Back to Journals » Journal of Pain Research » Volume 15
Comparison of the Effectiveness and Tolerability of Nabiximols (THC:CBD) Oromucosal Spray versus Oral Dronabinol (THC) as Add-on Treatment for Severe Neuropathic Pain in Real-World Clinical Practice: Retrospective Analysis of the German Pain e-Registry
Authors Ueberall MA , Essner U, Vila Silván C, Mueller-Schwefe GHH
Received 29 September 2021
Accepted for publication 23 December 2021
Published 2 February 2022 Volume 2022:15 Pages 267—286
DOI https://doi.org/10.2147/JPR.S340968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Michael A Ueberall,1 Ute Essner,2 Carlos Vila Silván,3 Gerhard HH Mueller-Schwefe4
1Center of Excellence in Health Care Research of the German Pain Association, Institute of Neurological Sciences, Nuernberg, Germany; 2Medical Department, O. Meany Consultancy GmbH, Hamburg, Germany; 3Global Medical Affairs, Almirall S.A., Barcelona, Spain; 4Medical Head, Schmerz- und Palliativzentrum Göppingen, Göppingen, Germany
Correspondence: Michael A Ueberall
Center of Excellence in Health Care Research of the German Pain Association, Institute of Neurological Sciences, Nordostpark 51, Nuernberg, 90411, Germany
Tel +49 911 21773760
Fax +49 911 21773761
Email [email protected]
Purpose: To compare the effectiveness and tolerability of add-on treatment with nabiximols (NBX: delta-9-tetrahydrocannabinol: cannabidiol) oromucosal spray or oral dronabinol (DRO: synthetic tetrahydrocannabinol) in patients with severe neuropathic pain poorly responsive to established treatments.
Methods: An analysis was conducted of anonymized, propensity score-matched real-world data from the German Pain e-Registry, using a sequential non-inferiority superiority approach, for adult outpatients with neuropathic pain who had initiated treatment with NBX or DRO between 10 March 2017 and 31 December 2019. The primary effectiveness variable was percent change from baseline in a 9-factor aggregated symptom relief (ASR-9) score, a composite index of nine distinct pain- and health-related parameters assessed using validated patient-reported instruments. Safety was assessed by the incidence of physician-confirmed treatment-related adverse events (TRAEs), and TRAEs leading to discontinuation.
Results: Propensity score-matched data were analyzed for 337 patients treated with NBX and 337 patients treated with DRO. Mean (standard deviation) THC dose over the 24-week evaluation period was 16.6 (6.5) mg for NBX and 17.2 (7.6) mg for DRO (p< 0.001). Median (standard error) improvement relative to baseline in the ASR-9 composite score was 55.4% (0.5) for NBX and 40.5% (0.5) for DRO (least squares mean difference, 14.0 (0.7), 95% confidence interval 12.6– 15.4; p< 0.001), and incidences of TRAEs (21.1 vs 35%) and TRAE-related discontinuations (5.9 vs 14.8%) were significantly lower with NBX than DRO (p< 0.001 for both), collectively indicating pre-specified non-inferiority and superiority of NBX. More NBX- than DRO-treated patients discontinued non-cannabinoid background pain medications and rescue analgesics, especially opioid analgesics (p< 0.001 for both).
Conclusion: Add-on treatment with cannabinoids is effective for treatment of severe neuropathic pain with inadequate response to established treatments. In daily practice, NBX had superior effectiveness and tolerability compared to DRO. The results emphasize the importance of combining CBD with THC in this patient population.
Keywords: nabiximols, oromucosal spray, dronabinol, neuropathic pain, real-world data, German Pain e-Registry
Introduction
Neuropathic pain is caused by a lesion or disease of the somatosensory system involving peripheral fibers and central neurons.1 Common causes of neuropathic pain include metabolic disorders (eg diabetes), viral infections (eg post-herpetic neuralgia, HIV), chemotherapy-induced peripheral neuropathies, autoimmune disorders affecting the central (eg multiple sclerosis [MS]) or peripheral (eg Guillain–Barre syndrome) nervous systems, and central post-stroke pain.1,2 The prevalence of pain with neuropathic characteristics in the general population is estimated at 7–10%,3 representing a substantial health resource burden.4
Symptoms of neuropathic pain are varied and patient-specific and include burning and electric-shock like sensations (dysesthesia), abnormal painful hypersensitivity to stimuli (hyperalgesia) and nociceptive responses to non-noxious stimuli eg light touching (allodynia).1,2 Individuals with neuropathic pain frequently suffer affective disturbances, anxiety and depression which can profoundly impair their quality of life.1,2
Neuropathic pain is difficult to treat and is often refractory to recommended treatments.5 German Society of Neurology guidelines recommend gabapentinoids (gabapentin, pregabalin) or antidepressants (duloxetine, amitriptyline) for first-line pharmacological management of neuropathic pain, with the option to switch drug class in case of inadequate response. Second-line options include combinations of first-line medications, and topical lidocaine and capsaicin (8%) patches which can also be regarded as primary treatment for localized peripheral neuropathic pain. Opioid analgesics are reserved for third-line therapy. Cannabinoids are also mentioned as a later-line option for patients with otherwise refractory pain.6
Evidence from preclinical animal models supports the role of cannabinoids and endocannabinoid system modulators in analgesia.7–9 Of the two main cannabinoids, delta-9-tetrahydrocannabinol (THC) mimics the effects of endogenous cannabinoids through its action as a partial agonist of cannabinoid 1 and 2 receptors. Cannabinoid 1 receptors are localized in several brain regions including those associated with pain processing and modulation, on peripheral primary afferent neurons, and in the dorsal horn of the spinal cord.8,10 Cannabidiol (CBD) does not interact directly with the endocannabinoid system but is associated with numerous other molecular targets such as ion channels, receptors, transporters and enzymes.11,12 In animal studies, CBD has been shown to reduce hyperalgesia and mechanical/thermal allodynia through various routes of administration.12 Concomitant administration of CBD and THC may potentiate the analgesic effects of THC while diminishing many of its adverse psychoactive effects.12
Nabiximols (Sativex®, NBX) oromucosal spray is a complex botanical mixture containing balanced quantities of THC and CBD, along with other cannabinoid and non-cannabinoid components.13 The medicine is approved in several world regions as add-on treatment in adults with moderate-to-severe MS-related spasticity who have not responded adequately to other antispasticity medications.14 NBX has also been investigated for treatment of neuropathic pain. Superior analgesic effects compared with placebo were demonstrated in randomized clinical trials (RCTs)15–19 and in a large observational study.20 A recent meta-analysis of nine RCTs involving 1289 participants concluded that NBX was superior to placebo in reducing chronic neuropathic pain, with a small but significant effect size.21
In the United States, dronabinol (DRO), a synthetic THC, is approved in adults for the treatment of anorexia-associated weight loss in AIDS patients and for cancer chemotherapy-associated nausea and vomiting.22,23 In Europe, DRO is approved in Austria, Denmark and Ireland for nausea and vomiting refractory to conventional treatment in cancer and palliative care; and additionally for cancer pain in Denmark and appetite stimulation in HIV in Ireland.24 Limited data are available for the efficacy of DRO in neuropathic pain and results are equivocal.25,26
Although neither NBX nor DRO is currently approved by the European Medicines Agency (EMA) for treatment of neuropathic pain, since March 2017, German physicians have been permitted to prescribe cannabis-based medicines for on-/off-label use in patients with severe disease resistant to available therapeutic options.27,28 The aim of the current study was to compare the effectiveness and tolerability of NBX with DRO for neuropathic pain by analyzing real-world data from the German Pain e-Registry (GPeR), a national web-based pain treatment registry that collects and stores data from around 230 pain centers and more than 800 pain specialists across the country.
Methods
Study Design
This was a cross-sectional, open-label, parallel-group, non-interventional, retrospective cohort analysis of anonymized real-world data provided by the GPeR to compare the effectiveness and tolerability of NBX oromucosal spray and oral DRO as add-on treatment in comparable patient populations with inadequate pain relief following recommended/established systemic therapy for peripheral neuropathic pain.
The GePR is a national web-based pain treatment registry developed by the Institute of Neurological Sciences on behalf of the German Pain Association.29 The GePR functions as a standard e-tool to capture patient-reported data about demography, history, pretreatment, pain characteristics, treatment response, etc., in daily practice; and fulfils physicians’ regulatory obligations according to the German Social Conduct of Law (V) for standardized (electronic) documentation of patients under treatment for severe/chronic pain. The GePR uses questionnaires recommended by the German Pain Association, German Pain Society and German Pain League which incorporate a broad spectrum of validated instruments addressing several pain- and health-related parameters. Under routine use of the GPeR, data are entered primarily by patients using electronic case report forms. Patient-entered data are then checked by physicians or other engaged healthcare professionals and supplemented by related physician information where appropriate and required to complete the dataset. After confirmation, the dataset for an individual timepoint is locked and cannot be further changed.
Inclusion and Exclusion Criteria
There was no formal sample size calculation for this study. Eligible GPeR datasets were those involving adult outpatients with medically confirmed peripheral neuropathic pain lasting for ≥3 months (based on explicit documentation in patients’ medical records and, as necessary, a painDETECT questionnaire [PDQ7] pain phenomenology score ≥19) and documented inadequate pain relief following recommended first- and second-line treatment for peripheral neuropathic pain. Patients were required to have newly initiated treatment with NBX or DRO between 10 March 2017 and 31 December 2019. The first dose of NBX or DRO during this period was defined as the treatment starting date. No previous use of NBX or DRO within 12 weeks of treatment initiation was permitted.
Datasets were excluded if they derived from patients with a diagnosis of cancer and/or cancer-related pain or chemotherapy-induced neuropathic pain; evidence of HIV and/or HIV-related pain; a diagnosis of osteoarthritis, complex regional pain syndrome, myofascial pain, trigeminal autonomic cephalalgia, or painful lesions of the cranial nerves not caused by post-herpetic neuralgia.
Data Analysis
Data from patients treated with NBX or DRO were propensity score matched using the nearest neighbor method (without replacement, caliper 0.15) for several baseline characteristics: age, gender, stage of chronification,30 severity of chronic pain,31 pain phenotype, pain duration, mean 24-hr pain intensity, previous and current pain medication (Anatomical Therapeutic Chemical [ATC] code, first 3 digits and/or medication group), concomitant illnesses (ICD-10, first 3 digits), comedications (ATC code, first 3 digits), and reasons for switching medication (ineffectiveness, poor tolerability, treatment-related adverse events [TRAEs]).
Study Treatments
NBX oromucosal spray and oral DRO were used under legislative conditions for use of cannabis as pain medicine enacted by the Parliament of the Federal Republic of Germany on 10 March 2017. All analgesic treatment followed medical requirements according to the previous decision of participating physicians and was based exclusively on individual patient needs with no external specifications other than those outlined in the prescribing information of respective products.
Outcome Measures
The primary effectiveness variable was the relative (percent) change from baseline in the 9-factor Aggregated Symptom Relief (ASR-9) score over the 24-week evaluation period. The ASR-9 is a composite measure of nine distinct pain- and health-related parameters assessed using validated patient-reported instruments aimed at broadly reflecting patients’ evolution; the ASR-9 composite score is calculated as the mean of relative (percent) change rates versus baseline for the individual components. The ASR-9 is the outcome measure used to inform management decisions in the routine care of pain patients enrolled in the GPeR and was reported previously.20
Secondary effectiveness outcomes were relative (percent) changes from baseline for individual components of the ASR-9: mean 24-h pain intensity index (PIX) reported on a 100 mm Visual Analogue Scale (VAS) from 0 (no pain) to 100 (worst pain conceivable); pain-related functional restrictions on activities of daily life (modified pain disability index [mPDI]) based on a 7-domain 100 mm VAS from 0 (none) to 100 (worst conceivable); physical and mental quality of life using the Veterans RAND 12-Item Health Survey (VR12-physical component score [PCS] and VR12 mental component score [MCS]);32 overall wellbeing (7-item Marburg questionnaire on habitual well-being [MFHW]);33 pain-related depression/anxiety/stress (21-item Depression, Anxiety and Stress Scale [DASS]);34 pain phenomenology assessed with the PDQ7.35,36
Treatment response was defined by a reduction of mean 24-h PIX equal to or greater than: the minimal clinically important difference (MCID) of 20 mm VAS; 50% pain relief versus baseline reported on the VAS; the tailored treatment target as defined by patients at baseline before the onset of treatment.
Each effectiveness parameter was evaluated at baseline and at 2-weekly intervals during a 24-week evaluation period.
The safety and tolerability of NBX and DRO were assessed by analyzing the frequency and spectrum of TRAEs, and TRAEs leading to discontinuation. TRAEs were defined as events that were newly reported or reported to worsen in severity after initiation of NBX and DRO and were evaluated as being possibly, probably or definitely related to treatment. TRAEs were classified according to Medical Dictionary for Regulatory Activities (MedDRA) system organ class (SOC) and summarized by treatment cohort.
Statistical Analyses
Analyses were conducted using propensity score matched data37 from the modified intent-to-treat population which was defined as patients who had (a) documented intake of at least one dose of NBX or DRO and (b) at least one post-baseline/post-dose measure.
The primary effectiveness outcome, relative (percent) change from baseline in the ASR-9 composite score, was analyzed using a mixed-model repeated measures (MMRM) covariance analytic method. The least squares means (LSM) difference in the ASR-9 score between treatments (NBX minus DRO) during the 24-week evaluation period (at 2-weekly intervals) and 95% confidence intervals (CI) were calculated using the model. Non-inferiority of NBX to DRO was concluded if the upper boundary of the 95% CI did not exceed +5.0. This narrow margin was selected to prevent super-interpretation of minor differences. If non-inferiority was demonstrated, a superiority analysis of the primary endpoint was to follow. Superiority was rejected if a) the 95% CI for the primary endpoint measure of both treatment cohorts overlapped; and/or b) the 95% CI of the LSM difference for the primary efficacy variable between both treatment groups included “0”; and/or c) the upper limit of the 95% CI was greater than −5.0; and/or d) ≥ one ASR-9 parameter failed to meet any of the above criteria. Using a multidimensional approach, the superiority of NBX was confirmed if a) all the above efficacy criteria were fulfilled and b) the incidence of treatment discontinuations due to TRAEs was significantly less in the NBX cohort than in the DRO cohort.
Descriptive statistics for continuous variables are summarized by number of patients, mean, standard deviation (SD), standard error (SE), 95% CI, median and range. Categorical and ordinal variables are summarized by frequency and percentage. Where applicable, 95% CI, Student’s t-test and Pearson’s chi-squared tests were used to compare groups with continuous or categorical variables. Differences in discontinuation rates between NBX and DRO treatment groups were evaluated using Fisher’s exact test. The baseline observation carried forward (BOCF) method was used to impute data missing due to treatment discontinuations in response to TRAEs or analgesic ineffectiveness, and the last observation carried forward (LOCF) method was used for all other missing values, except for dosage data which was analyzed as reported. All statistical tests were conducted using 2-tailed tests and a significance level of 0.05. No adjustments were made for multiple testing.
Ethical Considerations
This non-interventional study was conducted in line with the principles of the Declaration of Helsinki, and conformed to relevant national and regulatory requirements. The present study was approved by the ethics committees of the German Pain Association and German Pain League. Patients and physicians provided written informed consent prior to participation in the GPeR and patients agreed to use of their anonymized data for healthcare research purposes. The study was registered in the electronic database of the EMA for non-interventional studies (ENCEPP: EUPAS 33014). All analyses were performed using anonymized data to comply with national guidelines on protection of data privacy and the EU General Data Protection Regulation. Use of the electronic documentation platform iDocLive® and access to the GPeR was free of charge for members of the German Pain Association and for all patients regardless of their insurance status.
Results
Baseline Characteristics
Of 260,013 cases entered into the GPeR up to 31 December 2019, 6580 (2.5%) were patients who had newly started treatment with a cannabinoid-based medicine, mainly NBX (45.4%) or DRO (35%). Of these, 1315 NBX-treated cases and 389 DRO-treated cases met the study inclusion and exclusion criteria. Following propensity score matching, datasets for 337 patients per cohort were available for comparison (Figure 1). In each cohort, mean (SD) age was 45.8 (9.6) years, 57.3% were female, mean duration of pain was ~2.7 years, 57.9% had chronic pain (MPSS stage III) and 93.5% had severe dysfunctional pain (von Korff grade 3 or 4). Cohorts were evenly matched for number of comorbidities and comedications, type of peripheral neuropathic pain, previous and current analgesic treatment, current rescue analgesics, tailored treatment target, and all pain- and health-related measures (Table 1).
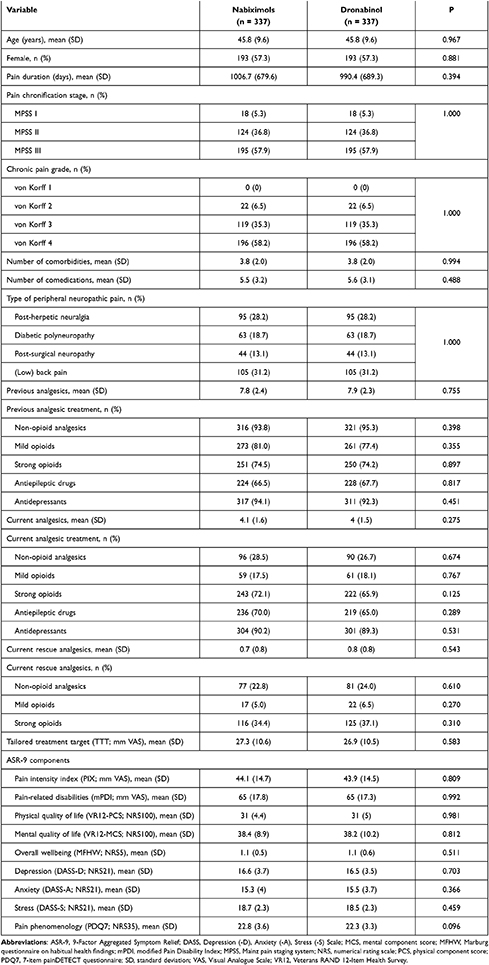 |
Table 1 Patient Demographics and Baseline Characteristics |
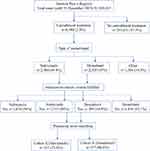 |
Figure 1 Patient flow chart. |
Patients who initiated treatment with NBX or DRO reported prior treatment with a mean (SD) of 7.8 (2.4) and 7.9 (2.3) analgesics and/or co-analgesics, respectively: antidepressants (94.1 vs 92.3%), non-opioid analgesics (93.8 vs 95.3%), mild opioids (81.0 vs 77.4%), strong opioids (74.5 vs 74.2%) and antiepileptic drugs (66.5 vs 67.7%). NBX and DRO were prescribed as add-on therapy to a current analgesic background of a mean (SD) of 4.1 (1.6) and 4.0 (1.5) medications, respectively: antidepressants (90.2 vs 89.3%), strong opioids (72.1 vs 65.0%), anticonvulsants (70.0 vs 65.0%), non-opioids (28.5 vs 26.7%), and mild opioids (17.5 vs 18.1%); and to a current rescue analgesic background of a mean (SD) of 0.7 (0.8) and 0.8 (0.8) medications, respectively: strong opioids (34.4 vs 37.1%), non-opioids (22.8 vs 24.0%) and mild opioids (5.0 vs 6.5%) (Table 1).
Treatment Discontinuations
By week 24, 80 patients (23.7%) treated with NBX and 134 patients (39.8%) treated with DRO had discontinued treatment, most commonly due to ineffectiveness (9.2 vs 13.1%, p<0.001) and TRAEs (5.9 vs 14.8%, p<0.001). Nine of ten discontinuations in both cohorts (91.3 vs 91.8%) occurred within the first 10 weeks of treatment. No further discontinuations were reported beyond week 14 (Figure 2).
 |
Figure 2 Treatment discontinuation rates and reasons for discontinuation in (A) nabiximols and (B) dronabinol cohorts. Abbreviations: TRAEs, treatment-related adverse effects; W, week. |
Study Medication Exposure
During the 24-week evaluation period, the applied mean (SD) dose of THC was slightly (but significantly) lower in patients treated with NBX than DRO [16.6 (6.5) vs 17.2 (7.6) mg/day; p<0.001] (Table 2). This was mainly attributable to clinically relevant dosage differences until week 8, with higher THC dosages recorded in DRO- versus NBX-treated patients. After week 8 and to the end of week 24, between-cohort THC dosages were comparable. The mean (SD) CBD dose in the NBX cohort during the 24-week evaluation period was 15.4 (4.1) mg/day.
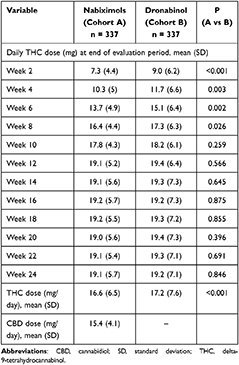 |
Table 2 THC Dose in Nabiximols and Dronabinol Groups Over 24 Weeks |
Effectiveness
Relative change from baseline over time in the ASR-9 composite score and individual components are shown in Figure 3. At the end of week 24, mean relative change (improvement) versus baseline in the ASR-9 composite score (primary effectiveness outcome) was significantly greater with NBX (Figure 3A) than DRO (Figure 3B): 60.8% vs 46.4% (LSM difference 14.4%; p<0.001; Cohen’s d effect size, 1.268). Mean relative change (improvement) rates at the end of week 24 for each ASR-9 component were significantly greater (all p<0.001) for patients treated with NBX (Figure 3A) than DRO (Figure 3B): 83.4 vs 75.9% for PIX, 76.0 vs 68.3% for mPDI, 27.8 vs 20.7% for VR12-PCS, 24.7 vs 18.4% for VR12-MCS, 64.1 vs 51.3% for MFHW, 70.5 vs 51.5% for DASS-D, 78.4 vs 52.3% for DASS-A, 80.7 vs 50.3% for DASS-S, and 40.8 vs 31.8% for PDQ7. The largest between-cohort differences in favor of NBX versus DRO at the end of week 24 were observed for stress (30.5%; effect size: 3.313), anxiety (26.2%; effect size: 1.526), depression (19.0%, effect size: 0.862), and overall wellbeing (12.8%; effect size: 0.862); the smallest difference was observed for pain phenomenology (9.0%; effect size: 0.288) (Figure 3C and D). Figure 3 Continue. Figure 4 Continue.

Throughout the 24-week evaluation period, NBX and DRO were each associated with significant improvements compared with baseline for evaluated (lowest, medium, highest) 24-hour pain intensity scores and calculated 24-hour PIX (all p<0.001; Table 3). However, mean (SD) absolute [36.8 (13.1) vs 33.3 (12.4) mm VAS] and relative [83.4 (12.6) vs 75.9 (17.0) percent] PIX improvement from baseline was significantly higher with NBX than DRO (p<0.001 for both). Response rates for patients who reported an improvement from baseline in mean 24-h PIX equal to or greater than a) the MCID of 20 mm VAS (92.0 vs 86.9%; p=0.006), b) 50% improvement in VAS score (98.8 vs 95.3%; p=0.002), and c) the tailored treatment target (95.8 vs 89.6%; p<0.001) were clinically relevant for both treatments, although significantly higher with NBX versus DRO (Table 3).
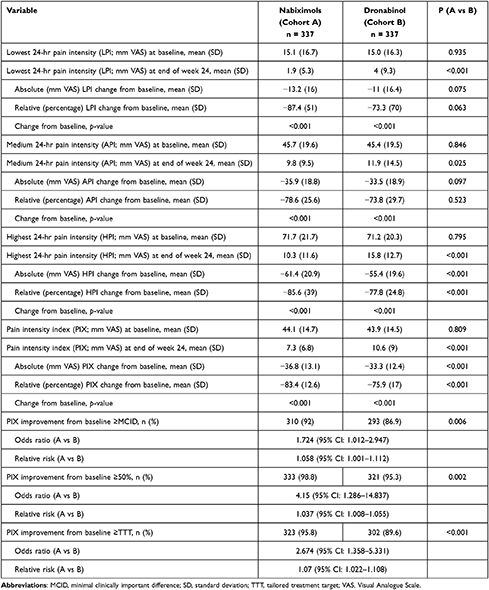 |
Table 3 Change in Pain Intensity from Baseline to Week 24 in Nabiximols and Dronabinol Groups |
Figure 4 shows response rates of patients treated with NBX (Figure 4A) or DRO (Figure 4B) who reported improvement from baseline in the ASR-9 composite score and individual components over the 24-week evaluation period, according to level of achievement (≥10%, ≥20%, etc.). An ASR-9 response of ≥50% relative to baseline was reported by 64.4 vs 22.8% of patients treated with NBX or DRO (p<0.001; odds ratio [OR]: 6.1, relative risk [RR]: 2.8; Figure 4C). Median percent ASR-9 improvement relative to baseline was 55.4 vs 40.5% (LSM difference 14.0; 95% CI: 12.6–15.4; Cohen’s d effect size: 0.9; Figure 4D). For any given response achievement level, and for all ASR-9 components, NBX was superior to DRO, with the highest between-cohort responder rate differences observed for stress (DASS-S: 45.5%), anxiety (DASS-A: 35.8%), and depression (DASS-D: 31.0%). Improvement rates of ≥50% relative to baseline were reported by 89.3 vs 78.6% of patients treated with NBX or DRO for PIX, 91.1 vs 75.1% for mPDI, 15.1 vs 7.8% for VR12-PCS, 10.9 vs 3.7% for VR12-MCS, 70.5 vs 51.3% for MFHW, 74.5 vs 43.6% for DASS-D, 79.2 vs 43.3% for DASS-A, 85.2 vs 39.8% for DASS-S, and 33.2 vs 24.0% for PDQ7. Responder rates were accompanied by comparable median percent improvements versus baseline in both treatment cohorts.
Mean effect sizes (Cohen’s d) for between-cohort differences reported from weeks 2 to 24 for relative improvement from baseline in the ASR-9 composite score and individual components are shown in Figure 5. According to Cohen’s d statistic, between-cohort differences in favor of NBX versus DRO were large (ie >0.8) for the ASR-9 composite score (0.893, 95% CI: 0.735–1.051), and for the individual components of stress (DASS-S: 1.323) and anxiety (DASS-A: 0.852). Effect sizes for depression (DASS-D: 0.598), mental component score (VR12-MCS: 0.546), and pain-related disability (mPDI: 0.538) were medium. Effect sizes for pain intensity (PIX: 0.320), physical component score (VR12-PCS: 0.400), overall wellbeing (MFHW: 0.436), and pain phenomenology (PDQ7: 0.288) were small.
Safety and Tolerability
Overall, 102 TRAEs were recorded by 71 patients (21.1%) treated with NBX, and 235 TRAEs were recorded by 118 patients (35.0%) treated with DRO (p<0.001; OR: 2.0, RR: 1.7, number need to harm [NNH]: 7.2; Table 4). A significantly lower proportion of NBX- versus DRO-treated patients reported ≥ two TRAEs (6.5 vs 21.1%, p<0.001; OR: 3.8, RR: 3.2, NNH: 6.8) or experienced TRAEs leading to treatment discontinuation (5.9 vs 14.8%; p<0.001; OR: 2.8, RR: 2.5, NNH: 11.2). By SOC, incidences of TRAEs were significantly lower with NBX than DRO for general disorders and administration site conditions (1.8% vs 5.9%; p<0.001), nervous system disorders (9.5 vs 19.9%; p<0.001) and psychiatric disorders (4.2% vs 14.8%; p<0.001).
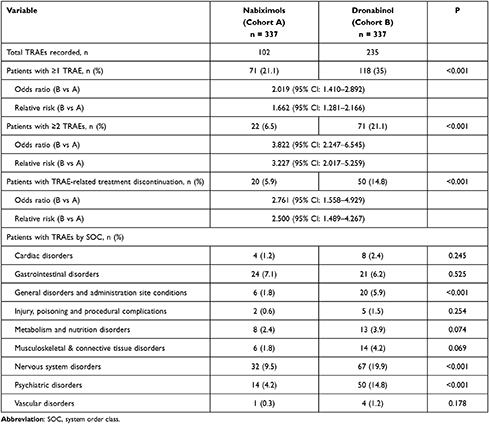 |
Table 4 Treatment-Related Adverse Events (TRAEs) in Nabiximols and Dronabinol Groups Over 24 Weeks |
Primary Endpoint Noninferiority Superiority Analysis
Both NBX and DRO significantly improved the ASR-9 composite score from baseline during the 24-week evaluation period (p<0.001 for both). Median (SE) ASR-9 improvement was 55.4% (0.502) and 40.5% (0.502), respectively. The LSM (SE) difference was 14.0 (0.711) with a 95% CI of 12.6 to 15.4 (p<0.001), thus fulfilling predefined criteria for noninferiority of NBX versus DRO. Given that the primary endpoint fulfilled all predefined prerequisites (ie no overlap of 95% CIs, 95% CIs of LSM did not include 0, upper 95% CI was > −5.0), a superiority analysis was performed, as defined in the statistical analysis plan. All 10 predefined superiority criteria were fulfilled, thus confirming the superiority of NBX over DRO.
Change of Concomitant Pain Medication
Add-on treatment with NBX or DRO was associated with a significant reduction in the use of current concomitant analgesia. The proportion of patients who discontinued all (41.8 vs 12.2%; p<0.001, OR: 5.2, RR: 3.4) or at least one (93.8 vs 73.3%; p<0.001; OR: 5.5, RR: 1.3) background analgesic was significantly greater in patients treated with NBX versus DRO (Figure 6A). Between-cohort differences (p<0.001 for all) were greatest for discontinuation of strong opioid analgesics (71.6 vs 33.8%), antidepressants (60.5 vs 24.9%), and anticonvulsants (70.3 vs 36.1%). In parallel, the proportion of patients stopping all (75.6 vs 45.9%; p<0.001, OR: 3.6, RR: 1.7) or at least one (83.7 vs 63.8%; p<0.001, OR: 2.9, RR: 1.3) rescue analgesic was significantly higher with NBX than DRO (Figure 6B), with between-cohort differences (p<0.001 for all) greatest for discontinuation of mild (82.4 vs 27.3%) and strong (75.9 vs 46.4%) opioids.
Discussion
This retrospective observational study used real-world data provided by the GPeR to compare the effectiveness and tolerability of NBX oromucosal spray with oral DRO, administered as add-on treatment to underlying analgesia in patients with severe neuropathic pain. Propensity score matching of patients for multiple baseline characteristics to eliminate confounding factors enabled a study design that was similar to a RCT.37 Cohorts were well matched for demographics and a series of clinically relevant pain-, treatment- and health-related characteristics.
Both treatments significantly improved pain parameters from baseline and allowed a substantial proportion of patients to reduce their background and rescue pain medication. In comparison to DRO, a significantly higher proportion of NBX-treated patients reported clinically relevant improvement compared with baseline over the entire 24-week evaluation period, as well as significantly fewer TRAEs and TRAE-related treatment discontinuations. Based on fulfilment of the 10 predefined superiority criteria and a TRAE-related discontinuation rate in favor of NBX, treatment with NBX proved to be superior to DRO. Effect size analyses confirmed the clinical relevance of this difference for daily practice. Relative improvements with NBX in effectiveness outcomes occurred despite comparable mean THC dosages during the 24-week evaluation period (17.2 vs.16.6 mg/day), suggesting the benefit of combining CBD with THC in the NBX formulation, compared with THC alone in the DRO formulation.
Responder rates were significantly higher in NBX- versus DRO-treated patients for all nine individual components of the ASR-9, although effect sizes assessed with Cohen’s d varied. Large effects (ie >0.8) were found for stress (DASS-S) and anxiety (DASS-A), possibly attributable to the specific effects of CBD as shown in studies of patients suffering from posttraumatic stress disorder.38,39 Medium effects (ie >0.5) were seen for pain-related disabilities in daily life activities (mPDI), mental QoL (VR12-MCS) and depression (DASS-D). Small effects (ie >0.2) were observed for pain intensity (PIX), pain phenomenology (PDQ7), overall wellbeing (MFHW) and physical QoL (VR12-PCS). In general agreement with the results for PIX, a small effect size for the change in mean pain score from baseline (using an 11-point numerical rating scale [NRS]) was reported for NBX versus placebo in a meta-analysis of RCTs in patients with chronic neuropathic pain: the mean difference on the 11-point NRS was –0.40 (95% CI: –0.59 to –0.21; p<0.0001).21
Surprisingly, between-cohort differences for NBX versus DRO treatment at the end of study (week 24) in patients with neuropathic pain were not driven predominantly by dramatic differences in biological parameters such as pain relief (respective improvement of 83.4% vs 75.9%; mean effect size of 0.513 indicating moderate clinical relevance) or pain phenomenology (respective improvement of 40.8% vs 31.8%; effect size of 0.288 indicating moderate clinical relevance), but rather by psychological parameters (especially stress and anxiety) and a significantly better tolerability profile of NBX vs DRO. There is preclinical and clinical evidence to suggest that CBD can counteract or attenuate some of the undesirable effects of THC, although additional clinical research is required in this area.40,41
In common with NBX, DRO significantly reduced pain intensity from baseline at 24 weeks. This finding was interesting as data for DRO in neuropathic pain are scarce. A small 3-week crossover RCT in 24 patients with MS reported lower median spontaneous pain intensity (11-point NRS) with DRO 10 mg compared with placebo (4 vs 5; p = 0.02) for central neuropathic pain in the last week of treatment.25 Elsewhere, a 16-week placebo-controlled RCT in 240 MS patients with central neuropathic pain found no significant difference in pain intensity reduction (11-point NRS) from baseline between DRO (–1.92 points) and placebo (–1.81 points).26 Our findings suggest that THC has a role in neuropathic pain as previously demonstrated in several placebo-controlled RCTs of NBX.15–19 Formulation appears to be key to patients benefitting from the synergy between THC and CBD, and possibly other phytotherapeutic substances (eg terpenoids) found in the Cannabis sativa plant.42
A higher proportion of patients discontinued treatment with DRO than NBX (39.8% vs 23.7%), mainly due to TRAEs (14.8% vs 5.9%), which occurred most frequently in the SOCs of general disorders and administration site conditions, nervous system disorders and psychiatric disorders. Common AEs in RCTs of NBX in neuropathic pain were dizziness, dysgeusia, nausea, fatigue, dry mouth and somnolence, especially during the first few weeks of treatment.15–19 The current European approved label lists dizziness (nervous system disorder) and fatigue (general disorder) as very common adverse drug reactions (ADRs; frequency ≥ 1/10) with possible causality to NBX.14 In a RCT of DRO in 240 patients with neuropathic pain, common ADRs were dizziness, vertigo, fatigue, and dry mouth, occurring most often in the initial 4 weeks of treatment during up-titration to the maximum tolerable dose.26 The FDA’s Prescribing Information for DRO lists dizziness, euphoria, paranoid reaction, somnolence, thinking abnormal, abdominal pain, nausea and vomiting as the most common ADRs.22,23 The mitigating effects of CBD on the psychoactive effects of THC12 might explain the similarities and differences in the safety profiles of NBX and DRO.
A higher proportion of NBX- than DRO-treated patients were able to discontinue non-cannabinoid background analgesics, possibly due to greater symptomatic improvement in several individual components of the ASR-9. Discontinuation rates were significantly higher with NBX for each class of background medication and most types of rescue pain medication. Importantly, both cannabinoid treatments were able to reduce patients’ requirement for long-acting opioid analgesics as background medication and especially immediate-release/rapid-onset opioid analgesics as rescue medication, which are known to carry a significant risk in patients with chronic (neuropathic) pain.
In common with all real-world registry analyses, the study is limited by its retrospective, non-randomized observational design, although propensity score matching allowed us to compare two patient cohorts with similar clinical features. While propensity bias may not have been fully addressed by the range of selected factors, all factors are established predictors of pain and are relevant for between-cohort comparisons. The primary endpoint in this analysis was the relative (percent) change from baseline in the ASR-9 composite score; this outcome measure was first reported in a previous 12-week exploratory analysis of the GPeR database.20 Although the ASR-9 is not a scientifically developed and validated instrument, it is a composite measure of nine individual validated patient-reported tools that measure the impact of pain across multiple dimensions. As such, the ASR-9 may represent a more integrated and holistic approach to pain management. Strengths of the study are the large sample size and representative patient population. Prospective collection and systematic analyses of GPeR data minimizes the potential for missing values as often occurs in retrospective studies. As participation in the GPeR is nationwide, our findings may have broad applicability across Germany.
Conclusion
Treatment with cannabinoids appears to be an effective alternative option for patients with severe neuropathic pain that is poorly responsive to established treatments. Although both cannabinoids evaluated in this analysis were effective, NBX proved to be superior to DRO due to significantly better improvement rates in all biopsychosocial dimensions of neuropathic pain and significantly lower rates of TRAEs and associated discontinuation rates. The results underline the importance of combining CBD with THC in this patient group.
Declaration of Financial/Other Relationships
The concept for evaluating routine data collected via the GPeR was developed by Dr Ueberall at the Institute of Neurological Sciences on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin) and German Pain League (Deutsche Schmerzliga). Its realization has been funded in part (~25%) by an unrestricted scientific grant from Almirall Hermal, Germany.
Dr Ueberall and Dr Mueller-Schwefe are physicians and independent of any significant/relevant financial or other relationship to the sponsor, apart from minor reimbursements for occasional lecture or consulting fees. Both are honorary members of the management boards of the German Pain Association and German Pain League. Dr Essner is a veterinarian and works as a scientific and medico-legal consultant for various pharmaceutical companies. Dr Vila Silván is a physician and works as the Global Medical Advisor CNS for Almirall SA (Barcelona, Spain).
The GPeR is hosted by an independent contract research organization by order of the German Pain Association, is under control of the Institute of Neurological Sciences, and has been collecting standardized real-world data from daily routine medical care since January 2000.
Acknowledgments
Editorial assistance was provided by Robert Furlong and Kerry Dechant on behalf of Content Ed Net (Madrid, Spain) with funding from Almirall S.A. (Barcelona, Spain).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Analyses were funded by an unrestricted grant from Almirall S.A.
Disclosure
MAU has received financial support and/or expenses in form of research funds, consultancy fees and/or renumerations for lecture activities from Allergan, Almirall, Amicus Therapeutics, Aristo Pharma, Bionorica, Glaxo Smith Kline, Grünenthal, Hapa Medical, Hexal, IMC, Kyowa-Kirin, Labatec, Mucos, Mundipharma, Nestle, Pfizer, Recordati, Servier, SGP-Pharma, Shionogi, Spectrum Therapeutics, Strathmann, Teva, and Tilray. He is also an honorary member of the management boards of the German Pain Association and the German Pain League, Medical Director of the private Institute of Neurological Sciences and CEO of O.Meany-MDPM GmbH, all of which are working on/with the German Pain e-Registry. UE has received honoraria for consultancy services from Almirall Hermal GmbH and Granzer Regulatory Consulting & Service. CVS is a full-time employee of Almirall S.A. GHHM-S has received financial support and/or expenses in the form of research funds, consultancy fees and/or remunerations for lecture activities from Allergan, Almirall, Grünenthal, Mundipharma, Pfizer, PharmAllergan, ProStrakan, and Teva. The authors report no other conflicts of interest in this work.
References
1. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi:10.1038/nrdp.2017.2
2. Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383. doi:10.1177/2058738419838383
3. Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–662. doi:10.1016/j.pain.2013.11.013
4. Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–2843. doi:10.1016/j.pain.2011.09.014
5. Gilron I, Baron R, Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 2015;90(4):532–545. doi:10.1016/j.mayocp.2015.01.018
6. Schlereth T. Guideline “diagnosis and non interventional therapy of neuropathic pain” of the German Society of Neurology (deutsche Gesellschaft für Neurologie). Neurol Res Pract. 2020;2:16. doi:10.1186/s42466-020-00063-3
7. Vučković S, Srebro D, Vujović KS, Vučetić Č, Prostran M. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;9:1259. doi:10.3389/fphar.2018.01259
8. Finn DP, Haroutounian S, Hohmann AG, Krane E, Soliman N, Rice AS. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain. 2021;162(Suppl 1):S5–S25. doi:10.1097/j.pain.0000000000002268
9. Soliman N, Haroutounian S, Hohmann AG, et al. A systematic review and meta-analysis of cannabis-based medicines, cannabinoids and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury-related or pathological persistent pain. Pain. 2021;162(Suppl 1):S26–S44. doi:10.1097/j.pain.0000000000002269
10. Donvito G, Nass SR, Wilkerson JL, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43(1):52–79. doi:10.1038/npp.2017.204
11. Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730. doi:10.1007/s13311-015-0377-3
12. Mlost J, Bryk M, Starowicz K. Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Int J Mol Sci. 2020;21(22):8870. doi:10.3390/ijms21228870
13. Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66(2):234–246. doi:10.1016/j.mehy.2005.08.026
14. emc. Sativex oromucosal spray. summary of product characteristics; 2020. Available from: https://www.medicines.org.uk/emc/product/602/smpc#gref.
15. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112(3):299–306. doi:10.1016/j.pain.2004.09.013
16. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65(6):812–819. doi:10.1212/01.wnl.0000176753.45410.8b
17. Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133(1–3):210–220. doi:10.1016/j.pain.2007.08.028
18. Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260(4):984–997. doi:10.1007/s00415-012-6739-4
19. Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18(7):999–1012. doi:10.1002/j.1532-2149.2013.00445.x
20. Ueberall MA, Essner U, Mueller-Schwefe GH. Effectiveness and tolerability of THC:CBD oromucosal spray as add-on measure in patients with severe chronic pain: analysis of 12-week open-label real-world data provided by the German Pain e-Registry. J Pain Res. 2019;12:1577–1604. doi:10.2147/JPR.S192174
21. Dykukha I, Malessa R, Essner U, Überall MA. Nabiximols in chronic neuropathic pain: a meta-analysis of randomized placebo-controlled trials. Pain Med. 2021;22(4):861–874. doi:10.1093/pm/pnab050
22. Marinol (dronabinol) prescribing information; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
23. Syndros (dronabinol) prescribing information; 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205525s000lbl.pdf.
24. Krcevski-Skvarc N, Wells C, Häuser W. Availability and approval of cannabis-based medicines for chronic pain management and palliative/supportive care in Europe: a survey of the status in the chapters of the European Pain Federation. Eur J Pain. 2018;22(3):440–454. doi:10.1002/ejp.1147
25. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329(7460):253. doi:10.1136/bmj.38149.566979
26. Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol. 2017;78(5–6):320–329. doi:10.1159/000481089
27. Gesetz zur Änderung betäubungsmittelrechtlicher und anderer Vorschriften [Law amending narcotics law and other regulations]. Bundesgesetzblatt; 2017. Available from: https://www.bgbl.de/xaver/bgbl/start.xav?start=%2F%2F*%5B%40attr_id%3D%27bgbl117s0403.pdf%27%5D#__bgbl__%2F%2F*%5B%40attr_id%3D%27bgbl117s0403.pdf%27%5D__1537108060099.
28. Lips J, Witt J. Cannabis law and legislation in Germany; 2021. Available from: https://cms.law/en/int/expert-guides/cms-expert-guide-to-A-legal-roadmap-to-cannabis/germany.
29. Deutsche Gesellschaft für Schmerzmedizin e.V. Dokumentationsplattform iDocLive®.[German Society for Pain Medicine registered association documentationplatform iDocLive®]. Available from: https://www.dgschmerzmedizin.de/.
30. Nagel B, Gerbershagen HU, Lindena G, Pfingsten M. Entwicklung und empirische Überprüfung des Deutschen Schmerzfragebogens der DGSS [Development and evaluation of the multidimensional German pain questionnaire of the DGSS]. Schmerz. 2002;16(4):263–270. doi:10.1007/s00482-002-0162-1
31. von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–149. doi:10.1016/0304-3959(92)90154-4
32. Kazis LE, Miller DR, Skinner KM, et al. Patient-reported measures of health: the Veterans Health Study. J Ambul Care Manage. 2004;27(1):70–83. doi:10.1097/00004479-200401000-00012
33. Basler HD. Marburger Fragebogen zum habituellen Wohlbefinden. Untersuchung an Patienten mit chronischem Schmerz [The Marburg questionnaire on habitual health findings–a study on patients with chronic pain]. Schmerz. 1999;13(6):385–391. German. doi:10.1007/s004829900047
34. Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck depression and anxiety inventories. Behav Res Ther. 1995;33(3):335–343. doi:10.1016/0005-7967(94)00075-u
35. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi:10.1185/030079906X132488
36. Cappelleri JC, Bienen EJ, Koduru V, Sadosky A. Measurement properties of painDETECT by average pain severity. Clinicoecon Outcomes Res. 2014;6:497–504. doi:10.2147/CEOR.S68997
37. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
38. Bitencourt RM, Takahashi RN. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: from bench research to confirmation in human trials. Front Neurosci. 2018;12:502. doi:10.3389/fnins.2018.00502
39. Elms L, Shannon S, Hughes S, Lewis N. Cannabidiol in the treatment of post-traumatic stress disorder: a case series. J Altern Complement Med. 2019;25(4):392–397. doi:10.1089/acm.2018.0437
40. Niesink RJM, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130. doi:10.3389/fpsyt.2013.00130
41. Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9-tetrahydrocannabinol. Neuropsychopharmacology. 2018;43(1):142–154. doi:10.1038/npp.2017.209
42. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–1364. doi:10.1111/j.1476-5381.2011.01238.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




