Back to Journals » Drug Design, Development and Therapy » Volume 12
Comparisons of therapeutic efficacy and safety of ipilimumab plus GM-CSF versus ipilimumab alone in patients with cancer: a meta-analysis of outcomes
Authors Chen P , Chen FC, Zhou BH
Received 16 October 2017
Accepted for publication 11 April 2018
Published 4 July 2018 Volume 2018:12 Pages 2025—2038
DOI https://doi.org/10.2147/DDDT.S154258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Peng Chen,1,* Fuchao Chen,2,* Benhong Zhou1,3
1Department of Pharmacy, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, People’s Republic of China; 2Department of Pharmacy, Dongfeng Hospital, Hubei University of Medicine, Shiyan, Hubei, 442008, People’s Republic of China; 3School of Pharmaceutical Sciences, Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China
*These authors contributed equally to this work
Background: Recent clinical studies have shown that initial therapy with combined cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blockade and granulocyte-macrophage colony-stimulating factor (GM-CSF)-based immunotherapies can enhance the antitumor efficacy of this approach. A key unanswered question is whether systemic GM-CSF enhances CTLA-4 blockade. Thus, the objective of this study was taking a meta-analysis of randomized controlled trials to compare the effect of ipilimumab plus GM-CSF versus ipilimumab alone on overall response, overall survival, and progression-free survival, as well as the risk of adverse events (AEs) in patients with cancer.
Materials and methods: Searches were made in electronic databases PubMed and Embase, and conference abstracts published by the American Society of Clinical Oncology from 2000 to 2017. Statistical analyses were carried out using either random-effects or fixed-effects models according to the heterogeneity of eligible studies.
Results: Six trials comprising of 445 patients were included in the meta-analysis. Combination group was superior to the ipilimumab alone in overall response rate, progression-free survival, and overall survival rate (combined relative risk [RR]=1.34, 95% CI: 1.24–1.45, P=0.09; combined hazard ratio [HR]=0.57, 95% CI: 0.32–1.02, P=0.06; combined HR=0.70, 95% CI: 0.60–0.82, P<0.001). Patients with combination therapies had a lower incidence of AEs including high-grade diarrhea (combined RR=0.27, 95% CI: 0.11–0.70, P=0.007), nausea (combined RR=0.25, 95% CI: 0.07–0.89, P=0.03), colitis (combined RR=0.34, 95% CI: 0.13–0.86, P=0.02), and fatigue (combined RR=0.91, 95% CI: 0.37–2.2.3, P=0.84) compared to the group having ipilimumab alone.
Conclusion: These data suggested that the combination of ipilimumab and GM-CSF was associated with a significant improvement in overall survival and lower high-grade toxicities, but there is no difference in overall response rate and progression-free survival among the cancer patients. Therefore, large-scale and well-designed studies are needed to summarize and analyze the data to draw a more convincing conclusion.
Keywords: ipilimumab, sargramostim, efficacy, safety, survival, meta-analysis
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF; Leukine® [sargramostim]) is a cytokine that increases antigen presentation by dendritic cells and enhances activities of T- and B-lymphocyte antitumor functions, and has been approved by the US Food and Drug Administration (FDA) for this purpose.1 Systemic administration of GM-CSF is being evaluated in multiple tumor types including melanoma and other cancers because of its benefits to prostate and ovarian carcinoma.2 The clinical properties of GM-CSF are somewhat controversial as several studies have suggested that the negative regulatory immune responses could be induced by itself. GM-CSF also plays a role in pulmonary and mucosal homeostasis and may modulate some forms of autoimmunity, especially involving the gastrointestinal (GI) tract.3
Yervoy® (Ipilimumab), a fully human immunoglobulin G1 monoclonal antibody that inhibits cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), was the first agent reported, in a randomized trial, to demonstrate survival advantages in patients with metastatic melanoma and was approved by the FDA in 2011 for the treatment of unresectable or metstatic melanoma.4 Since its approval, it has been studied in combination therapy regimens to build on these promising results and to obtain information about the immunologic mechanisms and improve clinical outcomes.5 In multiple preclinical models, lots of clinical data have suggested that the combination of CTLA-4-antibody blockade with GM-CSF-secreting tumor cell vaccines shows therapeutic synergies. An opportunity to investigate a regimen combining CTLA-4 blockade and GM-CSF-secreting tumor cell vaccines was provided by the fact of clinical benefits observed in melanoma, prostate cancer, and ovarian carcinoma having been validated as a therapeutic approach in the same patient population.6
In recent years, clinical benefits of combining ipilimumab with GM-CSF were initially seen in several randomized trials, which found that it not only can reduce the incidence of high-grade immune-related adverse events (AEs) and GI events, including colitis and diarrhea, but also improve the progression-free survival (PFS) and overall survival (OS) than with ipilimumab alone in patients with cancers.7 Importantly, there has been no systematic to synthesized toxicity data of these agents and taking into consideration the fact that the combination of ipilimumab and GM-CSF is increasingly evaluated in cancers with diverse indications, we think that proper knowledge of the different toxicities of these agents is of paramount significance to practicing oncologists.8 Thus, the chief purpose of our analysis was to assess the therapeutic efficacy and safety for the combination of systemic GM-CSF plus ipilimumab and ipilimuab treatment alone to get a more credible result.
Materials and methods
Search strategy
The electronic databases of PubMed, Embase, and Cochrane Library from January 2000 to January 2017 were searched using the key words “Ipilimumab”, “Sargramostim”, “granulocyte-macrophage colony-stimulating factor”, “GM-CSF”, and “cancers”. Additionally, the abstracts presented at major meetings from the American Society of Clinical Oncology, the European Society for Medical Oncology, and the World Lung Cancer Conference were manually searched. Finally, full publications (not abstracts) from the Web of Science database were also searched to ensure that there were no additional studies.
Study selection
We considered for inclusion studies meeting the following criteria: 1) prospective Phase I, II, and III clinical trials and expanded-access (ie, outside clinical trials) programs; 2) clinical investigations in patients with cancers (melanoma, pancreatic cancer, prostate cancer, spongioblastoma, non-small-cell carcinoma, and so on) and the participants have been assigned to ipilimumab and GM-CSF combination therapy; 3) efficiency measures including overall response rate (ORR), PFS, OS, and AEs have been recorded. The exclusion criteria were as follows: 1) investigations in patients of original studies unrelated to the study drug; 2) original studies that met criterion 1 but in which the required information such as the overall response, OS, and AEs were not available.
Data extraction and quality assessment
We extracted data from the included trials that included these information: the first author, number of patients enrolled in the study, treatment information, and characteristics of the participants to understand the baseline of all included studies. We evaluated the methodologic quality of the included literature, according to randomized controlled trial (RCT) quality evaluation standards of the Cochrane review manual 5.3.0: 1) generation of the random allocation scheme (random sequence generation); 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessment; 5) incomplete outcome data; 6) selective reporting; and 7) other bias. Any disagreements were resolved by discussion among our investigators or referencing the original publication.
Definition of main outcomes
The definition of complete response was that all symptoms and signs of all measurable diseases disappeared for at least 4 weeks and new lesions did not appear during the time.9 The definition of partial response was that the sum of the products of the perpendicular diameters of all measurable lesions reduced 50% for at least 4 weeks and new lesions did not appear or existing lesions did not enlarge during the time. Overall response included complete response and partial response and was defined as the proportion of patients with confirmed complete response or partial response.10 The PFS was defined as a type of measurement that can be used in a clinical study or a trial to help determine whether a new treatment is effective. It refers to the probability that a patient will remain alive, without the disease getting worse. We defined the OS as the time from the date of randomization to the date of death from any cause, or the date of last follow-up of a living patient. The version of the response criteria, RECIST 1.0, was used.11 Meta-regression was used to determine whether the rates of ORR were significantly affected by the histologic pattern. As for the safety outcomes, we referred to the trial authors’ definitions. We collected the number of the frequent toxicity events (diarrhea, nausea, colitis, and so on) to enter into the meta-analysis.
Statistical analysis
The statistical analysis was carried out using software of Review Manager Version 5.3 provided by the Cochrane Collaboration. The effect size of categorical outcomes was determined by the pooled relative risk (RR) and hazard ratio (HR) along with 95% CIs. The between-study heterogeneity was assessed using the chi-squared test. If I2 was <50% (P>0.1), the fixed-effect model was used, if not (I2>50%, P<0.1), the random-effect model was used, and we tried to find the cause of heterogeneity. Egger’s test was used for evaluation of the presence of publication bias. P-values of <0.05 or 0.01 were considered significant.
Results
Search results
Study selection
Of the studies initially identified, we excluded reports that did not meet the inclusion criteria after first screening the study titles and abstracts. At last, six studies12–17 were ultimately included in the meta-analysis. Figure 1 illustrates how the six studies were obtained from the literature search. The six selected studies (six RCTs), comprising a total of 445 participants, were published between 2009 and 2015, including two Phase I studies13,16 and four Phase II studies.12,14,15,17 In all studies, the starting dose and schedule of ipilimumab and GM-CSF were based on US FDA guidelines (3 or 10 mg/kg ipilimumab; 250 or 125 μg/m2 GM-CSF). The main characteristics and qualities of included trials are listed in Table 1. Of all the RCTs included in this study, one mentioned a specific random method,15 one was not blinded,13 and all did not conduct allocation concealment. The quality evaluations of the included studies are shown in Figures 2 and 3.
  | Figure 1 Flow diagram of the study selection process. |
  | Table 1 Summary of the characteristics of the studies included in the meta-analysis |
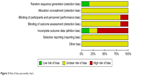  | Figure 2 Risk of bias percentile chart. |
  | Figure 3 Risk-of-bias assessment of randomized controlled trials included in meta-analysis. |
Effectiveness
In our meta-analysis, four comparisons were used to analyze the ORR, and five comparisons to analyze the PFS and OS.
Overall response rate
For the overall response, testing for interstudy heterogeneity gave significant results (χ2=1.58, df=3 [P=0.66], I2=0%); therefore, RR and 95% CI were calculated by a fixed-effects model. Our fixed-effect model analysis revealed that combination therapy improved the ORR in comparison to monotherapy but the difference was not significant (RR: 1.34 [95% CI: 1.24–1.45], P=0.09) as shown in Figure 4A.
Progression-free survival
For the PFS, the heterogeneity of studies for analysis was not significant (P=0.99; I2=0%), so we used fixed-effects model for meta-analysis. The result showed an increase in the pooled HR of PFS by the fixed-effects model meta-analysis for combination therapy versus monotherapy but the difference was not significant (HR: 0.57 [95% CI: 0.32–1.02], P=0.06) as shown in Figure 4B.
Overall survival
In total, six studies12–17 that reported that the combination therapy provided significant advantage in OS over monotherapy were included in the meta-analysis. The result showed that the combination of ipilimumab and sargramostim gave a significant increase in the pooled HR for oversurvival compared to the ipilimumab alone according to the fixed-effects model (HR: 0.70 [95% CI: 0.60–0.82], P<0.00001; heterogeneity: χ2=0.24, df=3 [P<0.00001], I2=0%) as shown in Figure 4C.
Safety
Adverse events
In this study, all-grade AEs were analyzed in our meta-analysis. To analyze the risk of all-grade AEs associated with the combination of ipilimumab and GM-CSF versus ipilimumab alone, we collected data from a total of 21 different types of AEs that were dealt with in combination therapy, to enter into the meta-analysis.
Our results showed that the combination of ipilimumab and sargramostim afforded an advantage in toxicity over ipilimumab alone. The results of the risk of AEs with combination therapy versus monotherapy have been listed in Table 2.
  | Table 2 Relative risk of adverse events between combination targeted therapy and monotherapy alone |
Subgroup analysis
RR of all-grade AEs
As meta-analysis results about risk of all-grade AEs revealed heterogeneity, the subgroup analysis was further performed by us according to the treatment regimen. All the studies were classified into three subgroups: 1) ipilimumab (10 mg/kg) + GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg); 2) ipilimumab (10 mg/kg) + GM-CSF (125 μg/m2) versus ipilimuab (10 mg/kg); 3) ipilimumab (3 mg/kg) + GM-CSF (250 μg/m2) versus ipilimumab (3 mg/kg). The pooled RRs of developing all-grade AEs (diarrhea, nausea, colitis, fatigue, hypophysitis, and dry skin) with combination therapy versus controls were 0.90 (95% CI: 0.53–1.54), 0.69 (95% CI: 0.27–1.79), 0.64 (95% CI: 0.33–1.26), 1.00 (95% CI: 0.28–3.55), 0.91 (95% CI: 0.06–12.66), and 1.03 (95% CI: 0.56–1.88), respectively (Table 3).
By subgroup analysis of the relative risk (RR) of all-grade AEs for the combination of ipilimumab and GM-CSF versus ipilimumab alone, we found the following: different RRs for diarrhea were observed in the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) (RR=0.91, 95% CI: 0.50–1.66), combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) (RR=0.87, 95% CI: 0.27–2.74; Figure 5A).
In regard to RR of nausea events, we found that the much higher RR of all-grade nausea was associated with the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) was 0.72 (95% CI: 0.17–3.14), followed by the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) (RR=0.67, 95% CI: 0.20–2.32; Figure 5B).
Concerning the RR of colitis, the higher RR of all-grade colitis was observed in patients associated with the combination of ipilimumab (3 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (3 mg/kg) (RR=1.00, 95% CI: 0.16–6.25), followed by the combination of ipilimumab (10 mg/kg) with GM-CSF (250 μg/m2) versus combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) (RR=0.72, 95% CI: 0.34–1.55; RR=0.16, 95% CI: 0.01–2.58; Figure 5C).
Furthermore, the higher RR of all-grade fatigue was observed in patients associated with the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) (RR=1.44, 95% CI: 0.68–3.05), followed by the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) (RR=0.47, 95% CI: 0.02–11.87; Figure 5D).
Concerning the all-grade hypophysitis events, their RR was more higher in patients associated with the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) (RR=2.89, 95% CI: 0.31–27.27), followed by the combination of ipilimumab (3 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (3 mg/kg) (RR=0.20, 95% CI: 0.01–3.86; Figure 5E). Finally, the RR of all-grade dry skin was much higher in patients associated with the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) (RR=1.08, 95% CI: 0.32–3.71), followed by the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) (RR=1.02, 95% CI: 0.51–2.02; Figure 5F).
RR of high-grade AEs
Using the random-effect or fixed-effect model, the pooled RRs of the combination of ipilimumab and GM-CSF versus ipilimumab-associated high-grade AEs from all included trials (diarrhea, nausea, colitis, and fatigue) were RR=0.27, 95% CI: 0.11–0.70, P=0.007; RR=0.25, 95% CI: 0.07–0.89, P=0.03; RR=0.34, 95% CI: 0.13–0.86, P=0.02; and RR=0.91, 95% CI: 0.37–0.23, P=0.84, respectively (Table 4).
Of the six RCTs12–17 included for RR analyses, four trials12–14,16 examined the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg), one trial15 examined the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg), and one trial17 examined the combination of ipilimumab (3 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (3 mg/kg). By subgroup analysis of the RR of high-grade AEs for combination therapy versus controls, we found the following: different RRs observed in the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) showed a significant difference (Z=2.51; P=0.01, RR=0.25, 95% CI: 0.09–0.74), while no differences between RRs of high-grade nausea were found between the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) (Z=0.99; P=0.32, RR=0.36, 95% CI: 0.05–2.72; Figure 6A).
In regard to RR of high-grade nausea events, no significant differences were found between RR of high-grade nausea observed in the combination of ipilimumab (10 mg/kg) with GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) and the combination of ipilimuab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) (Z=1.69; P=0.09, RR=0.30, 95% CI: 0.07–1.21; Z=1.30; P=0.19, RR=0.81, 95% CI: 0.01–2.58; Figure 6B).
Concerning the RR of high-grade colitis, the combination of ipilimumab (10 mg/kg) with GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) resulted significant (Z=2.01; P=0.04, RR=0.28, 95% CI: 0.08–0.97) while no differences between RRs of high-grade nausea were found between the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) and the combination of ipilimumab (3 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (3 mg/kg) (Z=1.30; P=0.19, RR=0.16, 95% CI: 0.01–2.58; Z=0.00; P=1.00, RR=1.00, 95% CI: 0.16–6.25; Figure 6C).
As for fatigue, the comparison between RR of high-grade fatigue in studies with the combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) versus ipilimumab (10 mg/kg) and the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) versus ipilimumab (10 mg/kg) showed no significant differences (Z=0.59; P=0.55, RR=1.38, 95% CI: 0.47–4.05; Z=0.20; P=0.84, RR=1.00, 95% CI: 0.91–0.37; Figure 6D).
Publication bias
Publication bias was assessed using funnel plot produced by Review Manager 5.3 software. The funnel plot shows a certain asymmetry (Figure 7), indicating that there is a certain degree of publication bias. However, the number of studies included is only six, and the funnel plots may not be very significant. Egger’s test revealed that publication bias was not significant in both the incidence (P=0.29) and RR (P=0.51) of AEs.
  | Figure 7 Funnel plot analysis for publication bias assessment. |
Discussion
To the best of our knowledge, this is the first meta-analysis focusing specifically on therapeutic efficacy and safety of ipilimumab plus GM-CSF versus ipilimumab alone in patients with cancers. The evidence that the addition of GM-CSF to ipilimumab therapy improved OS in patients with cancers can be supported by our study.18 These results are consistent with the preclinical animal experiment and preliminary clinical study of combining CTLA-4 blockade with GM-CSF-secreting whole-cell vaccines.19 The result of our meta-analysis showed that the addition of sargramostim to ipilimumab did not affect the PFS in cancer patients and it is similar to those reported previously for patients receiving second-line ipilimumab monotherapy or first-line ipilimumab therapy with dacarzine.20 The pathogenesis of the combination of ipilimumab with GM-CSF not enhancing the PFS versus ipilimumab is still unclear at present but we can suggest that its pathophysiologic mechanism may be related with the repeated subclinical traumas of ipilimumab.21 Many studies have confirmed that the lack of correlation between OS and PFS in this study presents challenges to clinical management and drug development because conventional radiographic criteria have not proven reliable for determining patient benefit.22 Several mechanisms of the improved efficacy found in our meta-analysis have been described in many studies and corroborated in tumor specimens obtained from patients. It has been confirmed that the benefits to OS of combination therapy may closely associate with improved antigen presentation with GM-CSF through recruitment of dendritic cells and macrophages, or to counteracting immune regulatory cells with ipilimumab.23
In previous studies of adjuvant therapy of melanoma and other cancers, it has been demonstrated that the combination of GM-CSF-secreting tumor cell vaccines with CTLA-4 blockade can stimulate an increase in the ratio of tumor-infiltrating CD8+ cytotoxic T cells to Fox P3+ regulatory T cells because the populations of myeloid and monocytic-derived suppressor cell could be stimulated by GM-CSF alone, and T cells were regulated by Fox P3+ resulting in a limit of antitum or immunity. More importantly, the addition of CTLA-4 blockade may overcome the potential tolerizing effects of the cytokine and favor instead the development of protective T-cell responses.24
In addition to the improvement in OS, our meta-analysis was able to demonstrate that the combination therapy afforded an advantage in toxicity over ipilimuab alone. Based on our further subgroup analysis, it is not difficult to find that the higher risks of combination of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) therapy were more common for all-grade diarrhea and colitis compared to the combination of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2) therapy.25 Moreover, subgroup analysis also showed that the combination therapy of ipilimumab (10 mg/kg) and GM-CSF (250 μg/m2) may carry lower risks of all-grade nausea and fatigue than the combination therapy of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2). Interestingly, the subgroup analysis in high-grade AEs showed that the combination therapy of ipilimuab (10 mg/kg) and GM-CSF (250 μg/m2) could bring a higher risk of nausea, colitis, and fatigue than the combination therapy of ipilimumab (10 mg/kg) and GM-CSF (125 μg/m2). The improvement of toxicity must be seen as a contribution to the improved survival even though the survival advantage of patients who have stopped the combination therapy due to toxicity is still present are excluded.26 Most notably, our meta-analysis demonstrated that the combination therapy significantly decreased the incidences of serious GI toxicities, particularly colonic perforation. Previous clinical studies have shown that some patients with Crohn’s disease get a lot of benefits from the systematic GM-CSF. Interestingly, a subset of patients with Crohn’s disease have an amount of antibodies that neutralize GM-CSF function, and a proportion of patients with inflammatory bowel disease show that the level of GM-CSF receptors was reduced. In preclinical models, severe colitis was developed under the management of GM-CSF in GM-CSF knockout mice, responsible for accelerated mucosal repair.27 GM-CSF is necessary for the generation of dendritic cells in the gut lamina propria to induce intestinal regulatory cells. Therefore, GM-CSF can promote the homeostasis of the GI tract by protecting and promoting the healing of mucosa. Consistent with this, the results of our meta-analysis also demonstrated that GM-CSF could improve antitumor activity and favorable toxicity profile when combined with ipilimumab because of the role of it in the tumor microenvironment and intestine.28
However, the reported success of these agents still comes at the cost of a set of other AEs, which significantly can affect therapeutic effect and quality of life of patients, and can lead to infection, discomfort, and bring some mental burden for patients to a certain degree.29 The studies included for meta-analysis have discussed the management of AEs and these measures are topical therapies for symptom relief, temporary treatment interruption, and dose modification of ipilimumab and GM-CSF in severe cases.30 In general, for AEs of grade 1 or 2, it is not recommended that patients should modify the dose according to the protocol. For most clinically significant AEs of grade 3 or higher, dosing of either ipilimumab or GM-CSF, or both drugs, was interrupted depending on the nature of the event, for example, abnormal liver function test results for ipilimumab. And the dose of ipilimumab and GM-CSF can be resumed at the same or a lower amount when grade 2 toxicity occurred at the first time.31
Limitations
There are several limitations in our analysis. First, because only six RCTs were included, the number of studies is small, and the patients in our study are also insufficient, so it could potentially make the conclusion less convincing. Second, researchers mostly adopted the personal experience to diagnose the toxicities in the clinical trials, and there were different judgments based on the same signs that varied with different researchers.
Third, the treatment regimens and doses of drugs are different among our studies including meta-analysis, so it could lead to significant heterogeneity in the data.32 Fourth, AEs and outcome of the study vary according to the dosage regimen and other concomitant medications, so it is difficult for us to correlate our data with the dose delays/interruptions or discontinuations secondary to AEs in the analysis. Finally, the publication bias might have occurred and it could not be completely excluded based on the funnel plot. Therefore, it is necessary to carry out more large-scale and high-quality RCTs to summarize and analyze the data to confirm this conclusion.
Conclusion
Patients treated with the combination of ipilimumab and GM-CSF achieved a longer OS compared to patients treated with ipilimumab alone and lower toxicity, but no difference in ORR and PFS. In addition, prompt and effective management of these AEs might allow for the safety when using combination therapy. We hope that our results could provide a reference point for physicians in clinical practice and ensure the safety and efficacy of the combination therapy of ipilimumab and GM-CSF for cancer patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Luke JJ, Donahue H, Nishino M, et al. Single institution experience of ipilimumab 3 mg/kg with sargramostim (GM-CSF) in metastatic melanoma. Cancer Immunol Res. 2015;3(9):986–991. | ||
Simmons SJ, Tjoa BA, Rogers M, et al. GM-CSF as a systemic adjuvant in a phase II prostate cancer vaccine trial. Prostate. 1999;39(4):291–297. | ||
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015;195(4):1341–1349. | ||
Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10(6):411–412. | ||
Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. | ||
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. | ||
Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. | ||
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from Phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. | ||
Daud A, Gill J, Kamra S, Chen L, Ahuja A. Indirect treatment comparison of dabrafenib plus trametinib versus vemurafenib plus cobimetinib in previously untreated metastatic melanoma patients. J Hematol Oncol. 2017;10(1):3. | ||
Larkin J, Chiarionsileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma-NEJM. N Engl J Med. 2015;373(1):23–34. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Patel RK, Ko AH, Onners B, et al. A phase 2, multicenter study of FOLFIRINOX followed by ipilimumab in combination with allogeneic GM-CSF transfected pancreatic tumor vaccine in the treatment of metastatic pancreatic cancer. J Clin Oncol. 2014;32(15 Suppl):1–4. | ||
Fong L, Kwek SS, O’Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69(2):609–615. | ||
Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312(17):1744–1753. | ||
Kwek SS, Kahn J, Greaney SK, et al. GM-CSF and ipilimumab therapy in metastatic melanoma: clinical outcomes and immunologic responses. Oncoimmunology. 2015;5(4):e1101204–e1101233. | ||
Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36(7):382–389. | ||
Luke JJ, Donahue H, Nishino M, et al. Single institution experience of ipilimumab 3 mg/kg with sargramostim (GM-CSF) in metastatic melanoma. Cancer Immunol Res. 2015;3(9):986–991. | ||
Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a Phase III trial. J Clin Oncol. 2015;33(10):1191–1197. | ||
Tsai KK, Daud AI. Nivolumab plus ipilimumab in the treatment of advanced melanoma. J Hematol Oncol. 2015;8(1):123–127. | ||
Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234–240. | ||
Puzanov I, Milhem MM, Minor D, et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(22):2619–2626. | ||
Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17(11):1558–1568. | ||
Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368–375. | ||
Araujo PB, Coelho MC, Arruda M, Gadelha MR, Neto LV. Ipilimumab-induced hypophysitis: review of the literature. J Endocrinol Invest. 2015;38(11):1159–1166. | ||
Taylor M, Antonia S, Bendell J, et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Immunother Cancer. 2015;3(Suppl 2):P376. | ||
Dummer R, Daud A, Puzanov I, et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J Transl Med. 2015;13(Suppl 1):O5. | ||
Johnson AS, Crandall H, Dahlman K, Kelley MC. Preliminary results from a prospective trial of preoperative combined BRAF, and MEK-targeted therapy in advanced BRAF, mutation-positive melanoma. J Am Coll Surg. 2015;220(4):581–593. | ||
Ribas A, Gonzalez R, Pavlick A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF (V600)-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15(9):954–965. | ||
Johnson DB, Sarangaperry V, Lavin PJ, et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol. 2015;33(33):e122–e126. | ||
Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015;3(1):23. | ||
Kwek SS, Lewis J, Zhang L, et al. Preexisting levels of CD4 T cells expressing PD-1 are related to overall survival in prostate cancer patients treated with ipilimumab. Cancer Immunol Res. 2015;3(9):1008–1016. | ||
Khanna R, Zou G, D’Haens G, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment Pharmacol Ther. 2015;41(1):77–86. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





