Back to Journals » Infection and Drug Resistance » Volume 15
Comparison of the Diagnostic Accuracy of Xpert MTB/RIF and CapitalBio Mycobacterium RT-PCR Detection Assay for Tuberculous Pericarditis
Authors Yu G , Wang L, Shen Y , Fang L, Yang J, Ye B, Xu K, Zhong F
Received 28 January 2022
Accepted for publication 9 April 2022
Published 22 April 2022 Volume 2022:15 Pages 2127—2135
DOI https://doi.org/10.2147/IDR.S360064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Guocan Yu,1,* Linhua Wang,2 Yanqin Shen,1 Likui Fang,1 Jun Yang,1 Bo Ye,1 Kan Xu,1 Fangming Zhong1,*
1Zhejiang Tuberculosis Diagnosis and Treatment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Hospital Infection, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guocan Yu; Fangming Zhong, Zhejiang Tuberculosis Diagnosis and Treatment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected]; [email protected]
Purpose: We evaluated CapitalBio Mycobacterium RT-PCR assay diagnosing tuberculous pericarditis (TBP), performed a head-to-head comparison with Xpert MTB/RIF, and assessed the impact of a parallel test (positive result for either of these two tests).
Methods: We reviewed suspected TBP patients with Xpert MTB/RIF, CapitalBio Mycobacterium RT-PCR assay, and Mycobacterium tuberculosis (MTB) culture. We analyzed sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC).
Results: Seventy-four patients were included. Overall sensitivity, specificity, PPV, NPV, and AUC of CapitalBio Mycobacterium RT-PCR assay compared with culture were 50%, 91.1%, 64.3%, 85%, and 0.71, respectively. Overall sensitivity, specificity, PPV, NPV, and AUC of Xpert MTB/RIF for TBP were 61.1%, 91.1%, 68.8%, 87.9%, and 0.76. Parallel test values were 72.2%, 91.1%, 72.2%, 91.1%, and 0.82. The diagnostic accuracy of Xpert MTB/RIF was higher than CapitalBio Mycobacterium RT-PCR assay but was not significant (P > 0.05). The parallel test could improve diagnostic accuracy, but it was not significant compared to single tests (P > 0.05).
Conclusion: CapitalBio Mycobacterium RT-PCR assay had a moderate diagnostic accuracy, similar to Xpert MTB/RIF. The parallel test maximized diagnostic efficacy, but differences were not significant. CapitalBio Mycobacterium RT-PCR assay and Xpert MTB/RIF for TBP could be an initial option for early diagnosis.
Keywords: tuberculosis pericarditis, polymerase chain reaction, Xpert MTB/RIF, CapitalBio, accuracy
Introduction
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis (MTB), remains a major threat to public health.1 TB is usually divided into two main categories depending on the MTB infection site: pulmonary TB (PTB) and extrapulmonary TB (EPTB).2 Tuberculous pericarditis (TBP) is a relatively rare form of EPTB, but it is usually severe and could lead to cardiac tamponade, constrictive pericarditis, and even death.3 Due to the AIDS epidemic, the incidence of TBP has increased and is the leading cause of pericarditis in areas with a high TB burden.4 TBP is prone to adverse outcomes, with a death rate of 17% to 40% over six months after onset.5 Early and effective TBP treatment is beneficial to prognosis, and the prerequisite for treatment is a precise diagnosis, which is still extremely challenging, especially in terms of the microbiological diagnosis.6 Adenosine deaminase (ADA) is the most used indicator for diagnosing TBP in pericardial fluid,7 but it is not microbiological evidence since other causes, such as bacterial infections, can elevate ADA in effusions.8 Other TB detection tests, such as interferon-γ release (T-SPOT.TB), cannot directly detect MTB and provide indirect evidence.9 On the other hand, the sensitivity of conventional acid-fast bacilli (AFB) smear (3.8%) in pericardial fluid is unsatisfactory,10 and MTB culture is time-consuming, both of which limit early diagnosis of TBP.11
Nucleic acid amplification tests (NAATs) are a major advance in the diagnosis of TB and can detect MTB DNA or RNA, providing microbiological evidence.12 The Xpert MTB/RIF assay is one of the most widely used NAATs for diagnosing TB and has demonstrated good diagnostic performance in PTB and EPTB.13,14 It can obtain test results within 2 hours to meet the need for rapid diagnosis.15 In TBP, Xpert MTB/RIF also showed relatively good performance with a sensitivity of 63.8%.16 The CapitalBio Mycobacterium real-time polymerase chain reaction (RT-PCR) assay is a new NAAT developed by CapitalBio that can detect both MTB and non-tuberculous mycobacteria (NTM) in a single test.17 CapitalBio Mycobacterium RT-PCR detection assay is more widely used in clinical practice and has shown diagnostic efficacy in PTB and EPTB that is not inferior to Xpert MTB/RIF.18,19 It can also detect NTM, which is impossible with Xpert MTB/RIF.18 The role of CapitalBio Mycobacterium RT-PCR detection assay in the diagnosis of TBP is currently unreported. The purpose of this study was to evaluate the efficacy of the CapitalBio Mycobacterium RT-PCR detection assay in the diagnosis of TBP and to perform a head-to-head comparison with Xpert MTB/RIF, and assess the impact of a parallel test (a positive test for either of the two tests was considered a positive parallel test) on the diagnosis of TBP.
Materials and Methods
Study Design
This retrospective study was conducted at the Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, the TB diagnosis and treatment center of Zhejiang Province. We reviewed 83 patients with suspected TBP hospitalized between June 1, 2019, and June 31, 2021, tested for TBP with the Xpert MTB/RIF, CapitalBio Mycobacterium RT-PCR assay, and MTB culture using pericardial fluid. Patients with TB-related clinical symptoms (such as chest tightness, recurrent low-grade fever), computed tomography or ultrasound findings of pericardial effusion, pericardial thickening and/or calcification, history of TB or contact with TB patients, positive gamma interferon release test or purified protein derivative test, or other sites involved of TB were considered suspected TBP. These patients were not on regular anti-TB treatment before diagnosis. All patients signed written informed consent for pericardiocentesis to obtain a specimen of pericardial fluid for testing. Patients with a small amount of pericardial fluid (width of pericardial effusion less than 1 cm) who could not undergo pericardiocentesis and in who Xpert MTB/RIF, CapitalBio Mycobacterium RT-PCR detection assay, and MTB culture were not performed at the same time, or who had inconclusive results were excluded from this study. We used the results of MTB culture as the reference standard for this study. All patients signed written consent forms. The present study complied with the Declaration of Helsinki. Ethical approval was obtained from the Human Research Ethics Committee of the Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine (2022-YS-012).
Diagnostic Specimen Collection and Handling
Pericardial fluid specimens were used for the relevant tests. Pericardial fluid was obtained by ultrasound-guided pericardiocentesis. The fresh pericardial fluid specimens were divided into three equal portions for the Xpert MTB/RIF, the CapitalBio Mycobacterium RT-PCR detection assay, and MTB culture.
MTB Culture
Lowenstein–Jensen solid medium and liquid culture medium (BACTEC MGIT 960 Mycobacteria Culture System, BD Diagnostic Systems, Sparks, MD) were used for MTB culture according to the manufacturer’s instructions at 37°C for 2–6 weeks.
CapitalBio Mycobacterium RT-PCR Detection Assay
According to the manufacturer’s instructions for the conductions of this test, at least 3 mL of pericardial fluid was centrifuged at 3000 g for approximately 15 minutes. After discarding the supernatant, the sediment was obtained. Subsequently, the sediment and nucleic acid extraction solution were added to the nucleic acid extraction tube. After subjecting the samples to vibration and incubation in a 95°C-water bath for 10 min, the centrifuged samples were used to extract MTB DNA. The extracted MTB DNA was used to perform the CapitalBio Mycobacterium RT-PCR detection assay (CapitalBio Technology Inc., Beijing, China; http://www.capitalbiotech.com) according to the manufacturer’s instructions. An RT-fluorescence quantification PCR instrument (SLAN-96S Real-Time PCR System ZEESAN Xiamen, CN) was used for nucleic acid amplification to detect IS6110 and HSP65 multicopy elements for MTB and NTM, respectively. The test results were obtained within 3 hours.14
Xpert MTB/RIF
As with the CapitalBio Mycobacterium RT-PCR detection assay, the Xpert MTB/RIF used at least 3 mL of pericardial fluid. The pericardial fluid was first centrifuged at 3000 g for 15 minutes. After discarding the supernatant, 2 mL of sample treatment solution was added and mixed thoroughly by shaking. Finally, 2 mL of the treated sample was added to the first generation Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) reaction cassette. Semi-quantitative estimation of MTB load was determined by Xpert MTB/RIF as “high”, “medium”, “low”, or “very low”. The system performs automatic testing and reports the results within 2 hours.20
Data Processing and Statistical Analysis
Microsoft Excel 2019 was used to manage the data. SPSS 24.0 (IBM Corp., Armonk, NY, USA) was used to calculate mean values, standard deviations, proportions and determine true positive (TP), false positive (FP), false negative (FN), and true negative values in cross-tabulations. Using TP, FP, FN, and TN values, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) with a 95% confidence intervals were calculated for Xpert MTB/RIF and CapitalBio Mycobacterium RT-PCR detection assay using MedCalc Statistical v15.2.2 (MedCalc Software Bvba, Ostend, Belgium; http://www.medcalc.org) with MTB culture as the reference standard to evaluate the diagnostic accuracy for TBP. All patients were included in the diagnostic accuracy test, with sensitivity calculated in MTB culture-positive patients and specificity calculated in culture-negative patients. We further evaluated the diagnostic accuracy of a parallel test (a positive test for either Xpert MTB/RIF and CapitalBio Mycobacterium RT-PCR detection assay was considered a positive parallel test). McNemar’s test was used to compare paired data, and a Chi-square test or Fisher’s exact test was used to compare proportions. A Z-test was used for AUC comparisons. An online interactive tool (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to generate a Venn diagram. Differences with a P-value <0.05 were considered statistically significant.
Results
We screened the clinical data of patients with suspected TBP using pericardial effusion for relevant tests. Finally, 74 patients hospitalized between June 1, 2019, and June 31, 2021, were included. Each included patient contributed one pericardial fluid specimen for a total of 74 specimens. The mean age of the 74 patients was 59.4 ± 17.1 years, of which 48 (64.9%) were men. All patients tested negative for human immunodeficiency virus antibody by enzyme-linked immunosorbent assay. Fifteen patients had coexisting PTB. Forty specimens were tested with AFB smear, and all were negative. No patient received a diagnosis of NTM infection. Table 1 shows the clinical characteristics of the included patients.
 |
Table 1 Clinical Characteristics of the Included Patients |
Figure 1 illustrates the classification of patients using culture as the reference standard. Eighteen patients were diagnosed with TBP, and the remaining 56 patients were non-TBP. Among all 74 patients, there were 18, 16, 14, and 18 positive MTB culture, Xpert MTB/RIF, CapitalBio Mycobacterium RT-PCR detection assay, and parallel test values, respectively. Cycle threshold (Ct) values for CapitalBio Mycobacterium RT-PCR detection assay -positive specimens ranged from 24 to 34, with a mean Ct value of 31. The semi-quantitative results for the positive Xpert MTB/RIF specimens were as follows: 6.3% (1/16) “high”, 37.5% (6/16) “medium”, 50.0% (8/16) “low”, and 6.3% (1/16) “very low”. The distribution and overlap of the positive MTB culture, Xpert MTB/RIF, CapitalBio Mycobacterium RT-PCR detection assay, and parallel test are shown in Figure 2. Among all specimens, 7 were positive with the CapitalBio Mycobacterium RT-PCR detection assay, Xpert MTB/RIF, and MTB culture; 12 were positive with the CapitalBio Mycobacterium RT-PCR detection assay and Xpert MTB/RIF; 9 were positive with the CapitalBio Mycobacterium RT-PCR detection assay and MTB culture; 11 were positive with the Xpert MTB/RIF and MTB culture, and 5 were positive for MTB culture only.
 |
Figure 1 Flow and classification of patients included in this study. |
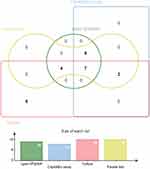 |
Figure 2 Venn diagram of positive tests for tuberculosis pericarditis patients. |
Among the TBP group, there were 11, 9, and 13 positive Xpert MTB/RIF, CapitalBio Mycobacterium RT-PCR detection assays, and parallel test values, respectively; and in the non-TBP group, the values of positive results for these tests were all 5. The positive and negative rate for each index and reference test is shown in Table 2.
 |
Table 2 The Positive and Negative Rate for Each Index and Reference Test |
Diagnostic Accuracy of CapitalBio Mycobacterium RT-PCR Detection Assay, Xpert MTB/RIF, and Parallel Test
The overall sensitivity, specificity, PPV, NPV, and AUC of the CapitalBio Mycobacterium RT-PCR detection assay using pericardial fluid for the diagnosis of TBP compared with MTB culture were 50.0% (26.0–74.0%), 91.1% (80.4–97.0%), 64.3% (35.1–87.2%), 85.0% (73.4–92.9%), and 0.71 (0.59–0.81), respectively. The overall sensitivity, specificity, PPV, NPV, and AUC of Xpert MTB/RIF for TBP were 61.1% (35.8–82.7%), 91.1% (80.4–97.0%), 68.8% (41.3–89.0%), 87.9% (76.7–95.0%), and 0.76 (0.65–0.85), respectively. These values for the parallel test were 72.2% (46.5–90.3%), 91.1% (80.4–97.0%), 72.2% (46.5–90.3%), 91.1% (80.4–97.0%), and 0.82 (0.71–0.90), respectively. These results are shown in Table 3.
 |
Table 3 Accuracy of CapitalBio Mycobacterium RT-PCR Assay, Xpert MTB/RIF, and Parallel Test for Tuberculous Pericarditis Compared with Culture Using Pericardial Fluid |
Comparison of the Diagnostic Accuracy of the CapitalBio Mycobacterium RT-PCR Detection Assay, Xpert MTB/RIF, and Parallel Test
The diagnostic accuracy of the Xpert MTB/RIF in diagnosing TBP using pericardial fluid was higher than the CapitalBio Mycobacterium RT-PCR detection assay, but the difference did not reach statistical significance (P > 0.05, Table 4). The parallel test could improve diagnostic accuracy compared to single tests, but the difference in comparison to single tests also did not reach statistical significance (P > 0.05, Table 4).
Discussion
Tuberculous pericarditis (TBP) is a severe form of TB, accounting for about 4% of all pericarditis in developed countries.21 In contrast, in areas with a high burden of TB, usually developing countries, TBP can account for up to 60%.22 Early, rapid and precise diagnosis and treatment are urgently needed to improve the prognosis of this group of patients, especially in areas with a high TB burden. Biochemical changes in pericardial effusion are an important aspect of the diagnosis of TBP, but they do not provide a microbiological basis. In this study, the biochemical parameters of pericardial effusion such as adenosine deaminase and lactate dehydrogenase were higher in the TBP group than in the non-TBP group, and these alterations also supported the diagnosis of TBP. Although traditional acid-fast bacilli (AFB) smear is easy and inexpensive, the positive rate is extremely low and easily leads to missed diagnoses.10 In this study, although not all pericardial fluid specimens underwent AFB smear testing, no positive cases were found in the specimens in which this test was performed. This result might be polarized and related to specimen selection, but it also suggests that the positive rate of AFB smear in the pericardial fluid is so low that in clinical practice, clinicians were prone to ignore tests with such low sensitivity and thus prefer other relatively better tests, especially when specimen volume is limited. MTB culture is the gold standard for the diagnosis of TB. For TBP, it is important to evaluate the diagnostic accuracy of the relevant tests using MTB culture as the gold standard. In this study, MTB culture had the highest positive rate (18/74), followed by Xpert MTB/RIF (16/74) and finally CapitalBio Mycobacterium RT-PCR detection assay (14/74). This result suggested that MTB culture is still essential in diagnosing TBP, but culture took a considerable amount of time (2–6 weeks), which did not meet the need for early and rapid diagnosis. These results were also similar to previous studies.23
To improve the diagnostic accuracy of TB, researchers continue to develop and innovate new diagnostic techniques, among which NAATs are the shining light.24 NAATs improve the detection of MTB by amplifying specific gene fragments in MTB DNA or RNA, which can be considered direct microbiological evidence of MTB because it is direct detection of MTB gene fragments. Clinical practice has shown that NAATs effectively improve the detection of TB compared to conventional AFB smears, regardless of PTB or EPTB.25,26 Xpert MTB/RIF is recommended by the World Health Organization to diagnose TB, and its diagnostic accuracy in TBP still varies from study to study.23 A recent meta-analysis assessing the diagnostic accuracy of Xpert MTB/RIF in TBP compared with MTB culture showed that the sensitivity of Xpert MTB/RIF in diagnosing TBP ranged from 25% to 100%, and the specificity ranged from 86% to 100% in different studies.23 The sensitivity of Xpert MTB/RIF for diagnosing TBP in this study was 61.1% and the specificity 91.1%; the overall diagnostic efficacy was similar to the results of the aforementioned meta-analysis.23
CapitalBio Mycobacterium RT-PCR detection assay is a novel fluorescent RT-PCR-TaqMan -based NAAT that, like Xpert MTB/RIF, is commercially available.18 It differs from Xpert MTB/RIF in that the CapitalBio Mycobacterium RT-PCR detection assay is a semi-automatic test that requires manual extraction of MTB nucleic acid, which may increase the potential for contamination. In contrast, Xpert MTB/RIF is fully automated and does not require manual nucleic acid extraction. CapitalBio Mycobacterium RT-PCR detection assay has a limit of detection (LOD) of 5000 colony forming units (CFU)/mL, whereas Xpert MTB/RIF is 131 CFU/mL.14 CapitalBio Mycobacterium RT-PCR detection assay can detect both MTB and NTM infections, whereas Xpert MTB/RIF can only detect MTB infections. Pericardial fluid with NTM infection was not detected in this study, so the advantage of simultaneous detection of two types of mycobacteria by the CapitalBio Mycobacterium RT-PCR detection assay was not reflected in this study. The CapitalBio Mycobacterium RT-PCR detection assay has an advantage over Xpert MTB/RIF in the diagnosis and differential diagnosis of pulmonary mycobacterial infections.18 Although the LOD of the CapitalBio Mycobacterium RT-PCR detection assay is higher than Xpert MTB/RIF in practical clinical applications, the CapitalBio Mycobacterium RT-PCR detection assay showed a diagnostic efficacy not weaker than Xpert MTB/RIF for TB. In TBP, the diagnostic accuracy of the CapitalBio Mycobacterium RT-PCR detection assay was not evaluated when testing with pericardial fluid, and its head-to-head comparison with Xpert MTB/RIF was never performed. This study showed that the sensitivity of the CapitalBio Mycobacterium RT-PCR detection assay in diagnosing TBP in pericardial fluid was 50%, which was not very satisfactory and was lower than the 61.1% of Xpert MTB/RIF. However, the difference between the two was not statistically significant and could be considered similar. This finding might be related to the limited volume of specimens. The specificity of both was consistent, both being 91.1%. In this study we found 5 patients positive for Xpert MTB/RIF and CapitalBio Mycobacterium RT-PCR detection assay, while MTB culture was negative, which might be related to the fact that these molecular tests were detected by PCR amplification, which might lead to false negative results.
Combined detection of two NAATs (parallel test) was also very rare in TBP. Our study showed that parallel test improved the corresponding diagnostic accuracy, and the diagnostic accuracy was the highest. This result was because the CapitalBio Mycobacterium RT-PCR detection assay and Xpert MTB/RIF were inconsistent. A patient with a negative CapitalBio Mycobacterium RT-PCR detection assay result may have a positive Xpert MTB/RIF result and vice versa. Combining the two tests can maximize the detection rate. However, the differences when comparing the parallel test to any single test did not reach statistical significance, which might be limited by the volume of specimens. When conditions permit, parallel test can still be beneficial compared to a single test.
This study inevitably had some limitations. First, patient selection was subject to bias, an unavoidable drawback of retrospective studies. Second, this study was conducted in a high TB burden area, and the findings might not be appropriate for low TB burden areas. Third, the volume of specimens included in this study was still limited due to the incidence of TBP. Fourth, these molecular tests need to be performed in specialized laboratories and require professional testers to operate. Fifth, the limited number of patients included in this study, especially MTB culture-positive patients, may have led to biased findings, which need to be further confirmed by studies with larger specimen sizes, and the current results need to be treated with caution. Finally, the CapitalBio Mycobacterium RT-PCR detection assay does not detect drug resistance; therefore, this study did not include relevant drug resistance analyses.
Conclusions
When applying pericardial fluid for CapitalBio Mycobacterium RT-PCR detection assay testing, it had a moderate diagnostic accuracy but limited sensitivity for TBP, similar to Xpert MTB/RIF. The parallel test maximized diagnostic efficacy, but differences were not statistically significant. The CapitalBio Mycobacterium RT-PCR detection assay, the same as the Xpert MTB/RIF for TBP, could be an initial option for early diagnosis.
Data Sharing Statement
Corresponding authors will provide data upon reasonable request.
Acknowledgments
We would like to express our feelings to the patients and colleagues in our department.
Funding
Guocan Yu, 20201203B183, Hangzhou Science and Technology Bureau, http://kj.hangzhou.gov.cn. Linhua Wang, 2020ZB185, Zhejiang Administration of Traditional Chinese Medicine, http://www.zjtcm.gov.cn. The funders do not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2021; 2021.
2. Sağıroğlu P, Atalay MA. Evaluation of the performance of the BD MAX MDR-TB test in the diagnosis of Mycobacterium tuberculosis complex in extrapulmonary and pulmonary samples. Expert Rev Mol Diagn. 2021;21(12):1361–1367. doi:10.1080/14737159.2021.1997594
3. Wiysonge CS, Ntsekhe M, Thabane L, et al. Interventions for treating tuberculous pericarditis. Cochrane Database Syst Rev. 2017;9(9):Cd000526. doi:10.1002/14651858.CD000526.pub2
4. Naicker K, Ntsekhe M. Tuberculous pericardial disease: a focused update on diagnosis, therapy and prevention of complications. Cardiovasc Diagn Ther. 2020;10(2):289–295. doi:10.21037/cdt.2019.09.20
5. Yu G, Ye B, Chen D, et al. Comparison between the diagnostic validities of Xpert MTB/RIF and interferon-γ release assays for tuberculous pericarditis using pericardial tissue. PLoS One. 2017;12(12):e0188704. doi:10.1371/journal.pone.0188704
6. Isiguzo G, Du Bruyn E, Howlett P, Ntsekhe M. Diagnosis and management of tuberculous pericarditis: what is new? Curr Cardiol Rep. 2020;22(1):2. doi:10.1007/s11886-020-1254-1
7. Hu X, Xing B, Wang W, et al. Diagnostic values of Xpert MTB/RIF, T-SPOT.TB and adenosine deaminase for HIV-negative tuberculous pericarditis in a high burden setting: a prospective observational study. Sci Rep. 2020;10(1):16325. doi:10.1038/s41598-020-73220-y
8. Chang SA. Tuberculous and infectious pericarditis. Cardiol Clin. 2017;35(4):615–622. doi:10.1016/j.ccl.2017.07.013
9. Xu HY, Li CY, Su SS, et al. Diagnosis of tuberculous pleurisy with combination of adenosine deaminase and interferon-γ immunospot assay in a tuberculosis-endemic population: a prospective cohort study. Medicine. 2017;96(47):e8412. doi:10.1097/MD.0000000000008412
10. Saeed M, Ahmad M, Iram S, Riaz S, Akhtar M, Aslam M. GeneXpert technology. A breakthrough for the diagnosis of tuberculous pericarditis and pleuritis in less than 2 hours. Saudi Med J. 2017;38(7):699–705. doi:10.15537/smj.2017.7.17694
11. Yu G, Zhong F, Shen Y, Zheng H, Naing C. Diagnostic accuracy of the Xpert MTB/RIF assay for tuberculous pericarditis: a protocol of systematic review and meta-analysis. PLoS One. 2021;16(5):e0252109. doi:10.1371/journal.pone.0252109
12. Acharya B, Acharya A, Gautam S, et al. Advances in diagnosis of tuberculosis: an update into molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep. 2020;47(5):4065–4075. doi:10.1007/s11033-020-05413-7
13. Yu G, Zhong F, Ye B, Xu X, Chen D, Shen Y. Diagnostic accuracy of the Xpert MTB/RIF assay for Lymph node tuberculosis: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:4878240. doi:10.1155/2019/4878240
14. Zheng H, Zhong F, Yu G, Shen Y. Comparison of the diagnostic efficacy of the CapitalBio Mycobacterium real-time polymerase chain reaction detection test and Xpert MTB/RIF in smear-negative pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2021;40(5):969–977. doi:10.1007/s10096-020-04113-1
15. Yu G, Wang X, Zhu P, Shen Y, Zhao W, Zhou L. Comparison of the efficacy of metagenomic next-generation sequencing and Xpert MTB/RIF in the diagnosis of tuberculous meningitis. J Microbiol Methods. 2021;180:106124. doi:10.1016/j.mimet.2020.106124
16. Pandie S, Peter JG, Kerbelker ZS, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-γ in a high burden setting: a prospective study. BMC Med. 2014;12:101. doi:10.1186/1741-7015-12-101
17. Sun L, Yao L, Fu G, Lin L, Zhu E, Huang J. A comparison of the accuracy of the CapitalBio Mycobacterium real-time polymerase chain reaction and the Xpert MTB/RIF assay for the diagnosis of tuberculous meningitis. Int J Infect Dis. 2021;104:92–96. doi:10.1016/j.ijid.2020.12.044
18. Shen Y, Fang L, Xu X, Ye B, Yu G. CapitalBio Mycobacterium real-time polymerase chain reaction detection test: rapid diagnosis of Mycobacterium tuberculosis and nontuberculous mycobacterial infection. Int J Infect Dis. 2020;98:1–5. doi:10.1016/j.ijid.2020.06.042
19. Yu G, Shen Y, Ye B, Chen D, Xu K. Comparison of CapitalBio™ Mycobacterium nucleic acid detection test and Xpert MTB/RIF assay for rapid diagnosis of extrapulmonary tuberculosis. J Microbiol Methods. 2020;168:105780. doi:10.1016/j.mimet.2019.105780
20. Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2021;1(1):Cd012768. doi:10.1002/14651858.CD012768.pub3
21. Lima NA, Stancic C, Vos D, et al. Hospital admissions for tuberculous pericarditis in the United States 2002–2014. Int J Mycobacteriol. 2019;8(4):347–350. doi:10.4103/ijmy.ijmy_150_19
22. Mayosi BM, Wiysonge CS, Ntsekhe M, et al. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S Afr Med J. 2008;98(1):36–40.
23. Yu G, Zhong F, Shen Y, Zheng H, Quinn F. Diagnostic accuracy of the Xpert MTB/RIF assay for tuberculous pericarditis: a systematic review and meta-analysis. PLoS One. 2021;16(9):e0257220. doi:10.1371/journal.pone.0257220
24. Shen Y, Fang L, Ye B, Yu G, Naing C. Meta-analysis of diagnostic accuracy of nucleic acid amplification tests for abdominal tuberculosis: a protocol. PLoS One. 2020;15(12):e0243765. doi:10.1371/journal.pone.0243765
25. Wu CW, Wu YK, Lan CC, et al. Impact of nucleic acid amplification test on pulmonary tuberculosis notifications and treatments in Taiwan: a 7-year single-center cohort study. BMC Infect Dis. 2019;19(1):726. doi:10.1186/s12879-019-4358-8
26. Pormohammad A, Nasiri MJ, McHugh TD, Riahi SM, Bahr NC, Kraft CS. A systematic review and meta-analysis of the diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis. J Clin Microbiol. 2019;57(6). doi:10.1128/JCM.01113-18
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

