Back to Journals » Infection and Drug Resistance » Volume 16
Comprehensive Assessment of Colistin Induced Nephrotoxicity: Incidence, Risk Factors and Time Course
Authors Rabi R , Enaya A, Sweileh MW, Aiesh BM, Namrouti A, Hamdan ZI , Abugaber D, Nazzal Z
Received 24 February 2023
Accepted for publication 28 April 2023
Published 15 May 2023 Volume 2023:16 Pages 3007—3017
DOI https://doi.org/10.2147/IDR.S409964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Razan Rabi,1,* Ahmad Enaya,1,* Mamoun W Sweileh,1 Banan M Aiesh,2 Ashraqat Namrouti,1 Zakaria I Hamdan,1,3 Dina Abugaber,1,3 Zaher Nazzal3
1Department of Internal Medicine, An-Najah National University Hospital, Nablus, Palestine; 2Infection Control Department, An-Najah National University Hospital, Nablus, Palestine; 3Department of Medicine, Faculty of Medicine and Health Sciences, An-Najah National University, Nablus, Palestine
*These authors contributed equally to this work
Correspondence: Zaher Nazzal; Dina Abugaber, Faculty of Medicine and Health Sciences, An-Najah National University, P.O. Box 7, Nablus, Palestine, Tel +970599545421, Fax +97092342905, Email [email protected]; [email protected]
Purpose: In recent years, the emergence of multidrug-resistant (MDR) microorganisms had caused the resurgence of colistin use after it was previously abandoned due to its side effects, nephrotoxicity in particular. However, the specific incidence of colistin-induced nephrotoxicity varies in reports with different populations. This study aims to assess the incidence of colistin-associated nephrotoxicity and the associated risk factors.
Patients and Methods: This study was on 178 patients who received colistin for more than 48 hours during the years 2019– 2022, who were followed up for 14 days after the initiation of colistin, and demographic and clinical data were gained from medical reports. Logistic regression was used to assess the relationship between nephrotoxicity and study variables.
Results: The incidence of nephrotoxicity was 44.9% (95% confidence interval (CI); 37% to 53%), and the overall mortality was 33%, with a significantly higher level among patients with nephrotoxicity. The significant risk factors for nephrotoxicity after adjustment were; higher weights (OR = 1.1, 95% CI; 0.03– 1.2), P-value: 0.006, and the combination with carbapenem showed a significant protective effect (OR = 0.09, 95% CI; 0.01– 0.8), P-value: 0.03. The severity, according to KDIGO classification, was stage 1 (47%), stage 2 (21%), and stage 3 (31%). Higher stages had earlier onset acute kidney injury, a lower percentage of returning to baseline, and exposure to a higher colistin dose.
Conclusion: Colistin-induced nephrotoxicity was a frequent issue associated with higher weights, mitigated by the combination with carbapenems. While higher colistin dosages, and earlier onset AKI, were linked to the progression to higher AKI stages and the need for dialysis.
Keywords: acute kidney injury, nephrotoxicity, colistin, multi-drug resistance organisms
Introduction
Colistin belongs to the family of polymyxin, which is a cationic polypeptide, that can interact with the bacteria’s outer membrane, leading to cell wall destruction, and subsequently destroying the organism. It was first discovered in the late 1940s and had FDA approval in 1962.1 However, it was abandoned shortly after its marketing began, after the rising reports of potentially serious adverse events, in terms of nephrotoxicity and neurotoxicity. However, since the beginning of the 21st century, colistin use has resurged, chiefly due to the emergence of gram-negative multi-drug resistance organisms, including Pseudomonas aeruginosa, Klebsiella Pneumoniae, Enterobacteriaceae, and Acinetobacter baumannii.2,3
The reintroduction of this compound was after the proven effectiveness and less toxicity than was reported in older studies. The reason for the lower rate of toxicity in recent studies compared to previous ones, from a side, is due to the lack of pharmacokinetic and pharmacodynamic data back then that the dosage regimen is now being designed to have the maximum efficacy with less toxicity.1 Furthermore, this nephrotoxicity is reversible and rarely causes permanent damage, in which the percentage of cases that necessitate renal replacement therapy and dialysis was about 1.1% and 2.16%, respectively.4 Though the benefit of fighting life-threatening infection outweighs colistin risk, its use is still restricted as a last resort, owing to its concerning side effects, primarily nephrotoxicity.5 For this point, research has been conducted to assess the incidence rate of colistin-related nephrotoxicity, the burden of its use in comparison with its benefits, assessing the optimal dosing and the risk factors for a higher nephrotoxicity rate.
Since the reintroduction of colistin, there is dissension about the exact incidence of its nephrotoxicity, which is widely ranged between 10% and 60% according to the literature.6 Different definitions of acute kidney injury (AKI) had been used, these include: Acute Kidney Injury Network, Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease (RIFLE), and Kidney Disease Improving Global Outcomes (KDIGO). Nephrotoxicity incidence is influenced by many risk factors including dosage, and patient-related characteristics such as age, preexisting renal disease, diabetes, hypoalbuminemia, and other concomitant nephrotoxic compounds exposure.7,8
The incidence of acute kidney injury in Palestine was assessed for other nephrotoxic medications, such as Amphotericin B,9 and among oncology patients,10 but not for colistin. This was the first study in Palestine to investigate as many aspects of colistin-induced nephrotoxicity as possible, including incidence, risk factors, confounders’ effect, and antibiotic combinations’ effect. Its findings are anticipated to provide a complete picture of the topic, aid in prevention planning, and improve prognosis. Therefore, this study aimed to provide more understanding of colistin nephrotoxicity in terms of its incidence, to weigh the magnitude of this matter. Additionally, assessing the confounding risk factors for nephrotoxicity, and the effect of colistin dosage regimens.
Materials and Methods
Design and Setting
The study adopted a retrospective cohort design that was conducted at AL-Najah National University Hospital during the years 2019–2022, by reviewing the hospital’s medical reports retrospectively. The study included all adult patients who received colistin for more than 48 hours while it excluded those who had received inhaled colistin, pregnant women, patients who had AKI at baseline and end-stage renal disease (ESRD) patients. For those who had colistin more than once, only the first time was considered. A sample size of 164 patients was needed to have an 80% power, with a 95% confidence level, assuming the incidence of colistin nephrotoxicity is 50% based on previous reports.7,11–14 We excluded a total of 85 patients who received colistin, resulting in a final sample size of 178. Patients who received colistin were excluded for a variety of reasons based on the criteria mentioned earlier, the most common of which were a history of end-stage renal disease, acute kidney injury at baseline, and having received colistin for less than 48 hours.
The Institutional Review Board (IRB) of An-Najah National University [Reference: Med. August, 2022/36] and the hospital’s local committee approved the research. The informed consent was waived by the IRB because the study utilized secondary data. The collected data was stored in password-protected computers, was accessible only to the research team, and was used solely for research purposes. The recruited patients were followed up for 14 days after the initiation of colistin.
Measures
Data that was recorded for all patients were: demographic data (age, gender, and weight), as well as hospitalization-related data, such as the cause of admission and length of stay. Comorbidities include: hypertension, diabetes mellitus, cardiovascular diseases, cerebrovascular accidents, malignancies (whether hematological or solid tumors), chronic pulmonary diseases and chronic liver disease. Concurrent use of medications, especially nephrotoxic medications (like furosemide, aminoglycosides, vancomycin, amphotericin B, Piperacillin-tazobactam, tigecycline and acyclovir), as well as exposure to contrast media. Sepsis-related data, including; the presence of septic shock or other shock types, the need for invasive ventilation or vasopressor medications, site of infection and causative microorganism. Data about colistin, such as its dose, frequency per day, duration of use and cumulative dose were recorded. The dose of colistin in our setting was given based on weight and adjusted for creatinine clearance. For normal kidney function, the usual dose was 2.5–5 mg/kg/day divided 2–3 times. The dose reported in million international units (1 million IU (MIU), is approximately equal to 80 mg colistimethate sodium or 33.3 mg colistin base activity (CBA).15
The primary outcome was defined as the occurrence of AKI after the initiation of colistin, in which the KDIGO criteria were chosen for defining and classification of AKI, that is an increase in serum creatinine by ≥0.3 mg/dl (≥26.5 mmol/l) within 48 h, or increase in serum creatinine to ≥1.5 times baseline (day zero of administering the colistin) that occurred within the previous seven days. The definition includes urine output as a marker for AKI, but as documentation for urine output was not readily available, it was not possible to collect data on urine output. Accordingly, baseline creatinine was recorded, in addition to the time of AKI since colistin started, maximum creatinine value, time of returning to baseline, and whether the patient had a persisting renal injury or ended on having renal replacement therapy.
Analysis Plan
We used the IBM SPSS Statistics software version 21 (IBM Corp., Armonk, NY, USA) for data entry and analysis. All variables were illustrated through percentages, frequencies or mean ± standard deviation. Univariate analysis was used to compare differences between dependent and independent variables (nephrotoxicity and its risk factors), with categorical data using the Pearson chi-squared test or Fisher’s exact test, while numerical data was based on normality testing using either the independent T-test or the Mann–Whitney U-test as appropriate. To account for the potential confounding influence of additional nephrotoxic causes, we performed multivariable analysis using the binary logistic regression model. Furthermore, the Kaplan–Meier curve was used to illustrate the time of AKI and compare empiric vs culture-based use of colistin. A p-value less than 0.05 was chosen as a significant cutoff.
Results
A total of 178 patients, who had colistin between the years 2019 and 2022, were included in the study. Among them, 53% were in the ICU, 38% were mechanically ventilated and 27% were in shock. The majority were patients with malignant diseases (62%), with hematological ones accounting for the majority (67% of total malignancies). The mean age ± SD was 48 ± 18 years, and the majority were males (58%). The three most reasons for colistin use were pneumonia, sepsis, and neutropenic fever accounting for 30%, 25%, and 12%, respectively. The infection site, based on culture and clinical picture, was respiratory in the first place (37%), then blood-stream (28%), urinary tract (11%) and wound (12%). Moreover, A. baumanni, K. pneumoniae and P. aeruginosa were the most organisms that colistin was indicated for. Besides, 28% of patients had an empirical colistin use (with negative culture). Baseline characteristics are illustrated in Table 1.
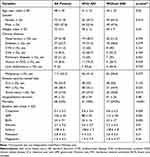 |
Table 1 Baseline Characteristics with Univariate Analysis for Comparison Between Patients with and without Acute Kidney Injury |
The incidence of AKI was 44.9% (95% confidence interval (CI); 37.0% to 53.0%) and its severity according to KDIGO classification was stage 1 (47%), stage 2 (21%) and stage 3 (31%). Among patients with AKI; 8% ended in dialysis, while 33.8% returned to their baseline creatinine (Figure 1). The overall mortality patients were 33% (Figure 1). Of the deceased patients, 70% had nephrotoxicity, which was significantly associated in univariate analysis, P-value <0.001 (Table 1), even after adjusting for age, shock state, underlying malignancy, mechanical ventilation and hypokalemia, the adjusted odds ratio for nephrotoxicity was 4.5 (95% CI; 2.1–9.4).
 |
Figure 1 Incidence of main outcomes among colistin patients. |
All baseline characteristics were compared between patients with and without AKI using univariate analysis (Table 1 and Table 2). Significant differences were found in: older ages (P-value: 0.02), male gender (P-value: 0.019), higher body weights (P-value: 0.04), history of chronic kidney disease (P-value: 0.029), subsequently higher baseline kidney functions, being mechanically ventilated (P-value: 0.03), being in shock (P-value: 0.019), use of furosemide (P-value: 0.038) and lower albumin levels (P-value: 0.01).
 |
Table 2 Infection, Colistin Dosing and Other Nephrotoxic Factors Related Data, and Comparison Between AKI and Non-AKI Group |
In multivariate analysis, as shown in Table 3, the significant variables after adjustment were only; higher weights (OR = 1.1, 95% CI; 0.03–1.2), P-value: 0.006, and the combination with carbapenem showed a protective significant effect (OR = 0.09, 95% CI; 0.01–0.8), P-value: 0.03.
 |
Table 3 Multivariate Analysis of Nephrotoxicity Risk Factors |
When comparing stages of AKI, the time to develop AKI in higher stages was earlier, as mean time in days were as the following 6.4 ± 3, 5.8 ± 2 and 5.4 ± 3 in stage 1, stage 2 and stage 3, respectively. In addition, returning to baseline creatine was less observed in stages 2 and 3 had (28% and 23% in stages 2 and 3 respectively) compared to stage 1 (52%). Moreover, patients with higher stages of AKI were exposed to a higher dose of colistin, for which the mean dose in stage 3 compared to the lower stages was 7.9 ± 1.5, 6.7 ± 2.4, with a P-value of 0.002 (Table 4).
 |
Table 4 Stages of Acute Kidney Injury and Related Data |
The mean time for AKI onset was 5.97 ± 2.93 days, while the mean time for returning to baseline was 7 ± 8 days (Figure 2). Nephrotoxicity tends to occur in days between two to nine of colistin utilization, as 30% occurred by day three, 50% by day five, and 90% by day 9. About 28% of all patients were treated empirically (who had negative cultures).
 |
Figure 2 Time course of colistin nephrotoxicity and time of creatinine normalization, form colistin start. |
The timing of AKI was compared between the empirically treated group and those with culture-based pathogens, the result showed that the time to develop AKI after treatment with colistin was 4.2+ 2 days versus 6.6 +0.39 (P-value 0.001) for patients with a negative culture result, and those who were treated with colistin with a positive culture result, respectively (Figure 3).
 |
Figure 3 Comparing the percentage of patients, who had no acute kidney injury over time, between patients treated with negative culture (empirically) and positive culture. |
Protective strategies were assessed in terms of their efficacy in reversing nephrotoxicity and results yielded no significance. These protective strategies were compared between stage 3 AKI and lower stages and showed, as well, no significant effect w except for the use of intravenous fluids (P-value = 0.04) (Table 5).
 |
Table 5 The Effect of Protective Strategies on Reversing Nephrotoxicity, the Severity of AKI and Mortality |
Discussion
The incidence of colistin-related nephrotoxicity in the literature ranged from 20% to 70%4,11–14 and this wide range in AKI incidence is attributed to many reasons. One was related to AKI definitions that were adopted in different studies, as the majority of older studies had used the RIFLE or the AKIN criteria for the definition of AKI, however, more recent studies the KDIGO criteria used. For this discrepancy in definitions, rates vary remarkably. So, if one compared the incidence in studies with different definitions, The AKI incidence in studies that used the RIFLE criteria ranged from 25% to 46%,6,12,14,16 which is comparable to studies that used the KDIGO criteria that showed an incidence from 50% to 70%.7,11,13 The higher incidence of AKI in the KDIGO studies is likely due to the lower threshold of AKI definitions, as a baseline increase in creatinine of 1.5 from baseline is considered as AKI stage 1, while it is considered AKI risk in the RIFEL definition.
This variation also existed, due to the underlying patients’ characteristics, in which a poorer condition of patients will reflect a higher incidence of AKI,8,14 as incidence reached 70% in a study conducted among a population of critically ill patients.11 In this study, the majority were patients with malignancies, half of them were in ICU, and a quarter of patients were in shock mostly septic. Moreover, the inclusion of preexisting renal impairment, as studies that had included this group in their analysis, had a higher AKI incidence.7 The incidence of nephrotoxicity in this study is a bit lower than the incidence range of the KDIGO studies, despite that our patients had a poor overall condition of patients and involved patients with chronic kidney disease.
The incidence of nephrotoxicity could not be attributed to colistin alone, as this patient’s population usually vulnerable to many nephrotoxic agents. In such instance, Garnacho-Montero et al found that the combination of colistin with vancomycin had a significantly higher risk for nephrotoxicity,17 while in other cohorts, it was similar to our finding, which showed no association.13,14,18 However, in this study, none of the other nephrotoxic factors showed a significant effect in the multivariate analysis, which could be attributed to the small sample size, a combined rather than independent effect in contributing to nephrotoxicity, or that these factors had no true association. The only factors related to higher nephrotoxicity in this study were weight, and combination with carbapenems. For the former, it would be explained that a higher weight would result in a higher dose of colistin. In Almutairy et al study, results showed for each 1 mg/kg/day of colistin, the odds of AKI would increase 1.6 times.19 Nevertheless, this explanation is not logical to our data, since the dose of colistin, whether the daily total dose or cumulative dose failed to show a significant effect in developing nephrotoxicity in our study. Whether this weight effect related to obesity and its association with higher morbidity and mortality, is possible, but for the lack of height data, this could not be studied. Yet, it may have an effect in progressing to a higher stage of AKI compared to patients with a low dose as presented in this study. Loading dose of colistin, was investigated in some studies, as in Katip et al results, the loading dose was found to be associated with a better clinical response, however, led to a higher incidence of nephrotoxicity,20 while this association with nephrotoxicity was not seen in other studies.13 Unfortunately, in our study, data about loading dose were not available. While other related factors, including older age, and having comorbidities were not significant, contrary to some studies, as other studies showed significant relation.7,8,14
Colistin combination with carbapenem appeared to decrease nephrotoxicity in this study, compared to colistin monotherapy. This was previously investigated in the literature, with no clear association established yet, though most studies agreed that it had no increased risk for nephrotoxicity,21,22 some reports had proven a significant protective effect from nephrotoxicity, as a meta-analysis, showed that this combination had significantly lowered the nephrotoxicity rate (OR = 1.57, 95% Cl 1.30–2.95: P = 0.001), while this effect not seen in other combinations such as sulbactam, aminoglycoside, glycopeptide or others.5 This combination may provide a synergistic effect against the multidrug organism, thus can decrease drug-resistant bacteria, for which it has significant improvement in clinical response and reduction in mortality,22,23 However the renal protective mechanism of this combination is unclear. But a possible explanation is that it leads to a better clinical response and improves the underlying sepsis that is worsening renal function. However, further studies are needed to assess the renal protective effect for this combination, and illustrate the underlying mechanism. Albumin level was assessed previously and showed a protective effect, as patients with high baseline serum albumin levels were 0.69 times less likely to experience nephrotoxicity compared to those with low serum albumin.4 One reason is that Polymyxins can bind to the partially unfolded intermediate forms of albumin and thus can decrease its side effects.24 In this study, although a difference was present, it could not reach a significance level, for that, a larger sample size study would better assess this relation.
The importance of determining the risk factors, especially the modifiable ones, and determining the appropriate dosing regimen, not only results in the reduction of nephrotoxicity risk, but also the overall mortality. However, this claim had been argued for many factors existed among this population of patients that would result in higher mortality itself. Therefore, in this study, we tried to adjust as much for common confounders, and yet the association was significant. This was also supported in that the discontinuing of colistin or reducing the dose was associated with less toxicity accordant with previous studies that had shown this association as well,13 though, in many reports, this association was lost after adjusting for confounders.11,14
The duration of therapy that led to nephrotoxicity, is not well determined; nonetheless, the longer duration would suggest a higher nephrotoxic risk, its high intrinsic tubular toxicity suggests that renal toxicity was supposed to occur rapidly. In literature, the time of AKI ranged between 5 and 12 days.1,7 However, a recent report found that 77% of renal toxicity occurred in the first 9 days, of which 32% were within 3 days and 58% were within 6 days. This study supports previous findings in that colistin-induced nephrotoxicity tended to occur within the first week, as the mean time was 6 ± 2 days.13 Despite this, a comparison study was made between short and long duration of colistin therapy, which showed no significant difference with nephrotoxicity, but a short duration was significantly associated with higher mortality.25
The reversibility of colistin-induced nephrotoxicity was not complete in this study, as only 34% of patients returned to their baseline creatinine. This was similar to the results of previous studies, as the reversibility rate of AKI in Miano et al's study was 44%,7 and in Çiftçi et al’s study was 31%.11 Colistin cause reversible renal damage according to literature, 11 but this low incidence, could be an underestimation of this value, as most patients were discharged and a follow-up creatinine was not gained to assess resolution. Furthermore, the underlying sepsis may lead to irreversible damage itself. Protective strategies, in the form of colistin discontinuation, dose reduction, or discontinuation of nephrotoxic medications, showed no effect in reversing renal injury in this study, indicating either irreversible damage that had occurred or other factors were playing a role in causing kidney injury. Moreover, these measures also failed to reverse the progression of kidney injury, except for the increasing the intravenous fluid, as it helps in any cause of pre-renal injury, and part of sepsis management, in which adequate hydration with prevention of volume overload, it is a known effective measure to prevent kidney injury in general among critically ill patients.26
This study was limited by the following points: First, this was an observational study, relying on medical records, for which it was exposed to missing data and is more prone to confounding. For instance, the arterial blood gas (ABG) paper result was not recorded electronically, for this reason, neither APACHE nor SOFA scores were possible to be calculated. Moreover, the loading dose of colistin was not recorded electronically so its effect was not studied. Secondly, the weight measures, among ICU patients were not so accurate, as were based on estimation rather than true weight, questioning their precision. Furthermore, the inclusion of patients with chronic kidney disease would overestimate the prevalence and the nature of patients being critically ill, many were immunocompromised and had severe sepsis, for which they were more prone for multiorgan failure. Additionally, the small sample size and the absence of a control group, being a single center study, its retrospective design, were also important limitations.
Conclusion
In conclusion, colistin-induced nephrotoxicity is a concerning matter with the rising incidence of multidrug-resistant pathogens that necessitated colistin use. Even with the risk of nephrotoxicity, it is essential for physicians and health-care professionals to be aware of its associated risk factors, and finding the appropriate measures to prevent it, as this would result in less morbidity, mortality, and providing the most effective therapy with the less risks. Our study showed an incidence of nephrotoxicity reaching 45% with a mean time of AKI about 6 days after colistin use and a return to baseline kidney function after a mean of 7 days. In this study, the appropriate dosing regimen, in combination with meropenem as appropriate would be recommended, with the need for further clinical trials.
Abbreviations
AKI, Acute Kidney Injury; ESRD, End-stage renal disease; IRB, Institutional Review Board; KDIGO, Kidney Disease Improving Global Outcomes; RIFLE, Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All procedures in this study were carried out in accordance with the Helsinki Declaration. The study was approved by An-Najah National University’s Institutional Review Board (IRB) [Reference: Med. August, 2022/36], and appropriate permissions were obtained from AL-Najah National University Hospital. Because the study used secondary data, the IRB waived informed consent. The confidentiality of participants’ data was ensured, and all data collected was used only for research purposes.
Acknowledgments
We would like to thank Pharmacy department at An-Najah National University Hospital for their assistance and contributions in facilitating the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding received for this study from any source.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Falagas ME, Kasiakou SK, Saravolatz LD. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis an off Publ Infect Dis Soc Am. 2005;40(9):1333–1341. doi:10.1086/429323
2. Panigrahi K, Pathi BK, Poddar N, et al. Colistin resistance among multi-drug resistant gram-negative bacterial isolates from different clinical samples of ICU patients: prevalence and clinical outcomes. Cureus. 2022;14(8):e28317. doi:10.7759/cureus.28317
3. Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22(6):535–543. doi:10.1097/QCO.0b013e328332e672
4. Sisay M, Hagos B, Edessa D, Tadiwos Y, Mekuria AN. Polymyxin-induced nephrotoxicity and its predictors: a systematic review and meta-analysis of studies conducted using RIFLE criteria of acute kidney injury. Pharmacol Res. 2021;163:105328. doi:10.1016/j.phrs.2020.105328
5. Chien H-T, Lin Y-C, Sheu -C-C, Hsieh K-P, Chang J-S. Is colistin-associated acute kidney injury clinically important in adults? A systematic review and meta-analysis. Int J Antimicrob Agents. 2020;55(3):105889. doi:10.1016/j.ijantimicag.2020.105889
6. Oliota AF, Penteado ST, Tonin FS, Fernandez-Llimos F, Sanches AC. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis. 2019;94(1):41–49. doi:10.1016/j.diagmicrobio.2018.11.008
7. Miano TA, Lautenbach E, Wilson FP, Guo W, Borovskiy Y, Hennessy S. Attributable risk and time course of colistin-associated acute kidney injury. Clin J Am Soc Nephrol CJASN. 2018;13(4):542–550. doi:10.2215/CJN.06980717
8. Özkarakaş H, Köse I, Zincircioğlu Ç, et al. Risk factors for colistin-associated nephrotoxicity and mortality in critically ill patients. Turkish J Med Sci. 2017;47(4):1165–1172. doi:10.3906/sag-1604-60
9. Abdel-Hafez Y, Siaj H, Janajri M, et al. Tolerability and epidemiology of nephrotoxicity associated with conventional amphotericin B therapy: a retrospective study in tertiary care centers in Palestine. BMC Nephrol. 2022;23(1):132. doi:10.1186/s12882-022-02770-2
10. Nazzal Z, Abdeljaleel F, Ashayer A, Salameh H, Hamdan Z. The rate and risk factors of acute kidney injury among cancer patients’ admissions in Palestine: a single-center study. Int J Nephrol. 2022;2022:2972275. doi:10.1155/2022/2972275
11. Ciftci A, Izdes S, Altintas ND. Factors determining nephrotoxicity and mortality in critical care patients receiving colistin. J Infect Dev Ctries. 2018;11(12):912–918. doi:10.3855/jidc.9443
12. Rashizal Sazli MR, Syed Mohamed AF, Wan Mazuan WM, Ling SM, Mahmud A, Amin Nordin S. Colistin-associated nephrotoxicity among patients in intensive care units (ICU) of hospitals in Selangor. Med J Malaysia. 2017;72(2):100–105.
13. Gunay E, Kaya S, Baysal B, Yuksel E, Arac E. Evaluation of prognosis and nephrotoxicity in patients treated with colistin in intensive care unit. Ren Fail. 2020;42(1):704–709. doi:10.1080/0886022X.2020.1795878
14. Hassan MM, Gaifer Z, Al-Zakwani IS. Incidence and risk factors of nephrotoxicity in patients on colistimethate sodium. Int J Clin Pharm. 2018;40(2):444–449. doi:10.1007/s11096-018-0607-y
15. Grayson ML, Cosgrove SE, Crowe S, et al. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic, and Antiviral Drugs. Vol. 3.
16. Hartzell JD, Neff R, Ake J, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis an off Publ Infect Dis Soc Am. 2009;48(12):1724–1728. doi:10.1086/599225
17. Garnacho-Montero J, Amaya-Villar R, Gutiérrez-Pizarraya A, et al. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy. 2013;59(3):225–231. doi:10.1159/000356004
18. Katip W, Oberdorfer P. Clinical efficacy and nephrotoxicity of colistin alone versus colistin plus vancomycin in critically ill patients infected with carbapenem-resistant Acinetobacter baumannii: a propensity score-matched analysis. Pharmaceutics. 2021;13(2):162. doi:10.3390/PHARMACEUTICS13020162
19. Almutairy R, Aljrarri W, Noor A, et al. Impact of colistin dosing on the incidence of nephrotoxicity in a tertiary care hospital in Saudi Arabia. Antibiotics. 2020;9(8):485. doi:10.3390/antibiotics9080485
20. Katip W, Uitrakul S, Oberdorfer P. Clinical efficacy and nephrotoxicity of the loading dose colistin for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients. Pharmaceutics. 2022;14(1):1266. doi:10.3390/pharmaceutics14010031
21. Falagas ME, Rafailidis PI, Kasiakou SK, Hatzopoulou P, Michalopoulos A. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin–meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin Microbiol Infect. 2006;12(12):1227–1230. doi:10.1111/j.1469-0691.2006.01559.x
22. Katip W, Uitrakul S, Oberdorfer P. A comparison of colistin versus colistin plus meropenem for the treatment of carbapenem-resistant Acinetobacter baumannii in critically ill patients: a propensity score-matched analysis. Antibiot. 2020;9(10):647. doi:10.3390/ANTIBIOTICS9100647
23. Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73(1):22–32. doi:10.1093/jac/dkx368
24. Poursoleiman A, Karimi-Jafari MH, Zolmajd-Haghighi Z, et al. Polymyxins interaction to the human serum albumin: a thermodynamic and computational study. Spectrochim Acta Part A Mol Biomol Spectrosc. 2019;217:155–163. doi:10.1016/J.SAA.2019.03.077
25. Katip W, Uitrakul S, Oberdorfer P. Short-course versus long-course colistin for treatment of carbapenem-resistant A. baumannii in cancer patient. Antibiot. 2021;10(5):484. doi:10.3390/ANTIBIOTICS10050484
26. Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the working group on prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43(6):730–749. doi:10.1007/s00134-017-4832-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
