up. Aminosalicylates (5-ASA) or Mesalazines; antibiotics; immunosuppressants such as azathioprine, methotrexate or cyclosporin/steroids; and especially antibody-based biological therapies, are the most common pharmacotherapies used to manage the disease.22 According to the practice guidelines for IBD, 5-ASA is used as an initial drug. In low to moderate cases, corticosteroids are used if the 5-ASA fails in treatment. Vedolizumab, anti-α4β7-integrin, Tofacitinib, a Janus kinase (JAK) inhibitor, are recommended if both 5-ASA and corticosteroids fail.20,23–26
Over the past decade, biologic agents, such as Ustekinumab, have emerged as important therapeutic options for moderate to severe IBD. Ustekinumab is a monoclonal antibody that selectively targets and binds strongly to interleukins IL-12 and IL-23, which are cytokines implicated in the pathogenesis of autoimmune diseases.27,28 Ustekinumab (Stelara®) is a biologic medication with indications for the treatment of moderate to severe plaque psoriasis for candidates for phototherapy or systemic therapy, and for the treatment of active psoriatic arthritis in patients 6 years old and over.29,30 Moreover, in 2019, the indication of Ustekinumab for ulcerative colitis was approved by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA).31 Therefore, Ustekinumab is indicated for moderate to severely active Crohn’s disease and ulcerative colitis in adults.32–34
Given the recent approval of Ustekinumab for the treatment of IBD, there is a growing interest in investigating its effectiveness and safety in this context. Consequently, the primary objective of this review is to comprehensively examine the utilization of Ustekinumab for IBD and delve into the specific characteristics of the clinical trials conducted to evaluate its efficacy and safety profile. In order to gain comprehensive insights into the utilization of Ustekinumab in IBD, we conducted an extensive review of the ClinicalTrials.gov registry. This registry serves as a valuable resource that provides information on ongoing and completed clinical trials, including those investigating the efficacy, safety, and optimal utilization of Ustekinumab for the management of IBD.
Methods
Data Sources and Extraction
A search of ClinicalTrials.gov was conducted to retrieve all the registered clinical trials on Ustekinumab for IBD that occurred up to and including December 30, 2022. Within the search engine of ClinicalTrials.gov, the keyword for the condition/disease was “IBD” and Ustekinumab was searched for as a treatment option. However, studies related to inflammatory bowel syndrome (IBS) were also included as it is a synonym for IBD. The main endpoints, sample size, duration of the study, and findings were also gathered.
The data were extracted manually and downloaded from the data reported in ClinicalTrials.gov, covering interventional, observational, and expanded access clinical trials in all their phases.
Data Categories
The trends and characteristics of the data and information obtained from the trials were classified by extracting and categorizing them. The criteria used for classification were status, estimated enrolment, study type, design and allocation, phase of study (for interventional studies), location, sponsors, and age eligibility criteria. In cases where multiple locations were involved in a study, the number of trials was calculated for each region based on the countries where the trials were conducted. However, the regions mentioned on ClinicalTrial.gov differed from the continents, resulting in the need for re-categorization into Africa, North America, Central America and the Caribbean, South America, East Asia, the Middle East, South Asia, Southeast Asia, Europe, and Oceania.
Only interventional studies were considered; observational studies and expanded access studies were excluded because they were not designed for the purpose that was of interest to the review. Drug interventions, excluding Ustekinumab, were classified according to the drug classes, and the number of drugs in each class was manually counted based on their generic names.
Results
Analysis of the Number of Research Registrations
A total of 69 registrations of studies related to Ustekinumab in IBD were found in the ClinicalTrials.gov database.
Trials Characteristics
From these 69 studies, there were 22,772 subjects in total. More than half of the studies (53.6%) were interventional studies and 27 (39.1%) were multi-centre interventional studies, with the remaining 44.9% being observational studies. The highest number of studies had been conducted in Europe, and the majority were conducted with participants 18 years old and older. Only 13 (18.8%) studies included children (17 years old and below). Six out of seven Phase 4 studies were still recruiting. A detailed description of the trials’ characteristics, including the current status, participant numbers, study type and allocation, geographical region, and funding bodies, are shown in Table 1, which shows the number of studies and percentages based on the total of 69 studies. Drug intervention and number of studies in which that intervention had been used were tabulated in Table 2.
 |
Table 1 Clinical Trial Characteristics |
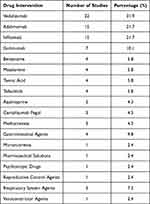 |
Table 2 Drug Intervention and Number of Studies in Which That Intervention Had Been Used |
Ustekinumab for Inflammatory Bowel Disease
Apart from Ustekinumab, 19 other drug classes had been used in the trials. The number of drugs used to manage the disease and symptoms are shown in detail in Table 3. However, a non-therapeutic placebo was given in 17 of the 69 trials (24.6%).
 |
Table 3 Drug Intervention and Number of Drugs in the Class Used to Manage the Disease |
Completed Studies
Out of the 69 studies initially identified for inclusion in our review, 22 studies had been successfully completed, forming a significant subset of our analysis. These completed studies have provided valuable insights into the various conditions affecting the digestive system, and a comprehensive breakdown of these conditions is presented in Table 4 for reference. Notably, approximately 54.5% of the completed studies (12 out of 22 trials) adopted an observational study design, with the remaining 10 studies taking an interventional approach. Among the observational studies, seven were cohort studies, shedding light on long-term trends and associations within the subject population. In contrast, the interventional studies offered a more proactive approach to understanding digestive system diseases, although it is important to mention that no phase 4 study within this category had reached completion at the time of our analysis; they were still in the recruitment phase. Of particular interest was that all the interventional trials were exclusively conducted among individuals with moderately to severely active IBD. This is a significant finding, as it highlights the focus on addressing the specific needs of patients who have encountered challenges with conventional IBD treatments. These individuals may have experienced treatment failure or intolerance to biologic therapy, or they may be new to biologic therapy altogether. The primary endpoints assessed across the interventional studies were clinical remission and response, which provide essential clinical insights into the effectiveness of the interventions. For a more detailed understanding of these interventional studies, including their specific objectives, target subjects, drug comparisons, phases, and key outcomes, please refer to the comprehensive information provided in Table 5. These findings collectively contribute to our understanding of the landscape of studies in the field of digestive system diseases, emphasizing the importance of both observational and interventional research in addressing the diverse needs of patients with IBD.
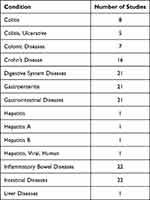 |
Table 4 Digestive System Diseases |
 |
Table 5 Completed Intervention Studies |
Discussion
This is the first article to analyse the usage of Ustekinumab in IBD and it provides details of the clinical trials that have been conducted on the disease. The number of patients diagnosed with IBD is rising around the world in all population ages. This has been confirmed by studies conducted in each region.35–40
Ustekinumab is a biologic medication that has demonstrated efficacy in managing chronic inflammatory conditions like psoriasis and Crohn’s disease. Its mechanism of action involves targeted inhibition of interleukins IL-12 and IL-23, resulting in immune response suppression and inflammation reduction. Ustekinumab is administered via subcutaneous injection, with a slow elimination rate allowing for less frequent dosing. The pharmacokinetic properties of Ustekinumab may vary depending on the specific indications and dosing regimens. Overall, Ustekinumab has demonstrated a favourable safety profile and has become an important therapeutic option in the management of chronic inflammatory diseases.
Inflammatory bowel disease, also called IBD, is a chronic condition related to gastrointestinal development that impacts patients’ usual life activities, lasts for years, and sometimes leads to serious disability, causing problems in physical activity, social interaction, work productivity and communication. The disorder also has limited pharmacological options.41–43
This paper has also revealed that the pharmacological treatment of IBD is being studied extensively around the world. However, the number of studies is not abundant and the information about the usage of Ustekinumab in paediatrics is still limited. The trials that have so far been conducted on this disease have been mainly conducted with adults. In terms of the study design, the number of interventional studies and observation studies was quite similar (53.6% vs 44.9%, respectively). Moreover, approximately 40% of all trials were randomized interventional trials, while around one-fifth of all studies were Phase 3 clinical trials. Nearly half of all the studies (47.8%) were conducted by universities or organizations. In the clinical trials, individuals treated with Ustekinumab reported a higher incidence of respiratory and gastrointestinal infections than those receiving a placebo.
Several completed trials evaluated the efficacy of Ustekinumab in inducing and maintaining remission in both Crohn’s disease and ulcerative colitis patients. The findings consistently demonstrated positive outcomes, with a significant proportion of patients achieving remission or showing improvements in disease activity scores. These results support the notion that Ustekinumab can be an effective treatment option for IBD patients, especially those who have failed to respond to conventional therapies or anti-TNF agents. In terms of safety, the completed trials consistently reported a favourable safety profile for Ustekinumab. Adverse events were generally mild and manageable, with the most common being upper respiratory tract infections, headaches, and injection site reactions. Serious adverse events, including serious infections, were rare but were reported in a small number of cases. These findings align with the known safety profile of Ustekinumab as observed in previous clinical trials and real-world studies. Moreover, some completed trials focused on specific patient subgroups, such as those who had previously failed to improve with anti-TNF agents. These trials provided evidence that Ustekinumab can be a viable treatment option for patients who have experienced treatment failure with anti-TNF agents, offering an alternative therapeutic avenue for this challenging-to-treat population. It is worth noting that the completed trials in the ClinicalTrials.gov registry encompassed various study designs and endpoints, which may have resulted in heterogeneity in the reported outcomes. Differences in patient populations, treatment regimens, and follow-up durations also contributed to the variability in the reported results. Nonetheless, the overall findings consistently support the efficacy and safety of Ustekinumab in IBD patients.
5-ASA is the standard therapy for mild to moderately active IBD as an initial treatment. Corticosteroids can be added or switched to, in cases of 5-ASA or sulfasalazine failure/refractory. Corticosteroids can be used in any stage of the disease. Immunomodulators and biologic therapy are recommended when corticosteroids and 5-ASA fail. The immunomodulators/immunosuppressants suggested in these guidelines are azathioprine and methotrexate. Biologic therapy can take the form of anti-INF therapy (infliximab, adalimumab, certolizumab, golimumab), vedolizumab, tofacitinib and currently Ustekinumab, which is recommended in moderate to severe IBD when the conventional therapy has failed, is inadequate or intolerable.20,44–47 According to the results for IBD, many anti-rheumatic agents have also been used as drug interventions. They were adalimumab, azathioprine, certolizumab pegol, infliximab, mesalamine, methotrexate, and mirikizumab. The top three most frequently used were vedolizumab, adalimumab and infliximab. The drug interventions found in this search also reflect the disease severity, management, and clinical practice guidelines behind the disease.
Following the results from the drug intervention section, anti-rheumatic agents contain anti-TNF, mesalamine (one of 5-ASA agents) and immunomodulators/ immunosuppressants. The drug interventions most frequently found were biologic therapy, immunomodulators/immunosuppressants and 5-ASA agents. It can be assumed that the trials for Ustekinumab and IBD were mainly conducted with patients with moderate to severe IBD, following the clinical practice guidance recommendation. For the completed studies, there was a predominance of intervention studies, and the outcomes of interest for these studies were clinical response and clinical remission. Moreover, all of them were conducted with patients with moderately to severely active disease, while the comparators were a comparison between different dosage regimens and a comparison between a placebo and biologic therapy drugs. It can be seen in the inclusion criteria of each trial that the population were patients who required biological therapy due to the failure of conventional therapy. This population might be naïve or have received biologic therapy before. However, two important characteristics were clearly observed. There was a limited number of interventional studies that had already been completed in paediatrics, and Crohn’s disease was more frequently studied than UC. One reason for this might be that the indication of Ustekinumab for Crohn’s disease was approved earlier than in ulcerative colitis (2009 vs 2019, respectively).33,34,48
It is important to have guidelines for policy and practice involving ustekinumab utilization in IBD, as this ensures consistent and evidence-based decision making. Based on current knowledge and clinical evidence, it is recommended that ustekinumab be considered as a treatment option in patients with moderate to severe IBD who have failed to improve with conventional therapies or anti-TNF agents. The decision to initiate ustekinumab treatment should be made on an individualized basis, taking into account factors such as disease severity, previous treatment responses, and the presence of IL-23-driven inflammation. Shared decision making between healthcare professionals and patients is crucial to determine the most appropriate treatment approach. Guidelines for policy and practice aim to ensure appropriate patient selection and monitoring, considering ustekinumab as a treatment option in suitable individuals. Continued research and surveillance are necessary to further enhance our understanding of ustekinumab’s long-term effects and refine its place in the treatment paradigm for IBD.
Despite the success of biologic therapies, there are still challenges in the management of IBD, including variability in responses to treatment, the potential side effects of medications, and issues related to long-term safety and cost effectiveness. Therefore, ongoing research and clinical trials, as well as personalized treatment approaches, are needed to further optimize the management of IBD and improve outcomes for patients with these chronic inflammatory conditions.
Limitations
It is important to note that the presented analysis has some limitations since the data from ClinicalTrials.gov has been recorded incorrectly in some instances. It is also possible that some data were misclassified during the selection and classification process. Additionally, there may be a bias in terms of the number of registered trials in recent years compared with the earlier period. However, our analysis is unique and important and provides researchers and clinicians with a picture of Ustekinumab usage in IBD.
Conclusion
Ustekinumab acts by inhibiting the interleukin-12 (IL-12) and interleukin-23 (IL-23) pathways, which play a crucial role in the pathogenesis of IBD. By targeting these pathways, ustekinumab helps to reduce inflammation and to control symptoms in patients with IBD. Extensive research and clinical trials have contributed to our understanding of the mechanism of action and the potential benefits of ustekinumab in the treatment of IBD. Efficacy studies have shown promising results regarding its use for patients with IBD. Clinical trials and real-world studies have demonstrated its efficacy in inducing and maintaining remission in both Crohn’s disease and UC. These findings suggest that ustekinumab can be considered as an alternative treatment option for patients who have failed conventional therapies or have not responded well to anti-TNF agents. Furthermore, ustekinumab has shown efficacy even in patients who have previously experienced treatment failure with anti-TNF agents, offering a new therapeutic option for these individuals. The findings from this study suggest that clinical trials for IBD using Ustekinumab as a treatment were limited. Approximately 53.6% of all trials were interventional trials. Among the 69 studies, almost one-fifth used Ustekinumab for IBD in paediatrics. All of the completed interventional studies focused on clinical response and remission with a comparison between non-therapeutic placebo and other biologic therapy for IBD. The completed trials investigated the use of Ustekinumab in moderately to severely active IBD, but mostly with Crohn’s disease. Researchers and clinicians can now access information about Ustekinumab usage from both interventional and observation trials, but there is still not a realistic picture of Ustekinumab treatment from phase 4 studies, hence this still needs further investigation and updates.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cleynen I, Linsen L, Verstockt S, et al. Inflammatory Bowel Disease (IBD)—A textbook case for multi-centric banking of human biological materials. Front Med. 2019;6:230. doi:10.3389/fmed.2019.00230
2. Gordon IO, Agrawal N, Willis E, et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922–939. doi:10.1111/APT.14526
3. Pimentel AM, Rocha R, Santana GO. Crohn’s disease of esophagus, stomach and duodenum. World J Gastrointest Pharmacol Ther. 2019;10:35. doi:10.4292/WJGPT.V10.I2.35
4. Almuntashri F, Binyaseen K, Alkhotani A, Almuntashri F, Binyaseen K, Alkhotani A. Chronic inflammatory demyelinating polyneuropathy in patients with Crohn’s disease on infliximab therapy. Cureus. 2021;13. doi:10.7759/CUREUS.19041
5. Bouchard D, Abramowitz L, Bouguen G, et al. Anoperineal lesions in Crohn’s disease: French recommendations for clinical practice. Techniq Coloproctol. 2017;21(9):683–691. doi:10.1007/s10151-017-1684-y
6. Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol. 2003;16:347–358. doi:10.1097/01.MP.0000064746.82024.D1
7. Schmid-Tannwald C, Agrawal G, Dahi F, Sethi I, Oto A. Diffusion-weighted MRI: role in detecting abdominopelvic internal fistulas and sinus tracts. J Magn Reson Imaging. 2012;35:125–131. doi:10.1002/JMRI.22804
8. Robert JR, Sachar DB, Greenstein AJ. Severe Gastrointestinal Hemorrhage in Crohn’s Disease. Ann Surg. 1991;213:207. doi:10.1097/00000658-199103000-00004
9. Fazio VW, Wilk P, Turnbull RB, Jagelman DG. The dilemma of Crohn’s disease: ileosigmoidal fistula complicating Crohn’s disease. Dis Colon Rectum. 1977;20:381–386. doi:10.1007/BF02587365/METRICS
10. Ephgrave K. Extra-Intestinal Manifestations of Crohn’s Disease. Surg Clin North Am. 2007;87:673–680. doi:10.1016/J.SUC.2007.03.003
11. Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585–595. doi:10.1038/nrgastro.2013.117
12. Alharbi MH, Alhazmi AH, Ujaimi MH, et al. The prevalence of irritable bowel syndrome and its relation to psychiatric disorders among citizens of Makkah region, Saudi Arabia. Cureus. 2022;14. doi:10.7759/CUREUS.32705
13. Yu YR, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349–355. doi:10.1053/J.SEMPEDSURG.2017.10.003
14. Coates MD, Lahoti M, Binion DG, Szigethy EM, Regueiro MD, Bielefeldt K. Abdominal pain in ulcerative colitis. Inflamm Bowel Dis. 2013;19:2207–2214. doi:10.1097/MIB.0B013E31829614C6
15. Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1474–1482. doi:10.1038/AJG.2012.260
16. Khayyat Y. Perception of symptomatic abdominal bloating in irritable bowel syndrome among Saudi Arabian patients. J Umm Al-Qura Univ Med Sci. 2020;6:1–3. doi:10.54940/MS84929526
17. Roda G, Chien Ng S, Kotze PG, et al. Crohn’s Disease. Nat Rev Dis Prim. 2020;6:1–19. doi:10.1038/s41572-020-0156-2
18. Sandle GI. Pathogenesis of diarrhea in ulcerative colitis: new views on on old problem. J Clin Gastroenterol. 2005;39:S49–S52. doi:10.1097/01.MCG.0000155520.04253.37
19. Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi:10.1128/CMR.15.1.79-94.2002
20. Okobi OE, Udoete IO, Fasehun OO, et al. A Review of four practice guidelines of inflammatory bowel disease. Cureus. 2021;13:8.
21. Crohn’s & Colitis FoundationThe Crohn’s & Colitis Foundation of America. THE FACTS ABOUT inflammatory bowel diseases 2014; 2014.
22. The National Health Service Inflammatory Bowel Disease. Available from: https://www.nhs.uk/conditions/inflammatory-bowel-disease/.
23. Kuhbacher T, Fölsch UR. Practical guidelines for the treatment of inflammatory bowel disease. World J Gastroenterol WJG. 2007;13:1149. doi:10.3748/wjg.v13.i8.1149
24. Almoallim H, Al-Ghamdi Y, Almaghrabi H, Alyasi O. Anti-tumor necrosis factor-α induced systemic lupus erythematosus. Open Rheumatol J. 2012;6:315. doi:10.2174/1874312901206010315
25. Arijs I, De Hertogh G, Lemmens B, et al. Effect of Vedolizumab (Anti-Α4β7-Integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut. 2018;67:43–52. doi:10.1136/GUTJNL-2016-312293
26. Liu E, Aslam N, Nigam G, Limdi JK. Tofacitinib and Newer JAK inhibitors in inflammatory bowel disease—where we are and where we are going. Drugs Context. 2022;11:1–17. doi:10.7573/DIC.2021-11-4
27. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. doi:10.1056/NEJMoa1602773
28. Bishop C, Simon H, Suskind D, Lee D, Wahbeh G. Ustekinumab in pediatric Crohn disease patients. J Pediatr Gastroenterol Nutr. 2016;63:348–351. doi:10.1097/MPG.0000000000001146
29. Reich K, Yasothan U, Kirkpatrick P. Ustekinumab. Nat Rev Drug Discov. 2009;8:355–357. doi:10.1038/nrd2878
30. Osuna CG, Gómez-Vila B, Pariente JA, et al. Ustekinumab drug survival in patients with psoriasis: a retrospective study of real clinical practice. Med. 2020;56:584. doi:10.3390/MEDICINA56110584
31. Ochsenkühn T, Tillack C, Szokodi D, Janelidze S, Schnitzler F. Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis. United Eur Gastroenterol J. 2020;8:91. doi:10.1177/2050640619895361
32. Weber J, Keam SJ. Ustekinumab. BioDrugs. 2009;23:53–61. doi:10.2165/00063030-200923010-00006
33. Johnson & Johnson Janssen Announces U.S. FDA Approval of STELARA® (Ustekinumab) for the treatment of adults with moderately to severely active ulcerative. Available from: https://www.jnj.com/janssen-announces-u-s-fda-approval-of-stelara-ustekinumab-for-The-treatment-of-adults-with-moderately-to-severely-active-ulcerative.
34. STELARA® (Ustekinumab). US FDA package insert; 2022.
35. Quaresma AB, Damiao AOMC, Coy CSR, et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in brazil: a large population-based study. Lancet Reg Heal. 2022;13:100298.
36. Juliao-Baños F, Kock J, Arrubla M, et al. Trends in the epidemiology of inflammatory bowel disease in Colombia by demographics and region using a nationally representative claims database and characterization of inflammatory bowel disease phenotype in a case series of Colombian patients. Medicine. 2021;100. doi:10.1097/MD.0000000000024729
37. Kuenzig ME, Fung SG, Marderfeld L, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. 2022;162:1147–1159.e4. doi:10.1053/j.gastro.2021.12.282
38. Singh S, Qian AS, Nguyen NH, et al. Trends in US health care spending on inflammatory bowel diseases, 1996–2016. Inflamm Bowel Dis. 2022;28:364–372. doi:10.1093/ibd/izab074
39. Su H-J, Chiu Y-T, Chiu C-T, et al. Inflammatory bowel disease and its treatment in 2018: global and Taiwanese status updates. J Formos Med Assoc. 2019;118:1083–1092. doi:10.1016/j.jfma.2018.07.005
40. Choe JY, Choi S, Song KH, et al. Incidence and prevalence trends of pediatric inflammatory bowel disease in the Daegu-Kyungpook province from 2017 to 2020. Front Pediatr. 2022;2022:1600.
41. Argyriou K, Kapsoritakis A, Oikonomou K, Manolakis A, Tsakiridou E, Potamianos S. Disability in patients with inflammatory bowel disease: correlations with quality of life and patient’s characteristics. Can J Gastroenterol Hepatol. 2017;2017. doi:10.1155/2017/6138105
42. Büsch K, Sonnenberg A, Bansback N. Impact of inflammatory bowel disease on disability. Curr Gastroenterol Rep. 2014;16:1–9. doi:10.1007/s11894-014-0414-0
43. Le Berre C, Peyrin-Biroulet L, Buisson A, et al. Impact of inflammatory bowel diseases on working life: a French nationwide survey. Dig Liver Dis. 2019;51:961–966. doi:10.1016/j.dld.2019.01.024
44. Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi:10.1136/gutjnl-2019-318484
45. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohn’s Colitis. 2020;14:4–22. doi:10.1093/ecco-jcc/jjz180
46. Singh S, Allegretti JR, Siddique SM, Terdiman JP. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158:1465–1496. doi:10.1053/j.gastro.2020.01.007
47. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn’s Colitis. 2022;16:2–17. doi:10.1093/ecco-jcc/jjab178
48. STELARA® (Ustekinumab). US FDA Package Insert 2016; 2016.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
