Back to Journals » International Journal of General Medicine » Volume 16
Comparisons of Electrolyte Balance Efficacy of Two Gelatin-Balanced Crystalloid for Surgery Patients Under General Anesthesia: A Multi-Center, Prospective, Randomized, Single-Blind, Controlled Study
Authors Duan G, Deng H, Fu H, Wang L, Yang H
Received 27 June 2023
Accepted for publication 18 October 2023
Published 12 December 2023 Volume 2023:16 Pages 5855—5868
DOI https://doi.org/10.2147/IJGM.S427904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Guangyou Duan,1,* Haibo Deng,2,* Hong Fu,3 Lingzhi Wang,4 Hanyu Yang5
1Department of Anesthesiology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing City, 400000, People’s Republic of China; 2Department of Anesthesiology, Huizhou Third People’s Hospital, Guangzhou Medical University, Guangzhou, 516002, People’s Republic of China; 3Department of Anesthesiology, Chongqing Emergency Medical Center, Chongqing University Central Hospital, School of Medicine, Chongqing University, Chongqing, 400014, People’s Republic of China; 4Department of Anesthesiology, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, 051026, People’s Republic of China; 5Department of Anesthesiology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, 510120, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guangyou Duan, Department of Anesthesiology, The Second Affiliated Hospital of Chongqing Medical University, No. 74, Linjiang Road, Chongqing City, 400000, People’s Republic of China, Email [email protected]
Purpose: This study aimed to compare the electrolyte balance efficacies of two Gelatin-Balanced Crystalloid in clinical applications.
Methods: A multi-center, prospective, randomized, single-blind, parallel controlled study was conducted among non-cardiac surgery patients, with clinical registration number ChiCTR2200062999. They were randomized into Succinylated Gelatin, Multiple Electrolytes and Sodium Acetate Injection (SG-MESAI) group (experimental group) and Succinylated Gelatin Injection (SGI) infusion group (control group). The same anesthetic induction technique, anesthetic method, and calculation method for the volume of colloid infusion were used in the two groups. Between-group differences in the changes in base excess (BE), Chloride ion (Cl−), bicarbonate radical (HCO3⁻) and other parameters were recorded at 15 min, 30 min after the infusion relative to the baseline. Hemodynamic indicators were determined at 30 min after colloid infusion. Safety follow-up was conducted by administering the following tests within 48 h± 12 h after surgery.
Results: A total of 225 subjects (full analysis set) were finally enrolled, with 110 subjects in the experimental group and 115 subjects in the control group. The baseline data were comparable between the two groups. At 15 min after infusion, the mean changes in BE, Cl− and HCO3⁻ concentration in the experimental group were smaller than those of the control group (P< 0.001). At 30 min after surgery, the mean changes in BE, Cl−, HCO3⁻concentration and pH value were smaller in the experimental group than in the control group (P< 0.05). The incidences of adverse events and adverse reactions in the experimental group was less than the control group, but the difference was not statistically significant (P≥ 0.05). Besides, no serious adverse events or adverse reactions were reported in any subjects.
Conclusion: Succinylated Gelatin, Multiple Electrolytes and Sodium Acetate Injection maintained the balance of BE, Cl−, HCO3⁻ and pH value in a better way than Succinylated Gelatin Injection in non-cardiac surgery patients under general anesthesia.
Keywords: anesthesia, non-cardiac surgery, fluid therapy, drug clinical trial, succinylated gelatin, multiple electrolytes and sodium acetate injection
Background
Acute diseases and surgeries can all cause hypovolemia and varying degrees of organ hypoperfusion, hypoxic state, or even shock.1,2 Appropriate fluid therapy can achieve the desired blood volume, ensure effective tissue perfusion, and reduce acid-base imbalance and postoperative complications, thus, assist in patient resuscitation after surgery.1,2
Succinylated Gelatin Injection (SGI), a plasma volume substitute containing 4% succinylated gelatin, is more commonly used in China, for which no toxicological clinical trials have been conducted in China. According to clinical safety studies of Succinylated Gelatin Injection, this intravenous colloid is an effective volume expander that maintains oxygen balance in the human body.3,4 However, impaired platelet aggregation may occur with profound hemodilution (>40%) using Succinylated Gelatin Injection.5 Normal saline is used clinically as a carrier solution for synthetic colloids. However, the Na+ and Chloride ion (Cl−) concentrations of normal saline are 154 mmol/L, which are significantly higher than the physiological levels of these two ions in the human body. Massive colloid infusion is inevitably accompanied by an injection of a large volume of normal saline, which, in turn, carries the risk of hyperchloremic metabolic acidosis.6 Succinylated Gelatin, Multiple Electrolytes and Sodium Acetate Injection (SG-MESAI) is a modification of SGI and contain 4% succinylated gelatin, sodium chloride, sodium acetate, potassium chloride, and magnesium chloride as active ingredients and sodium hydroxide and hydrochloric acid as excipients. The only difference is that SG-MESAI contains sodium acetate solution instead of sodium chloride solution as the carrier. Sodium acetate solution is composed of potassium chloride, magnesium chloride, and sodium acetate. Therefore, the electrolyte composition of SG-MESAI comes close to that of human blood while maintaining the original osmotic pressure for drug delivery. Previous reports show that SG-MESAI has a reduced risk of hyperchloremia after massive infusion, lower toxic and side effects, and better maintaining of fluid-electrolyte and acid-base balance than SGI.7–10
But in spite of the above, there are only a few clinical studies on SG-MESAI. Besides, the types of intravenous fluids are usually chosen based on national or local practical model rather than on evidence of clinical effectiveness.1,2 This study evaluated the efficacy and safety of SG-MESAI by comparing it with SGI for fluid therapy in non-cardiac surgery patients under general anesthesia.
Methods
Study Design
A multi-center, prospective, randomized, single-blind, parallel controlled study was conducted, involving 5 participating centers. The present study had been registered at Chinese Clinical Trial Registry (registration number: ChiCTR2200062999; registration time: 27/08/2022). The research process complied with the 2013 Version of the Declaration of Helsinki and Good Clinical Practice (GCP) issued by NMPA.
Participants
Non-cardiac surgery patients were enrolled from the above centers during the time period from September 2022 to March 2023. The first subject was enrolled on September 21, 2022, and the last on February 17, 2023.
All of the subjects were enrolled according to the following inclusion criteria: Aged ≥18 and ≤64, regardless of gender; body mass index (BMI) 18–29 kg/m2, Hb>100 g/L; estimated duration of surgery>2 h; volume of colloid infusion 500–1000 mL; assessed as grade I or II according to the American Society of Anesthesiologists (ASA) Physical Status Classification System; the patients or their guardians having consented to participate in the clinical trial and signed the written informed consent. The subjects were excluded if they met any of the following exclusion criteria: (1) severe hypernatremia, hyperchloremia, and hypercalcemia before surgery, defined as serum sodium>152 mmol/L, serum calcium>12 mg/dL (>3.00 mmol/L), or serum chlorine>1.06 mmol/L. Assessment was made by combining laboratory tests with clinical manifestations, including sleepiness, coma, delirium, and insanity; 2) pulmonary edema, cerebral edema, intracranial hemorrhage, and heart failure; (3) liver and kidney function indicators more than twice the upper limit of the normal range; 4) having a history of organic brain diseases or cerebral hemorrhage, having organic heart diseases or wearing a cardiac stent, and having a history of mental illnesses and disturbance of consciousness; (5) abnormal results on coagulation function test and electrolyte test (abnormalities assessed by investigators as clinically significant); (6) having received dialysis treatment within one month before surgery; (7) having been judged as lacking the capacity to give informed consent; (8) having participated in other drug clinical trials in the past three months; (9) having a known allergy to the investigational drug and/or its ingredients (eg, gelatin plasma substitute, α-galactose); (10) having a history of hypothyroidism; (11) pregnancy, lactation, or preparing for pregnancy; (12) scheduled for neurosurgery; (13) any other conditions that disqualified the subjects for the present study, as judged by the investigator.
The subjects were entitled to quit a clinical trial at any time for any reason. The investigator or sponsor could require the subjects to exit the clinical trial at any time due to safety concerns. Data were collected from those who quit the clinical trial following the standard workflow.
Test Method and Anaesthesia Management
Gelofusine (500mL: 20 g, Braun Medical (Suzhou) Co., Ltd., National Medicine Permit No.: H20113119) was used as the positive control drug (SGI) for the study. Gelaspan, manufactured by Braun Medical (Suzhou) Co., Ltd., has passed the consistency evaluation and has been approved for marketing in Chinese mainland on October 11, 2021 (number of product license: H20213785).11 The investigational drug was Gelaspan (500mL: 20g, Braun Medical (Suzhou) Co., Ltd., National Medicine Permit No. H20213785), used as the experimental drug (SG, MESAI) in the experimental group. The two kinds of drugs were packaged and coded in the same way. Coding was performed in the presence of statisticians and personnel managing the EDC system. As for the blind codes, “NPZ” represented experimental medication and “TPB” control medication. The coded medications were sent to each center at a 1:1 ratio between the experimental group and the control group. At each center, the investigators administered the medications at an appropriate dosage required by the subject.
The same anesthetic induction technique, anesthetic method, and calculation method for volume of colloid infusion were used during surgery to preclude the influence of intraoperative confounding factors. Patients in either group first received an intravenous infusion of normal saline 5 mL/kg after being wheeled into the operation room, followed by anesthetic induction via intravenous infusion of the agents below: sufentanil 0.2–1 μg/kg, propofol 1–2 mg/kg (or target-controlled infusion of propofol, with the target plasma concentration set to 1.5–3 ug/mL), vecuronium bromide 0.1–0.2 mg/kg or rocuronium bromide 0.6–0.9 mg/kg or cisatracurium 0.2mg/kg. Endotracheal intubation was performed for mechanical ventilation, with the ventilatory parameter PETCO2 set to 35–40 mmHg. Puncture catheterization was performed in the radial artery after successful anesthetic induction to collect arterial blood samples for arterial blood gas analysis. Colloid infusion began within 30 min after the anesthetic induction. Intravenous injection of the experimental drug and the control drug (15 mL/kg) was performed within 1.5 h. Maintenance of anesthesia: The technique for the maintenance of anesthesia was intravenous general anesthesia, or combined anesthesia consisting of intravenous anesthesia and sevoflurane inhalation, depending on patients’ conditions. During the course of surgery, intravenous muscle relaxant was given intermittently. The average initial infusion dose was 500~1000mL/per patient. Patients with severe blood loss can be given a higher dose according to the state of the patient.
In case of intraoperative hypotension (Systolic pressure [SBP] <80 mmHg), the investigator might choose from the following countermeasures as deemed appropriate: (1) Norepinephrine: 0.03–0.2 μg/kg/min; (2) Dopamine: 1–5 μg/kg/min, by intravenous pumping. Intraoperative hypertension (SBP> 160 mmHg) was preferably managed by increasing the concentration of sevoflurane inhaled. If this method failed, the investigators might choose from the following countermeasures: (1) Nitroglycerin: Intravenous drip starting from 5 μg/min, with an increment of 5 μg/min every 3–5 min if necessary, until reaching the maximum of 200–300 μg/min; (2) Urapidil: 10–50 mg urapidil injection was administered intravenously at a slow rate. Blood pressure changes were monitored, and an antihypertensive effect was expected within 5 min. If the effect was unsatisfactory, urapidil injection could be administered repeatedly; intravenous drip: 250 mg urapidil was added into an appropriate liquid medium (eg, normal saline, 5% GS). The maximum drug concentration was 4 mg of urapidil per litter. Routine method for preparing urapidil solution for use in the infusion pump: 50 mg + NS to 50 mL IV, with an initial rate of 6 mL/h (6 mg/h) and a subsequent adjustment based on blood pressure.
Episodes of bradycardia (heart rate [HR] <50 beats/min) could be managed by intravenous injection of atropine 0.3 mg; episodes of tachycardia (HR>100 beats/min) could be managed by intravenous injection of esmolol 10 mg. If SBP >140 mmHg or/and Diastolic pressure (DBP) >90 mmHg, the infusion rate should be reduced immediately. If SBP/DBP did not change or rise continuously, colloid infusion should be stopped. Episodes of hypotension, hypertension, bradycardia, and tachycardia occurring during the use of the investigational drug could be only managed using the specified drugs as instructed above. Besides, the administration time and dosage of such medications, response and improvements of patients should be recorded in detail.
During the trial, the subjects might suffer from fever, skin rash, sudden flushing of the face and neck, and a sudden fall in blood pressure, or might even evolve into shock and cardiac and respiratory arrest, which are rare. If there were any signs of immediate allergic reactions or signs indicative of immediate allergic reactions due to the infusion of the investigational drug, the infusion should be stopped immediately. First aid should be performed following standard procedures.12 All adverse events (AEs) should be tracked until resolution or at the end of the trial when the conditions of affected cases were reported for the last time. Any serious adverse events (SAEs) should be closely monitored until resolution even after the trial ended.
Clinical Observation Parameters
The assessment indicators included the following: general condition, physical check-up, routine blood test (RBC, WBC, PLT, Hb, NEUT#, NEUT%, HCT), routine urine test (urine RBC, urine protein, urine WBC), liver function test (ALT, AST, TBIL, TP, and ALB), kidney function test (BUN, Cr, Ccr), coagulation function test (PT, APTT, TT, FIB), electrolyte test (K+, Na+, Ca2+, Cl−), arterial blood gas analysis (PH, BE, HCO3-, Arterial oxygen saturation, Partial pressure of oxygen, Partial pressure of carbon dioxide), mean arterial pressure, fasting time (h), post-fasting volume of fluid infusion in the ward, volumes of crystalloid and colloid infusion, length of hospital stay, and incidence of AEs and SAEs.
Before colloid infusion, blood samples were collected following anesthesia and arterial catheterization. Arterial blood gas analysis and determination of hemodynamic indicators (pulse blood oxygen saturation, HR, SBP, DBP, mean arterial pressure) were performed. The fasting time (h), post-fasting volume of fluid infusion in the ward (mL), and volume of crystalloid infusion before colloid infusion (mL) were record. During colloid infusion, blood oxygen saturation, heart rate, blood pressure, hemodynamic indicators, and use of vasoactive agents were monitored and recorded once every 15 min. At 15min after colloid infusion, routine blood test, liver function test, kidney function test, coagulation function test, electrolyte test, arterial blood gas analysis, and determination of colloid infusion continued as before. The total volume of colloid infusion (mL) was determined. Hemodynamic indicators were determined at 30 min after colloid infusion. At 30 min after surgery, routine blood test, liver function test, kidney function test, coagulation function test, electrolyte test, arterial blood gas analysis, and determination of hemodynamic indicators were performed. The length of hospital stays (d) and volumes of colloid and crystalloid infusion (mL) were recorded. Safety follow-up was conducted by administering the following tests within 48 h±12 h after surgery if needed: routine blood test, routine urine test, liver function test, kidney function test, coagulation function test, electrolyte test.
Throughout the time period from the administration of the investigational drug to the end of follow-up, AEs, SAEs, and suspected unexpected serious adverse events (SUSARs) were observed and reported. The causal relationship between these events and investigational drug was assessed.
Efficacy Evaluation
Primary efficacy endpoints: Between-group differences in the changes in BE and Cl− concentrations at 15 min after the infusion relative to the baseline.
Secondary efficacy endpoints: Between-group differences in the changes in BE and Cl− concentrations at 30 min after surgery relative to the baseline, changes in hemodynamic indicators, arterial blood gas analysis indicators, volume of fluid infusion.
Safety endpoints: Electrolytes, coagulation function, kidney function, AEs, and SAEs, with extra attention given to such AEs as anaphylaxis, hyperchloremia, hypercalcemia, coagulation abnormalities, abnormal liver and kidney functions, and skin rash. All AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA).13 The severity of AEs was graded according to CTCAE v5, and all of the reported AEs were assessed. If an recurring episode of the same AE was considered to have a reduced severity, it should not be reported as a new AE. For intermittently occurring AEs, the time of onset and duration of each episode were recorded.
Sample Size
According to previous clinical study reports,7 the expected change in BE before and after the infusion was −2.59±2.25 in the experimental group and −4.79±2.38 in the control group; the expected change in Cl− concentration before and after the infusion was 2.4±1.9 in the experimental group and 5.2±3.1 in the control group. Double endpoints were assessed. Bonferroni correction was performed to test the two hypotheses simultaneously. α was set to 0.025, and the power of the test 1-β was 0.90. The ratio of sample size was 1:1 between the two groups. SAS 9.4, a tool for power and sample size analysis, was used to estimate the minimum sample size, which was 23 in each group. The total sample size was 46 in this study, according to this estimation. However, a statistical procedure tends to underestimate the sample size. Besides, individual differences might be more significant in a multi-center clinical trial, so the estimated sample size was inadequate to represent the entire population. Therefore, we followed the recommendations in “Administrative Measures for Drug Registration”, which say that at least 100 subjects should be enrolled in each group in a blind randomized controlled trial. Assuming that the dropout rate/elimination rate was below 20%, the sample size was set to 120 in each group, totaling 240 in this study.
Randomisation
Randomization was independently performed at each of the six participating centers. Different seeds were used at the six centers. A random grouping table was generated using SAS at a 1:1 ratio of sample size to assign subjects to different treatments. The enrollment was competent at different centers. When the total number of subjects enrolled at different centers reached 200, the number of subjects to be enrolled subsequently into each center was determined based on the number of patients scheduled for surgery later on. This enrollment scheme ensured that the final total number of enrolled subjects was at least 240 at different centers. Single blinding was adopted to avoid the impact of subjective factors on the research process and results and reduce biases caused by subjective factors on effect size measures. That is, the subjects were blinded to treatment assignment.
Statistical Analysis
Statistical analyses were conducted using SAS (version 9.4 or above). All statistical tests were two-sided, and the significance level was set to 0.05. The 95% confidence intervals of parameters were estimated. Statistical procedures were performed to describe subjects’ demographic variables (eg, gender, age), vital signs (body temperature, respiratory rate, blood pressure), past medical history, laboratory tests (coagulation function test, blood biochemistry test, routine blood test, and blood gas analysis), and other information.
Normality of measurement data was verified using the Shapiro–Wilk normality test. Continuous variables obeying a normal distribution were expressed as mean±standard deviation; otherwise, they were expressed using medians and interquartile ranges. Categorical variables were expressed as frequencies and percentages (N,%). Quantitative variables were compared between the two groups using the t-test or Wilcoxon rank-sum test relative to the baseline. Qualitative variables were compared between the two groups using the chi-square test or Fisher’s exact test relative to the baseline. Repeated measurements were analyzed by repeated-measures ANOVA. Given the intravenous route of drug administration during surgery, the medication compliance was generally good. As for concomitant medication, descriptive statistical analysis was performed for the drug name and reasons for medication by expressing categorical variables as frequencies and percentages (N, %).
Primary efficacy endpoints were analyzed using independent-samples t-test or non-parametric Mann–Whitney U-test. If the mean changes in the experimental group were significantly smaller than those in the control group, the medication in the experimental group was considered superior to that in the control group. The 95% confidence intervals were estimated for the mean changes in variables in the two groups. Between-group differences in secondary efficacy endpoints were analyzed by independent-samples t-test or non-parametric Mann–Whitney U-test. Numbers of episodes, frequencies, and incidence of AEs (SAEs included) and adverse reactions (ARs, SARs included) were described in the two groups. Between-group differences in the above indicators were analyzed by independent-samples t-test or Fisher’s exact test.
Since the present study was conducted at multiple centers, primary endpoints should be analyzed with consideration of the center effect. Continuous primary endpoints were analyzed by building a general linear model (GLM). Thus, a statistical model incorporating treatment, center as the dummy variable, and the product term of treatment and center as the dummy variable was established.
A P<0.05 was considered statistically significant.
Result
Baseline Data
A total of 248 subjects were preliminarily enrolled, and 225 subjects (Full Analysis Set, FAS) were considered eligible for the study based on the inclusion and exclusion criteria. There were 110 subjects assigned to the experimental group and 115 subjects to the control group. The Safety Set (SS) consisted of 236 subjects, with 117 subjects from the experimental group and 119 from the control group, Subject inclusion and exclusion for SS are shown in Figure 1. FAS had 78 males (35%) and 147 females (65%), who were aged 47.5 on average (standard deviation: 11.7). The youngest was aged 20 and the eldest 64. The group-based descriptive statistics of other baseline indicators are provided in Table 1. The two groups were comparable (Table 1).
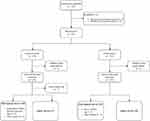 |
Figure 1 Subjects’ inclusion flow chart. |
 |
Table 1 Description of Baseline Parameters of Subjects in Two Groups (Full Analysis Set) |
Primary and Secondary Efficacy Endpoints
At 15 min after colloid infusion, the mean changes in base excess (BE) and Cl− concentration in the experimental group were smaller than those of the control group (BE: −0.37 (−0.7, −0.04) vs −1.53 (−1.97, −1.09), P<0.001; Cl−: 0.16 (−0.26, 0.57) vs 1.27 (0.89, 1.66), P<0.001, Table 2). For more details, see Table 2 below. The changes in K+(P=0.045) and Ca2+(P<0.001) concentrations relative to the baseline were also significantly different between the two groups (Table 2). The mean change in bicarbonate radical (HCO3⁻) concentration, determined as a secondary efficacy endpoint, was larger in the treatment group than in the control group (−0.13 (−0.47, 0.22) vs −1.17 (−1.61, −0.72), P<0.001, Table 2). No significant differences were found in other indicators (Table 2).
 |
Table 2 Comparison of Changes in BE, Cl−, and Other Indicators at 15 Min After Colloid Infusion Between the Two Groups (Full Analysis Set) |
At 30 min after surgery, the mean change in BE and Cl− concentration were smaller in the experimental group than in the control group (BE: −1.17 (−1.58, −0.76) vs −2.15 (−2.61, −1.69), P=0.002; Cl−: 0.49 (−0.02, 0.99) vs 1.19 (0.74, 1.64), P=0.039, Table 3). The mean changes in pH value and HCO3⁻concentration were smaller in the experimental group than in the control group (pH value: −0.05 (−0.06, −0.03) vs −0.07 (−0.08, −0.06), P=0.007; HCO3⁻concentration: −0.24 (−0.68, 0.20) vs −0.93 (−1.37, −0.48), P=0.032, Table 3). The changes in other efficacy endpoints relative to the baseline did not differ significantly between the two groups (Table 3).
 |
Table 3 Comparison of Changes in the Concentrations of BE, Cl− and Other Indicators at 30 Min After Surgery Between the Two Groups (Full Analysis Set) |
Occurrence of Adverse Events
In SS, there were 27 cases (42 episodes) of AEs from the experimental group and 28 cases (54 episodes) of AEs from the control group. The incidence of AEs and ARs was not significantly different between the experimental group and the control group, and no SAEs or SARs were reported in any group (P≥0.05, Table 4). As for the severity of AEs, No AEs of grade 5 occurred in any group (Table 5).
 |
Table 4 Description of Adverse Events in Two Groups (Safety Set) |
 |
Table 5 Distribution of Different Grades of Adverse Events in Two Groups (Safety Set) |
Among all AEs, the number of cases suffering from hypocalcemia (2 vs 6) and hypokalemia (5 vs 9) in the experimental group was smaller than that in the control group (Table 5). However, the number of cases of hyperchloremia in the experimental group was 0 while those cases in the control group 9 (Table 5). Besides, prolonged activated partial thromboplastin time (APTT) were found in both groups (10 vs 13, Table 6).
 |
Table 6 Comparison of the Incidence of Adverse Events in Two Groups (Safety Set) |
Center Effect Analysis
As shown by the center effect analysis, there were no significant treatment-by-center interactions in changes in Cl− and BE concentrations at 15 min after colloid infusion (P>0.05). Significant center effect was not observed (P>0.05). After the correction for center effect and the treatment-by-center interactions, the between-group differences were still of statistical significance (P<0.05).
Discussion
In this study, BE, Cl−, pH value, and HCO3⁻concentrations were kept in a significantly better balance in the experiment group than the control group. These results indicated that the electrolyte/acetic acid solution had no effect on the dilatation efficiency of succinyl gelatin. So that SG-MESAI achieved better effects than SGI, promoting fluid-electrolyte and base-acid balance in the perioperative period and facilitating rapid recovery from surgery. Furthermore, the incidence of AEs was similar in the two groups, neither of which caused SARs. These results indicated favorable short-term safety of SG-MESAI.
A growing number of studies have recommended restrictive fluid replacement to avoid potential risk factors in the perioperative period, reduce postoperative complications, and improve prognosis.14,15 Fluid loss can be induced by primary diseases, anesthesia, surgery, and bleeding. The volume of fluid infusion and the types of electrolytes replaced by infusion should be respectively matched with the volume and electrolyte composition of the fluid lost.16,17 At present, the use of fluid therapy is controversial in several fields. The type, volume, efficacy, and safety of the fluids infused and patients’ pathophysiological state all need to be considered when administering fluid therapy.
Fluids used in the fluid therapy are divided into three categories: crystalloids, colloids, and hypertonic saline or the mixture of hypertonic saline and colloids. The two commonly used crystalloids are normal saline and balanced salt solution. Massive infusion of normal saline may cause hyperchloremic metabolic acidosis due to its high chlorine content.
By contrast, balanced salt solution can reduce the postoperative incidence of hyperchloremic metabolic acidosis compared with normal saline.18 However, which is the preferred fluid for critically ill patients, normal saline, or balanced salt solution, is a disputable topic.19 Instead of improving prognosis, hypertonic saline and the mixture of hypertonic saline and colloids may even induce iatrogenic hypertonic state and hypernatremia.20,21 These two fluids are rarely used in clinical practice nowadays. One major difference between colloids and crystalloids is that the former have a specific colloidal osmotic pressure. Massive infusion of crystalloids during the resuscitation process may lead to a decrease in colloidal osmotic pressure and tissue edema. In China, normal saline is usually used as the carrier solution for SGI. Massive infusion of SGI is inevitably accompanied by an infusion of large amount of chlorine, thus increasing the risk of hyperchloremic metabolic acidosis.7,8 For SG-MESAI, sodium acetate solution, which contains potassium chloride, magnesium chloride, and sodium acetate, is used as the carrier instead of sodium chloride. Therefore, SG-MESAI can greatly reduce the risk of hyperchloremia after infusion while maintaining electrolyte-fluid and acid-base balance.9,10 Our results showed that at 15 min after infusion and at 30 min after surgery, the mean changes in BE and HCO3⁻ concentrations in the experimental group were less than those of the control group. However, the mean change in Cl− concentration was smaller in the experimental group than in the control group. Colloid infusion better maintained the stability of osmotic pressure and electrolyte-fluid and acid-base balance. We may say that SG-MESAI had an improved clinical efficacy in the perioperative period than SGI. Our finding agrees with the previous discovery. In real-world clinical scenarios, immediate and massive colloid infusion is usually required for those with massive blood loss, and SG-MESAI is a good option.
The most common ARs associated with SG-MESAI are abnormal laboratory tests, which usually imply metabolic abnormalities, nutritional disorders, and diseases of hematologic and lymphatic systems. According to the clinical trials of the branded drug Gelaspan®, SG-MESAI had a significantly smaller impact on BE and serum chlorides, compared with the control drug. However, the two drugs did not differ significantly in their impact on hemodynamics, kidney function, and coagulation function.7 Bradley et al9 found that SG-MESAI achieved similar effects in volume expansion and increasing cardiac output compared with a larger volume of crystalloids. In one study, Marx et al reported 15 cases of AEs. One of them suffered from SAE (increased creatine phosphokinase) after admission to ICU. This AE was later judged as possibly related to known peripheral arterial occlusive disease.7 But Md Nizar et al reported one case of hypotension in the SG-MESAI group that required ephedrine treatment.10 No SAEs occurred throughout the present trial. Neither were there significant differences in the incidence of AEs and ARs between the two groups, which indicated favorable safety of SG-MESAI in the perioperative period. After surgery, the changes in BE, HCO3⁻, and Cl− concentrations in those receiving SG-MESAI were less violent relative to the baseline levels. None of the subjects in the experiment group had hyperchloremia, which confirmed the superiority of SG-MESAI in clinical use.
In the present study, hypocalcemia and hypokalemia occurred more frequently in the two groups, compared with other AEs and ARs. One possible reason was that the amount of colloids infused (calculated by body weight in kilogram) in the study was greater than that given in clinical practice and the infusion speed was also higher. This study administered the patient at 15mL/kg, and a total of 500–1000mL/per patient. Patients with severe blood loss can be given a higher dose according to the state of the patient. As mentioned above, the volume of fluid infusion and the types of electrolytes replaced by infusion should be respectively matched with the volume and electrolyte composition of the fluid lost.16,17 Since the infusion volume was estimated solely based on body weight in this study, it might be greater than the actual amount needed by the subjects. While inflexible fluid management scheme is a less favored option,22 restrictive fluid therapy in the perioperative period may help reduce complications and shorten hospital stay.15,16,22,23 Furthermore, preoperative fasting and restricted drinking may cause pathological changes after surgery such as anemia, water and electrolyte disorders, and insufficient effective circulating blood volume, etc.24 Moreover, Succinyl gelatin has the side effect of electrolyte disturbance because it contains saline solution in which the level of CL− and Na+ is higher than that in normal plasma.5,6,25 The Ca2+, K+ levels can also be reduced for the sake of blood dilution, ion flow, and increased urine volume among the patients with operations under general anesthesia.5,6,25 Succinyl gelatin infusion at a dose of 10 mL/kg and a rate of 20 mL/min is shown to achieve effective dilatation without causing serious hemodynamic change and adverse events.22–27 Administered with up to 20% of blood volume with balanced intravenous solutions is safe in terms of their impact on human plasma electrolyte and metabolic equilibrium.25
In the present study, APTT was prolonged in both groups probably due to fast infusion within a short period of time, resulting in dilution of the clotting factor. Besides, high-dose gelatin infusion may increase the fibrinogen level.28 According to one study, colloid-induced hemodynamic changes during fluid resuscitation are only temporary but may induce acute kidney injury29 and coagulation disorders.28 The incidence of these AEs increases as the cumulative dose increases. Another study compared perioperative infusion of colloids and crystalloids in cardiac surgery patients and found no significant correlation between the infusion of either and postoperative acute kidney injury and mortality.30 Moreover, preoperative fasting and restricted drinking and the side effect of Succinyl gelatin may also cause hemodynamic change,5,6,25 which may prolong APTT. Therefore, individualized fluid management plan should be adjusted and optimized intraoperatively based on the monitoring of patients’ conditions and other indicators, so as to facilitate patient recovery and reduce complications.16,17,22–25 A clinical protocol for individualized fluid management of SG-MESAI is awaiting to be established.
However, we only discussed the clinical efficacy and safety of SG-MESAI and SGI in the perioperative period for the current study, but the further follow-up or long-term prognosis still need to be performed. In addition, SG-MESAI has been neither extensively applied in clinical practice nor adequately studied. An optimization of SG-MESAI based on patients’ needs requires an in-depth investigation.
Conclusion
Compared with Succinylated Gelatin Injection, Succinylated Gelatin, Multiple Electrolytes and Sodium Acetate Injection achieved the desired volume expansion more rapidly in non-cardiac surgery patients under general anesthesia with a better maintaining of the balance of BE, Cl−, pH value, and HCO3⁻concentration. The experimental medication and control medication shared similar safety profiles, and neither caused SAEs or SARs. However, hypocalcemia, hypokalemia and prolonged APTT were common AEs in the two groups. The long-term prognosis of patients was not studied in our trial, and an observation is needed in the long run.
Data Access Statement
The protocol and unidentified patient data can be acquired from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The five participating centers included in this study and the related ethical approval numbers were as follows: First Affiliated Hospital of Guangzhou Medical University (No. EC-2022-046(YW)-03), Second Affiliated Hospital of Guangzhou Medical University (No. Y2022-56-02), Fourth People’s Hospital of Chongqing University (also named: Chongqing University Central Hospital, No. 2023-Ethics-006)), Second Affiliated Hospital of Chongqing Medical University (No. 2023-Ethics-003), and Third People’s Hospital of Huizhou (No. AF/SW-07/01.1). All subjects signed their names on the written informed consent before participation in this study, and all procedures of this study were in compliance with the tenets of the Helsinki Declaration.
Consent for Publication
All patients gave written informed permission for the study’s publication.
Acknowledgments
We thank Haixiao Yang for his help and resources for this research.
Funding
This study received no funding for this study.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Raghunathan K, Murray PT, Beattie WS, et al.; ADQI XII Investigators Group. Choice of fluid in acute illness: what should be given? An international consensus. Br J Anaesth. 2014;113(5):772–783. PMID: 25326478. doi:10.1093/bja/aeu301
2. Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14(9):541–557. PMID: 30072710. doi:10.1038/s41581-018-0044-0
3. Awad S, Dharmavaram S, Wearn CS, Dube MG, Lobo DN. Effects of an intraoperative infusion of 4% succinylated gelatine (Gelofusine(R)) and 6% hydroxyethyl starch (Voluven(R)) on blood volume. Br J Anaesth. 2012;109(2):168–176. PMID: 22508964. doi:10.1093/bja/aes098
4. Zhou Z, Chen X, Zhou X, et al. Effects of intraoperative gelatin on blood viscosity and oxygenation balance. J Perianesth Nurs. 2019;34(6):1274–1281. PMID: 31492603. doi:10.1016/j.jopan.2019.05.136
5. Kam P, Varanasi S, Yang KX. The effects of haemodilution with succinylated gelatin solution on coagulation in vitro as assessed by thromboelastometry and impedance (multiple electrode) aggregometry. Anaesth Intensive Care. 2018;46(3):272–277. PMID: 29716485. doi:10.1177/0310057X1804600304
6. Zheng XQ, Zhou JK, Lin Y, Wu YD. Adverse reactions caused by succinyl gelatin and its potential risk. J Chin Prescription Drug. 2016;14(03):15–16.
7. Marx G, Meybohm P, Schuerholz T, et al. Impact of a new balanced gelatine on electrolytes and pH in the perioperative care. PLoS One. 2019;14(4):e0213057. PMID: 31034525; PMCID: PMC6488052. doi:10.1371/journal.pone.0213057
8. Raman S, Gibbons KS, Mattke A, et al. Effect of saline vs gluconate/acetate-buffered solution vs lactate-buffered solution on serum chloride among children in the pediatric intensive care unit: the SPLYT-P randomized clinical trial. JAMA Pediatr. 2023;177(2):122–131. PMID: 36534387; PMCID: PMC9857166. doi:10.1001/jamapediatrics.2022.4912
9. Bradley CR, Bragg DD, Cox EF, et al. A randomized, controlled, double-blind crossover study on the effects of isoeffective and isovolumetric intravenous crystalloid and gelatin on blood volume, and renal and cardiac hemodynamics. Clin Nutr. 2020;39(7):2070–2079. PMID: 31668721; PMCID: PMC7359406. doi:10.1016/j.clnu.2019.09.011
10. Md Nizar ND, Hassan SK, Mohamad Zaini RH, Hassan MH, Wan Hassan WMN, Mazlan MZ. Comparing the effects of pre-loading with gelatine 4% plasma volume expander and 6% hydroxyethyl starch solution before spinal anaesthesia for lower limb orthopaedic surgery. Malays J Med Sci. 2020;27(6):68–78. PMID: 33447135; PMCID: PMC7785271. doi:10.21315/mjms2020.27.6.7
11. National Medical Products Administration Drug Evaluation Center. Basic information of succinylated gelatin, multiple electrolytes and sodium acetate injection; 2021. Available from: https://www.cde.org.cn/hymlj/detailPage/7afac745282bc0df29b3fe98899699da.
12. Anesthesia Society of Chinese Medieal Association. Expert Consensus on Fluid Therapy During Anesthesia Surgery; 2014.
13. Medical Dictionary for Regulatory Activities. Side effects of drugs annual; 2009. Available from: https://www.meddra.org/.
14. Park J, Han SS, Park SJ, et al. Effect of perioperative fluid volume restriction on the incidence of complications following pancreaticoduodenectomy. ANZ J Surg. 2022;92(7–8):1797–1802. PMID: 35531886. doi:10.1111/ans.17751
15. Yokoi H, Chakravarthy V, Winkleman R, Manlapaz M, Krishnaney A. Incorporation of blood and fluid management within an enhanced recovery after surgery protocol in complex spine surgery. Global Spine J. 2022;23:21925682221120399. PMID: 35998380. doi:10.1177/21925682221120399
16. Scales K. NICE CG 174: intravenous fluid therapy in adults in hospital. Br J Nurs. 2014;23(8):S6, S8. PMID: 24763276. doi:10.12968/bjon.2014.23.Sup8.S6
17. Intravenous fluid therapy in adults in hospital. National Institute for Health and Care Excellence (NICE); 2017. PMID: 32101393
18. Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255(5):821–829. PMID: 22470070. doi:10.1097/SLA.0b013e31825074f5
19. Kopp BJ, Lenney M, Erstad BL. Balanced salt solutions for critically ill patients: nonplused and back to basics. Ann Pharmacother. 2022;56(12):1365–1375. PMID: 35392676. doi:10.1177/10600280221084380
20. Bulger EM, May S, Kerby JD, et al. ROC investigators. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011;253(3):431–441. PMID: 21178763; PMCID: PMC3232054. doi:10.1097/SLA.0b013e3181fcdb22
21. Bergmans SF, Schober P, Schwarte LA, Loer SA, Bossers SM. Prehospital fluid administration in patients with severe traumatic brain injury: a systematic review and meta-analysis. Injury. 2020;51(11):2356–2367. PMID: 32888722. doi:10.1016/j.injury.2020.08.030
22. Peltoniemi P, Pere P, Mustonen H, Seppänen H. Optimal perioperative fluid therapy associates with fewer complications after pancreaticoduodenectomy. J Gastrointest Surg. 2023;27(1):67–77. PMID: 36131201; PMCID: PMC9876870. doi:10.1007/s11605-022-05453-3
23. Weinberg L, Wong D, Karalapillai D, et al. The impact of fluid intervention on complications and length of hospital stay after pancreaticoduodenectomy (Whipple’s procedure). BMC Anesth. 2014;14(1):35. PMID: 24839398; PMCID: PMC4024015. doi:10.1186/1471-2253-14-35
24. Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to enhanced recovery after surgery (ERAS). Can J Anaesth. 2015;62(2):158–168. PMID: 25391735. doi:10.1007/s12630-014-0266-y
25. Krzych ŁJ, Czempik PF. Does fluid resuscitation with balanced solutions induce electrolyte and metabolic abnormalities? An in vitro assessment. Kardiol Pol. 2017;75(8):779–785. PMID: 28553873. doi:10.5603/KP.a2017.0079
26. Li Y, Wang SL, Yi XZ, Zhang J. Preinfusion dose analysis of succinyl gelatin injection in gastrectomy under general anesthesia. Shandong Med J. 2017;57(4):50–53.
27. Zhu QL, Deng YX, Yu BW, Zheng MH, Jin J. Acute hypervolemic infusion can improve splanchnic perfusion in elderly patients during laparoscopic colorectal surgery. Med Sci Monit. 2018;24:614–622. PMID: 29382813; PMCID: PMC5802329. doi:10.12659/msm.906155
28. Groene P, Wiederkehr T, Kammerer T, et al. Comparison of two different fibrinogen concentrates in an in vitro model of dilutional coagulopathy. Transfus Med Hemother. 2020;47(2):167–174. PMID: 32355477; PMCID: PMC7184830. doi:10.1159/000502016
29. Smart L, Boyd C, Litton E, et al. A randomised controlled trial of succinylated gelatin (4%) fluid on urinary acute kidney injury biomarkers in cardiac surgical patients. Intensive Care Med Exp. 2021;9(1):48. PMID: 34549356; PMCID: PMC8455786. doi:10.1186/s40635-021-00412-9
30. Koponen T, Musialowicz T, Lahtinen P. Gelatin and the risk of acute kidney injury after cardiac surgery. Acta Anaesth Scand. 2022;66(2):215–222. PMID: 34811729. doi:10.1111/aas.14004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
