Back to Journals » OncoTargets and Therapy » Volume 14
Complete Pathologic Response of Multiple Liver Metastases and Clinical Complete Response of Rectal Cancer in a Patient with Ataxia-Telangiectasia Mutated Gene Mutations After XELOXIRI Plus Bevacizumab: A Case Report
Authors Cheng Y, Wu G, Zhang S, Liu Y , Qu J, Qu X
Received 18 May 2021
Accepted for publication 7 July 2021
Published 15 July 2021 Volume 2021:14 Pages 4201—4209
DOI https://doi.org/10.2147/OTT.S320477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Yu Cheng,1– 4 Gang Wu,5 Simeng Zhang,1– 4 Yunpeng Liu,1– 4 Jinglei Qu,1– 4 Xiujuan Qu1– 4
1Department of Medical Oncology, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 2Key Laboratory of Anticancer Drugs and Biotherapy of Liaoning Province, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 3Liaoning Province Clinical Research Center for Cancer, Shenyang, People’s Republic of China; 4Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Ministry of Education, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 5Department of Hepatobiliary Surgery, The First Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Xiujuan Qu; Jinglei Qu People’s Republic of China
Tel +86-24-83282312
Fax +86-24-83282543
Email [email protected]; [email protected]
Background: Doublet or triplet chemotherapy plus or minus targeted drugs can achieve a high objective response rate (ORR) and are currently considered to be the backbone of conventional therapy for liver metastatic colorectal cancer (mCRC). However, current biomarkers (such as UGT1A1 and DPYD) are limited to the prediction of toxicity and there are no effective biomarkers to predict chemotherapy response. Therefore, personalized cancer chemotherapy underpinned by genomic alterations in mCRC has received increasing attention.
Case Presentation: We present a case of a 28-year-old female rectal cancer patient with multiple liver metastases (clinical risk score, CRS = 5 points). The patient underwent XELOXIRI plus bevacizumab regimen that consisted of irinotecan (150 mg/m2), oxaliplatin (100 mg/m2) on day 1, capecitabine (1700 mg/m2 per day from day 2 to 15), bevacizumab (7.5 mg/kg) on day 1 (on the second cycle), given every three weeks for eight cycles. After multi-disciplinary team (MDT) discussion, the patient underwent right hemihepatectomy, partial liver resection of segment IV and cholecystectomy. Surprisingly, the patient achieved a complete pathologic response (pCR) of the hepatic metastasis and clinical complete response (cCR) of the primary rectal lesion. A paired tumor molecular profile revealed somatic mutations in ataxia-telangiectasia mutated (ATM) genes that may explain why the patient achieved such a dramatic tumor response. Treatment was discontinued after eight cycles of a single oral dose of capecitabine and the patient started a follow-up program of physical and radiological examinations. To monitor the signs of recurrence, we also obtained blood samples to analyze circulating tumor DNA (ctDNA). To date, the patient has remained disease-free.
Conclusion: The XELOXIRI-bevacizumab regimen is a feasible and effective regimen for patients with mCRC. Mutations in the ATM genes may characterize a subset of patients with a better prognosis who are more sensitive to chemotherapy plus biological agents.
Keywords: XELOXIRI, bevacizumab, ATM, case report, rectal cancer
Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer-related death throughout the world and the incidence amongst adolescents and young adults has increased significantly in recent years.1 The liver is the dominant site of metastasis for CRC. Imai et al have reported that 79% of patients who underwent curative hepatectomy developed recurrence and the optimal cutoff point of early recurrence was eight months.2 When surgery is not possible, systemic chemotherapy is the preferred treatment option. Several recent studies have reported that chemotherapy regimens including (oxaliplatin, irinotecan, and 5-fluorouracil (5-FU)) plus bevacizumab or an anti-EGFR antibody can improve response rates as well as progression-free survival (PFS) and overall survival (OS) in mCRC. However, the triplet chemotherapy regimen is associated with significant levels of treatment-related toxicity. Therefore, a predictive biomarker that can help patients avoid ineffective and potentially toxic systemic chemotherapies would be highly beneficial.
Evidence from recent studies have demonstrated that K-RAS/N-RAS and BRAF mutations are associated with the efficacy of anti-EGFR agents,3 VEGF-A and SNPs of VEGF/VEGFR pathway may be used as predictors of clinical outcome to anti-VEGF treatment,4,5 and dMMR/MSI-H status is a biomarker predictive of response to anti–programmed death-1 (PD-1) checkpoint inhibitors in metastatic CRC.6 Pre-therapeutic genotyping of UGT1A1 and DPYD genotyping could potentially reduce the toxicities induced by irinotecan and 5-FU.7 In pancreatic cancer, the validity for targeting BRCA with platinum has been established; however, there are limited effective biomarkers for predicting chemosensitivity in CRC. Studies have shown that mutations in genes involved in DNA repair deficiency, including ATM, are associated with enhanced chemosensitivity in ovarian cancer.8 In ATM-deficient CRC, oxaliplatin-based therapy is associated with superior OS.9
In this case study, we report a K-RAS/ATM mutation rectal cancer patient with multiple liver metastases (CRS = 5 points) who had a pCR of hepatic metastasis and cCR of primary rectal cancer after XELOXIRI plus bevacizumab. The patient was disease-free at 37 months after treatment at the last follow-up in May 2021.
Case Description
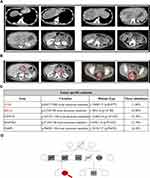
A 28-year-old female patient with a familial tumor history complaining of hematochezia was referred to our hospital in April 2018. Colonoscopy revealed ulcerative lesions in the rectum 7–10 cm from the anal verge with an increased CEA level of >1000 ng/mL. Pathological inspection demonstrated poorly differentiated adenocarcinoma. Enhanced abdominal computed tomography (CT) showed multiple lesions in the liver that were suspected as metastases (Figure 1A). We also found the enlargement of the liver portal and retroperitoneal lymph nodes (Figure 1B). The clinical stage was cT4aN+M1 (multiple liver metastases, liver portal and retroperitoneal lymph nodes metastasis) according to AJCC version 8.0.
A paired tumor molecular profile indicated microsatellite stability and somatic mutations of K-RAS, ATM, PARP1, MAP3K1 and FANCG but showed no mutations in N-RAS/PIK3CA/BRAF. We also detected no UGT1A1*6/UGT1A1*28/DPYD mutations and investigated family tumor history of the patient (Figure 1C and D). After a multi-disciplinary team (MDT) discussion, the patient was young in good performance status and surgical resection was not indicated. The treatment strategy was to minish burthen of the tumor and increase overall survival by systemic chemotherapy. The patient underwent a XELOXIRI plus bevacizumab regimen consisting of irinotecan (150 mg/m2), oxaliplatin (100 mg/m2) on day 1, capecitabine (1700 mg/m2 per day from day 2 to 15), bevacizumab (7.5 mg/kg) on day 1 (on second cycle), given every three weeks. The treatment was well tolerated and major toxicities during the whole treatment were G1 vomiting and neurotoxicity and G3 neutropenia. We administered recombinant human granulocyte colony stimulating factor (G-CSF) in preventing neutropenia during chemotherapy and the dose of TRIPLET regimen was not modified.
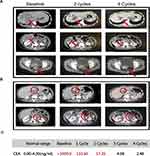
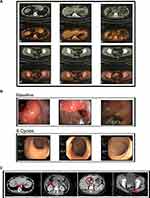
Two and four cycles after the beginning of therapy, enhanced abdominal CT showed significant tumor regression of liver metastasis and primary rectal lesions (Figure 2A). The sizes of the liver portal and retroperitoneal lymph nodes were stable (Figure 2B) and tumor markers (CEA) returned to normal (Figure 2C). After six cycles of treatment, positron emission tomography-computed tomography (PET-CT) showed that the tumor had continued to shrink with a maximum standard uptake value (SUV) of 8.1 for the liver metastasis and 2.1 for the rectal lesion (Figure 3A). There were no signs of other parts metastatic lesions. The patient then finished eight cycles of XELOXIRI plus bevacizumab. Colonoscopy showed thickening of the rectal mucosa with a slightly rough surface about 8 cm from the anal margin and the remaining mucosa was smooth (Figure 3B). Enhanced abdominal CT showed the size of the liver metastasis continued to shrink (Figure 3C).
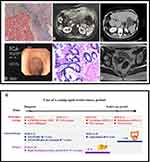
On November 20, 2018, after the decision of the MDT group, the patient underwent right hemihepatectomy, partial liver IV resection and cholecystectomy. The intraoperative findings were a 6.0×4.0 x 4.0 cm mass on the surface of sections VII, VIII and IV of the liver, and a 5.0×4.0 × 4.0 cm mass on the surface of section VI of the liver. Pathology showed the absence of neoplastic cells in all of the examined specimens (Figure 4A). Treatment was then discontinued after eight cycles of a single oral dose of capecitabine and the patient started a follow-up program with a physical examination and blood tests were performed every three months and an abdominal and chest CT every 3–6 months (Figure 4C). Colonoscopy and rectal or liver magnetic resonance imaging (MRI) were also performed every six months (Figure 4B and D–F). To monitor the signs of recurrence, we also obtained blood samples to analyze circulating tumor DNA (ctDNA) in May 21, 2019 and April 13, 2020. The results were negative. The patient remained free of disease and the duration of OS (from time of diagnosis to present) was more than three years (Figure 4G).
Discussion
To the best of our knowledge, this is the first case of the successful treatment in a patient with cT4aN+, multiple liver metastases, liver portal and retroperitoneal lymph nodes metastasis and K-RAS/ATM gene mutation rectal cancer. The treatment achieved a pCR of the liver metastasis and a cCR of the primary rectal cancer after eight cycles of treatment of XELOXIRI plus bevacizumab. After nearly three years, the patient currently shows no evidence of recurrence or progression of the disease.
We found tumor truncation mutants (p.R457*10th exon) in the ATM gene. ATM is a member of the highly conserved PI3K-related kinases, a master regulator of DNA damage response (DDR) and cell-cycle checkpoint activation, and it is mutated in approximately 15% of CRC.10 Tumors with DNA repair-deficient mutations such as ATM are predicted to have enhanced chemosensitivity to agents such as platinum salts or topoisomerase inhibitors.11 McGillivray et al recently reported a patient with locally advanced colon cancer who had a germline ATM mutation and exhibited a complete response to neoadjuvant FOLFOXIRI plus panitumumab.12 In mCRC, patients with ATM-mutant tumors are independently associated with longer OS.10 These studies suggest that ATM-mutant colorectal cancer may represent a subset of mCRC patients that have a better prognosis. Preclinical studies have demonstrated that ATR (ataxia telangiectasia and Rad 3-related, an essential DNA damage signaling kinase) inhibitors can synergize with cisplatin to kill ATM-deficient CRC cells.13 ATM may therefore be another predictive biomarker for response to ATR inhibitor therapy in CRC.
Compared to standard doublet chemotherapy, triplet chemotherapy (oxaliplatin, fluorouracil, irinotecan) was first evaluated in patients with metastatic colorectal cancer (mCRC) in the early 2000s and resulted in significant anti-tumor activity.14 This may have led to a considerably higher rate of successful resections particularly for R0 resections in liver-limited metastatic disease. Recently, systemic conversion therapy has emerged for the use of triplet chemotherapy agents alone or in combination with monoclonal antibodies targeting angiogenesis or agents that act on the EGFR signaling pathway. The FOLFOXIRI-bevacizumab combination has been assessed in two large Phase III randomized clinical trials. The Triplet plus Bevacizumab (TRIBE) and (TRIBE2) trial showed that the triplet combination plus bevacizumab regimen is associated with statistically significantly improved ORR as well as improved PFS and OS.15,16 The XELOXIRI regimen with 5-FU substituted by capecitabine has the advantages of being convenient and reducing complications related to the central venous catheters utilized in the FOLFOXIRI regimen.17 The XELOXIRI-bevacizumab regimen is also a feasible and active regimen for patients with mCRC regardless of RAS state.18 For the conversion therapy in patients with RAS/BRAF wild-type mCRC, the XELOXIRI-cetuximab regimen can also achieve a high ORR.11 Based on mutations in the KRAS gene, these lines of evidence and clinical experience, the patient was treated with XELOXIRI-bevacizumab for eight cycles. Surprisingly, the patient manifested a tremendous therapeutic effect and obtained a pCR of liver metastasis. More importantly, PET-CT revealed no signs of further metastasis.
Currently, we do not have an accurate technique to predict pCR in patients without surgical resection. The methods are mainly performed using rectal examination, MRI, endoscopy, biopsies and molecular markers.19,20 We used digital rectal examination, endoscopy, biopsies, MRI and blood plasma ctDNA to monitor the patients to determine whether they were achieving a pCR and benefit from a watch and wait strategy. The watch and wait strategy is a novel management strategy in patients with rectal cancer who have cCR after neoadjuvant chemoradiotherapy. The International Watch & Wait Database suggests that the risk of local regrowth and systemic recurrence in most patients with cT1-3 stage who have a sustained cCR for three years is 5% or less.21 Previous studies have shown that perioperative chemotherapy plus biological agents without radiation have a pCR of 6.6–25% and a low rate of local recurrence with good long-term outcomes for locally advanced rectal cancer (LARC).22,23 The patient did not have primary rectal cancer radiotherapy or resection for the following reasons: (1) the patient was diagnosed with rectal carcinoma with synchronous multiple liver metastases. After eight cycles of XELOXIRI plus bevacizumab, the patient achieved a pCR of liver metastasis and a cCR of rectal cancer and so we adopted a watch and wait strategy to monitor the patient; (2) the young patient was not pregnant and was interested in preserving fertility potential; (3) the young patient had a high and complete requirement on living quality. Furthermore, previous studies have evaluated the preferable prognostic factors after hepatectomy for colorectal liver metastases such as age <65 years, lymph node metastases (-), extrahepatic disease (-), prehepatectomy CEA ≤ 7.6 ng/mL, margin R0, BRAF mutation (-).24 We found two major poor prognostic markers in this case, tumor number (multiple) and KRAS mutation. Fortunately, the patient remained disease-free after more than three years.
Conclusions
We present the case of a 28-year-old female rectal cancer patient with multiple liver metastases and a K-RAS/ATM mutations that exhibited a high sensitivity to XELOXIRI plus bevacizumab therapy. Patients with ATM mutations are mainly associated with a better response to oxaliplatin, and in combination with the favorable genetics for irinotecan and 5-fu and are able to receive all cycles with full doses and achieve great results. However, this is just a case report and there are large individual differences in response to hypnosis. Further randomized Phase 2/3 studies are warranted to determine if ATM-mutant tumors are sensitive to chemotherapy plus biological agents, which could lead to more personalized approaches in the management of CRC.
Consent for Publication
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Funding
The present study was supported by National Natural Science Foundation of China (No. 81803092), China Postdoctoral Science Foundation (No. 2018M641747).
Disclosure
The authors declare that they have no competing interests.
References
1. Dekker E, Tanis P, Vleugels J, Kasi P, Wallace MJL. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi:10.1016/S0140-6736(19)32319-0
2. Imai K, Allard M, Benitez C, et al. Early recurrence after hepatectomy for colorectal liver metastases: what optimal definition and what predictive factors? Oncologist. 2016;21(7):887–894. doi:10.1634/theoncologist.2015-0468
3. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi:10.1093/annonc/mdw235
4. Papachristos A, Kemos P, Katsila T, et al. VEGF-A and ICAM-1 gene polymorphisms as predictors of clinical outcome to first-line bevacizumab-based treatment in metastatic colorectal cancer. Int J Mol Sci. 2019;20(22):22. doi:10.3390/ijms20225791
5. Loupakis F, Cremolini C, Yang D, et al. Prospective validation of candidate SNPs of VEGF/VEGFR pathway in metastatic colorectal cancer patients treated with first-line FOLFIRI plus bevacizumab. PLoS One. 2013;8(7):e66774. doi:10.1371/journal.pone.0066774
6. Overman M, Lonardi S, Wong K, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi:10.1200/JCO.2017.76.9901
7. Bruera G, Ricevuto E. Pharmacogenomic assessment of patients with colorectal cancer and potential treatments. Pharmgenomics Pers Med. 2020;13:601–617. doi:10.2147/PGPM.S253586
8. Pennington K, Walsh T, Harrell M, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–775. doi:10.1158/1078-0432.CCR-13-2287
9. Bakkenist C, Lee J, Schmitz J. ATM is required for the repair of oxaliplatin-induced DNA damage in colorectal cancer. Clin Colorectal Cancer. 2018;17(4):255–257. doi:10.1016/j.clcc.2018.09.001
10. Randon G, Fucà G, Rossini D, et al. Prognostic impact of ATM mutations in patients with metastatic colorectal cancer. Sci Rep. 2019;9(1):2858. doi:10.1038/s41598-019-39525-3
11. Sato Y, Hirakawa M, Ohnuma H, et al. A triplet combination with capecitabine/oxaliplatin/irinotecan (XELOXIRI) plus cetuximab as first-line therapy for patients with metastatic colorectal cancer: a dose escalation study. Cancer Chemother Pharmacol. 2017;80(6):1133–1139. doi:10.1007/s00280-017-3458-7
12. McGillivray E, Farma J, Savage M, Hall M, Luo B, Jain R. Pathologic complete response in patient with ATM mutation after neoadjuvant FOLFOXIRI plus panitumumab therapy for locally advanced colon cancer: a case report. Clin Colorectal Cancer. 2021;20(2):e96–e99. doi:10.1016/j.clcc.2020.09.004
13. Combès E, Andrade A, Tosi D, et al. ATR inhibition of ataxia-telangiectasia mutated and RAD3-related (ATR) overcomes oxaliplatin resistance and promotes antitumor immunity in colorectal cancer. Cancer Res. 2019;79(11):2933–2946. doi:10.1158/0008-5472.CAN-18-2807
14. Falcone A, Masi G, Allegrini G, et al. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J Clin Oncol. 2002;20(19):4006–4014. doi:10.1200/JCO.2002.12.075
15. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, Phase 3 TRIBE study. Lancet Oncol. 2015;16(13):06–15. doi:10.1016/S1470-2045(15)00122-9
16. Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(4):497–507. doi:10.1016/S1470-2045(19)30862-9
17. Kudo T, Takemasa I, Hata T, et al. A phase I study of neoadjuvant capecitabine, oxaliplatin, and irinotecan (XELOXIRI) in patients with locally advanced rectal cancer. Oncology. 2019;97(4):211–216. doi:10.1159/000500677
18. Sato Y, Ohnuma H, Hirakawa M, et al. A dose-escalation study of oxaliplatin/capecitabine/irinotecan (XELOXIRI) and bevacizumab as a first-line therapy for patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2015;75(3):587–594. doi:10.1007/s00280-014-2672-9
19. Ryan J, Warrier S, Lynch A, Heriot A. Ireland: assessing pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis. 2015;17(10):849–861. doi:10.1111/codi.13081
20. Hur H, Cho M, Koom W, et al. Nomogram for prediction of pathologic complete remission using biomarker expression and endoscopic finding after preoperative chemoradiotherapy in rectal cancer. Chin J Cancer Res. 2020;32(2):228–241. doi:10.21147/j.issn.1000-9604.2020.02.10
21. Fernandez L, São JG, Figueiredo N, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the international watch & wait database: a retrospective, international, multicentre registry study. Lancet Oncol. 2020;22(1):43–50. doi:10.1016/S1470-2045(20)30557-X
22. Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34(27):3300–3307. doi:10.1200/JCO.2016.66.6198
23. Schrag D, Weiser Martin R, Goodman Karyn A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–518. doi:10.1200/JCO.2013.51.7904
24. Margonis G, Buettner S, Andreatos N, et al. Prognostic factors change over time after hepatectomy for colorectal liver metastases: a multi-institutional, international analysis of 1099 patients. Ann Surg. 2019;269(6):1129–1137. doi:10.1097/SLA.0000000000002664
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.