Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Comparison of the Application of Vibrating Mesh Nebulizer and Jet Nebulizer in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta‐analysis
Authors Feng Z , Han Z, Wang Y, Guo H, Liu J
Received 28 November 2023
Accepted for publication 24 March 2024
Published 28 March 2024 Volume 2024:19 Pages 829—839
DOI https://doi.org/10.2147/COPD.S452191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Zhouzhou Feng,1 Zhengcai Han,1 Yaqin Wang,1 Hong Guo,1 Jian Liu1,2
1The First Clinical Medical College of Lanzhou University, Lanzhou City, People’s Republic of China; 2Gansu Maternal and Child Health Hospital/Gansu Central Hospital, Lanzhou City, People’s Republic of China
Correspondence: Jian Liu, Department of Clinical Medicine, the First Clinical Medical College of Lanzhou University, No. 1, Donggang West Road, Chengguan District, Lanzhou City, Gansu Province, People’s Republic of China, Tel +86 136 0935 4197, Email [email protected]
Objective: To comparison of the application of Vibrating Mesh Nebulizer and Jet Nebulizer in chronic obstructive pulmonary disease (COPD).
Research Methods: This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statements. The primary outcome measures analyzed included: The amount of inhaler in the urine sample at 30 minutes after inhalation therapy (USAL0.5), The total amount of inhaler in urine sample within 24 hours (USAL24), Aerosol emitted, Forced expiratory volume in 1 second (FEV1), Forced vital capacity (FVC).
Results: Ten studies were included with a total of 314 study participants, including 157 subjects in the VMN group and 157 subjects in the JN group. The data analysis results of USAL0.5, MD (1.88 [95% CI, 0.95 to 2.81], P = 0.000), showed a statistically significant difference. USAL24, MD (1.61 [95% CI, 1.14 to 2.09], P = 0.000), showed a statistically significant difference. The results of aerosol emitted showed a statistically significant difference in MD (3.44 [95% CI, 2.84 to 4.04], P = 0.000). The results of FEV1 showed MD (0.05 [95% CI, − 0.24 to 0.35], P=0.716), the results were not statistically significant. The results of FVC showed MD (0.11 [95% CI, − 0.18 to 0.41], P=0.459), the results were not statistically significant. It suggests that VMN is better than JN and provides higher aerosols, but there is no difference in improving lung function between them.
Conclusion: VMN is significantly better than JN in terms of drug delivery and utilization in the treatment of patients with COPD. However, in the future use of nebulizers, it is important to select a matching nebulizer based on a combination of factors such as mechanism of action of the nebulizer, disease type and comorbidities, ventilation strategies and modes, drug formulations, as well as cost-effectiveness, in order to achieve the ideal treatment of COPD.
Keywords: chronic obstructive pulmonary disease, aerosol, vibrating mesh nebulizers, jet nebulizers, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, poorly reversible and often progressive airflow obstruction characterized by persistent airway inflammation, the pathogenesis of which may be related to smoking, endothelial dysfunction, abnormalities in airway and alveolar structure and function, inflammation and genetic factors.1,2 Due to the exponential increase in morbidity and mortality, it places an enormous burden on society and the health system.3 Up to now, although there is no cure for COPD, effective treatments aim to stabilize the disease, mitigate its progression, and reduce the risk of acute exacerbations and hospitalizations.
Nebulizers, used primarily for aerosol therapy, agitate the liquid medication to create small aerosol droplets that rapidly deliver the medication directly to the lungs, allowing for topical drug delivery for the local treatment of respiratory diseases. Aerosol therapy is considered to be effective in the management of lung diseases, including asthma, COPD, cystic fibrosis or pneumonia, and its main advantages are rapid onset of action, lower doses, higher lung concentrations and lower systemic side effects.4,5 Based on their working principle, nebulizers can be classified into three types, ie, jet nebulizers (JN), ultrasonic nebulizers and vibrating mesh nebulizers (VMN).6
JNs are the standard and least costly devices for inhaled drugs, but they require a large amount of external medical gas to produce aerosols and are relatively wasteful, resulting in limited dosage, while VMN is not.7–9 It has been reported that >50% of the nebulized drug volume is retained in JNs and tubes with lower lung deposition, which proves the inefficiency of JNs.10,11 On the other hand, compressor-based jet nebulizer systems are inconvenient for patients as they require additional tubing, heavy compressors and longer treatment times.12,13
VMN is a new, quiet, portable, time-saving nebulizer that has been developed for invasive and NIV, high-flow nasal cannula, and also for outpatients.14 VMN has a high aerosol output efficiency and a high proportion of fine particles, a low residual drug volume15 and a larger inhalation dose in an in vitro invasive ventilation model, and has the advantage of being able to nebulize even at low drug volumes, unstable solutions, proteins/peptides, and other advantages.16,17 In ventilated patients, VMN does not alter pressure or air flow, therefore does not affect ventilator parameters, nor does it dilute the aerosol or detrimentally alter the pressure in the circuit or the volume delivered.18 However, the major disadvantages of VMN include: much higher cost, difficulty in cleaning, and the possibility of highly viscous solutions and suspensions clogging mesh pores.19,20
Despite the respective strengths and weaknesses of VMNs and JNs in the treatment of lung diseases, it remains unclear which specific nebulizer plays a greater therapeutic role in aerosol therapy for patients with COPD, to the best of our knowledge, no review has been conducted to evaluate the comparative therapeutic effects of VMNs and JNs in patients with COPD. Therefore, for the first time, we compared the two nebulizers by means of Meta analysis in order to provide a reliable evidence-based basis for the use of nebulizers in patients with COPD.
Methods
This systematic review and meta-analysis was registered at PROSPERO (http://www.crd.york.ac.uk/prospero; CRD: 42023463293). The study was designed as per the Cochrane Handbook for Systematic Reviews of Interventions21 and reported according to the PRISMA guidelines.22 See Supplementary materials Table S1 for details.
Data Sources and Searches
Search database: PubMed, Web of science, Cochrane Database, Embase. The date of searching the database was from the date of construction to October 30, 2023. The search combined subject and free words: Pulmonary Disease, Chronic Obstructive, Vibrating Mesh Nebulizer, Jet Nebulizer. The detailed search strategy is shown in Supplementary material Data S1.
Literature Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) Adults ≥40 years of age (2) Fulfilled diagnostic criteria for COPD and have acute exacerbation or require nebulization treatment. (3) Comparison of the Vibrating Mesh Nebulizer and the Jet Nebulizer, with no requirement for the specific manufacturer or model of the nebulizer (4) RCT or Controlled clinical trail studies (5) At least one data outcome of interest (6) Searchable literature in any language.
Exclusion criteria were as follows: (1) Patients younger than 40 years of age (2) Cardiopulmonary disease other than COPD (pneumonia, heart failure, myocardial infarction, pneumothorax) (3) Hemodynamic instability (heart rate >150 bpm and systolic blood pressure <90 mmHg) (4) Incomplete or unavailable data.
Types of Outcome Measures
The primary outcome indicator we selected is: The amount of inhaler in the urine sample at 30 minutes after inhalation therapy (USAL0.5). Secondary outcomes included: The total amount of inhaler in urine sample within 24 hours (USAL24), Aerosol emitted, Forced expiratory volume in 1 second (FEV1), Forced vital capacity (FVC).
Data Extraction and Quality Assessment
Two researchers independently reviewed the retrieved documents and based on the inclusion and exclusion criteria, the documents were initially screened by reading the titles and abstracts, and then the trials that met the inclusion criteria were selected by reading the full text and extracting from the articles the last name of the first author, the year of publication, the type of participant, the sample size, the ventilation strategy, the dose of bronchodilator, and the outcome metrics results. Quality assessment was performed using the Cochrane risk of bias tool to assess the quality of RCTs, including randomized sequence generation, allocation concealment, patient and intervener blinding, outcome measures blinding, incomplete outcome data, selective reporting, and other potential biases. Each item was rated as “low risk”, “high risk” or “unclear”. Any disagreements were resolved through arbitration by a third author.
Data Analysis
Statistical analyses were performed using REVMAN 4.5 and STATA MP 17, binary variables were represented by risks ratio (RR), and continuous variables were represented by mean difference (MD), and all effect sizes were expressed as 95% confidence intervals (CI). Heterogeneity between the results of the included studies was analyzed using the I2 test. When the heterogeneity test P ≥ 0.05, I2<50%, it can be considered that multiple similar studies are homogeneous, and a fixed-effects model was used; otherwise, a random-effects model was used. The Engauge Digitizer (version 4.1) graphical data extraction software was used to extract the data only provided by the images. Sensitivity analysis indicated the robustness of the results by excluding each study in turn.
Results
Study Retrieved and Characteristics
Initially, 172 related literatures were retrieved and screened according to the inclusion and exclusion criteria after reading the titles and abstracts of the literatures, and finally 10 qualified clinical studies were included,23–32 with a total of 314 study subjects, including 157 subjects in the VMN group and 157 subjects in the JN group. The screening process is shown in Figure 1, Reason of exclusion after full-text reading is shown in Supplementary material Data S2, and the basic characteristics of the included studies are shown in Table 1.
 |
Table 1 Characteristics of All Studies Included in Meta-Analysis |
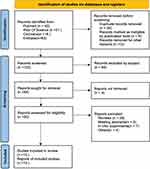 |
Figure 1 PRISMA (preferred reporting items for systematic reviews and meta-analysis) flow diagram. Adapted from Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:160. Creative Commons.22 |
Evaluation of Methodological Quality
Of the ten studies included, all were in English. Nine studies23–31 used standard RCT studies, four studies23,24,26,28 explicitly mentioned the method of generating the randomized sequences, and two of them24,28 were blinded. Three studies23,27,29 had a ventilation strategy of High-flow nasal cannula (HFNC), five studies25,26,28,30,32 were non invasive ventilation (NIV), one study31 was invasive mechanical ventilation (IMV), and one study24 did not provide a ventilation strategy. Six studies23,24,26–29 chose the bronchodilator salbutamol 2.5 mg, three studies30–32 chose salbutamol 5 mg, and one study chose Beclomethasone dipropionate. No other risk of bias was found in the results of all studies. Details are shown in Figure 2.
 |
Figure 2 Risk of bias across studies. |
Primary Outcome
Usal0.5
Five studies25,27,30–32 reported USAL0.5, with a heterogeneity test result of I2 = 77.1%, P = 0.002, and a statistically significant difference in MD (1.88 [95% CI, 0.95 to 2.81], P = 0.000) using a random effects model. (Figure 3) suggesting that VMN was more effective than JN in improving pulmonary drug absorption in COPD patients. However, the heterogeneity between the results was high, and we performed a sensitivity analysis, suggesting that the heterogeneity was significantly reduced by deleting Hassan’s study,32 which showed I2=49.8%, P=0.113, and MD (2.23 [95% CI, 1.49 to 2.97], P=0.000), (Figure 4) suggesting that this study was the main source of heterogeneity, but the overall results were unchanged. At the same time, we conducted subgroup analysis based on respiratory support strategies, medication, and dosage, and the results showed no changes. Details see in Supplementary materials Figure S1.
 |
Figure 3 Forest plot of USAL0.5. |
 |
Figure 4 Forest plot of USAL0.5 after excluding Hassan’s study. |
Secondary Outcomes
Usal24
Four studies25,27,30,32 reported USAL24, with a heterogeneity test result of I2 = 39.6%, P = 0.174, and using a fixed-effects model, the results showed a statistically significant difference in MD (1.61 [95% CI, 1.14 to 2.09], P = 0.000). It suggested that VMN is more effective than JN in improving systemic drug absorption in COPD patients. (Figure 5) Meanwhile, we conducted subgroup analysis based on respiratory support strategies, medication, and dosage, and the results showed no changes. Details show in Supplementary materials Figure S2.
 |
Figure 5 Forest plot of USAL24. |
Aerosol Emitted
Five studies25,27,28,31,32 reported Aerosol emitted, with a heterogeneity test result of I2 = 0.0%, P = 0.528, and using a fixed-effects model, the results showed a statistically significant difference in MD (3.44 [95% CI, 2.84 to 4.04], P = 0.000).(Figure 6) It suggested that VMN provided higher aerosols than JN. We conducted subgroup analysis based on respiratory support strategies, medication, and dosage, and the results showed no changes. Details see in supplementary materials Figure S3.
 |
Figure 6 Forest plot of aerosol emitted. |
Fev1
Four studies23,24,26,29 reported the results of FEV1, the test of heterogeneity resulted in I2=0.0%, P=0.925, and using a fixed-effects model, the results showed MD (0.05 [95% CI, −0.24 to 0.35], P=0.716), the results were not statistically significant. (Figure 7) It suggested that there was no difference between VMN and JN in improving airway function. We conducted subgroup analysis based on respiratory support strategies, and the results showed no changes. Details see in Supplementary materials Figure S4.
 |
Figure 7 Forest plot of FEV1. |
Fvc
Four studies23,24,26,29 reported the results of FVC, the test for heterogeneity resulted in an I2=0.0%, P=0.474, and using a fixed-effects model, the results showed an MD (0.11 [95% CI, −0.18 to 0.41], P=0.459), which showed no statistically significant difference. (Figure 8) It suggested that there was also no difference between VMN and JN in improving lung capacity. We conducted subgroup analysis based on respiratory support strategies, and the results showed no changes. Details see in Supplementary materials Figure S5.
 |
Figure 8 Forest plot of FVC. |
Discussion
Chronic Obstructive Pulmonary Disease is a common, exponentially increasing morbidity and respiratory disease that requires long-term management. Aerosols are considered to be a superior way to manage all lung diseases because of their advantages of rapid effect, lower dose, higher lung dose and lower systemic effects.33,34 Nebulizers are the primary device for generating aerosols and are widely used in both home and hospital settings, including during mechanical ventilation, during noninvasive ventilation and during high-flow nasal cannula, and they are also useful for pediatric, geriatric, and unconscious patients.35 The amount of drug delivered by nebulizers varies widely, and physiological factors such as breathing pattern and airway diameter depend on the type of disease and can affect the efficiency of drug delivery. In addition, particle size, output rate, residual volume and nebulization time depend on the nebulizer type.36,37
In the study, we compared the efficacy of two nebulizers, the jet nebulizer and the mesh nebulizer, in patients with COPD, and the results of the data analysis showed that the VMN 30 min urine was significantly higher than that of the JN, suggesting that the VMN has a greater dose of the drug delivered, which may be dependent on the VMN device advantage as the VMN has a minimal residual volume (<10%), whereas JN is up to 50%.38 The VMN also incorporates a breath drive feature to stop wasteful aerosol production during exhalation or to draw additional air into the device to minimize waste and improve drug delivery.39 It has been shown that if the nebulizer type is changed, it is recommended to adjust the drug dose to avoid possible toxicity, as the VMN may deliver five times the dose emitted by the JN.40 However, there was a large heterogeneity among the study results, which decreased significantly after we excluded Hassan’s study32 based on the results of the sensitivity analysis, probably due to the methodological differences in the literature, where randomized controlled methods were not strictly implemented. Meanwhile, the coefficient of variation for urinary salbutamol was reported to be approximately high, which may account for some of the insignificant differences. Regardless, the overall study results did not change, suggesting that the study results were more stable Meanwhile, our analysis of the USAL24 and Aerosol emitted outcome metrics also reflected higher output rates and higher patient uptake rates in VMN compared to JN, which also corroborated the above results, and the results of the study showed very good homogeneity, suggesting that the results of the study are more reliable.
Despite the above findings suggesting that mesh nebulizers provide greater drug delivery, interestingly, no statistical difference was found in the analysis of data on lung function FEV1 and FVC, suggesting that there is not much difference between VMN and JN in terms of improvement of lung function. The reason for this analysis may be the mutual result of the pharmacological effects of the bronchodilators themselves and the disease itself. It has been suggested that in patients with COPD treated with the bronchodilator salbutamol, the assessment of effective bronchodilation and FEV1 achieved in patients with COPD is underestimated due to the increased compliance of the airway wall within the chest cavity, which may be more collapsible at high thoracic pressures, limiting the improvement of the caliber of the airways.41 Bronchodilator therapy (especially for small airways) may benefit these patients even if there is no significant change in FEV1.42 It has also been shown that changes in FEV1 and FVC do not correlate well after bronchodilator administration, and that FVC can improve independently of FEV1, possibly because FEV1 is determined by the airflow at high to medium lung volumes, whereas FVC is mainly determined by airway narrowing and flow limitation at low lung volumes.43 In addition, VMN delivers higher doses of salbutamol than JN, and it is possible that higher salbutamol leads to lactic acidosis, and that the combination of high lactic acid and high doses of salbutamol may cause clinical deterioration, including exacerbation of dynamic hyperinflation, acidosis, tachycardia, apnea and anxiety, and even respiratory failure.44,45 Therefore, the selection of the optimal dose of bronchodilators is of particular importance, and the optimal threshold for their use needs to be determined in future studies, depending on the ventilation strategy, the nebulizer, and the type of drug. Notably, the choice of ventilation mode (IMV vs NIV vs HFNC), the interface (facial mask vs tracheal tube), ventilation mode (pressure support vs volume-controlled ventilation), and overtime variability of respiratory drive, as well as the degree of match between patient and ventilator, may also have some influence on aerosol delivery, and more studies are still needed to provide evidence of this effect and the choice of drug dose.46–48
The advantages of the VMN over the JN are obvious, but clinicians are concerned that vibrating mesh nebulizers may malfunction due to clogged or blocked mesh panels, resulting in the VMN being less widely used than the JN, but a recent study has shown that despite fuzzy or partially clogged mesh panels, nebulization time, residual volume, and particle size of uncleaned vibrating mesh nebulizers remained consistent and reliable after 28 days of repetitive use.49
In summary, with advances in medical device technology and inhaled drug administration methods, coupled with the continuous development of new compounds and the growing need for personalized approaches to managing chronic respiratory diseases, nebulizers have become valuable devices in the therapeutic hierarchy of respiratory diseases. In addition, they have evolved into an important platform for the development of new drugs. Our results show that VMNs are significantly superior to JNs in terms of drug delivery and utilization in the management of COPD patients, and have enormous advantages and a wide range of potential applications. However, in future use, it is important to select a matching nebulizer based on a variety of factors, such as the mechanism of action of the nebulizer, the type of disease and complications, ventilation strategies and modes, drug formulations, and cost-effectiveness, with a view to optimizing the therapeutic regimen.
Limitation
This study compares the treatment of VMN and JN in COPD patients with a comprehensive meta-analysis, which is clinically instructive, but there are still some limitations, including:1. Although we included RCT and Controlled clinical trail studies, the quality of all the included literature varied, and the sample sizes were generally small.2. Most of the literature was not blinded, probably because the appearance of the nebulizers had some differences, making it difficult to take a blinded approach.3. There are many types and models of nebulizers, and we did not categorize specific models and manufacturers.4. Graphical data extraction software was used to extract data provided only by images, which may have some errors. Therefore, future studies are still needed based on various aspects such as ventilation strategies and modes, nebulizer types, drug formulations, and the location of the nebulizer in the respiratory line, and of course, the studies should be high-quality, multi-sample, and multi-center randomized studies in order to further clarify the choice between VMN and JN in COPD management.
Conclusion
To summarize, based on the available clinical evidence, VMN is significantly better than JN in terms of drug delivery and utilization in the treatment of patients with COPD. However, in the future use of nebulizers, it is important to select a matching nebulizer based on a combination of factors such as mechanism of action of the nebulizer, disease type and comorbidities, ventilation strategies and modes, drug formulations, as well as cost-effectiveness, in order to achieve the ideal treatment of COPD.
Acknowledgments
The authors gratefully acknowledge all the authors who shared the results of the included studies, the support of the Clinical Medical Research Center of Lanzhou University First Hospital, (Lanzhou, China), and the First Clinical Medical College of Lanzhou University.
Funding
This work was supported by the Project for the First Hospital Fund of Lanzhou University (grant no. ldyyyn2019-66). Funder had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi:10.1183/13993003.00239-2023
2. Wedzicha JA, Ers C-C, Miravitlles M, et al. Management of COPD exacerbations: a European respiratory society/American thoracic society guideline. Eur Respir J. 2017;49(3):1600791. doi:10.1183/13993003.00791-2016
3. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3
4. Lexmond A, Forbes B. Drug delivery devices for inhaled medicines. Handb Exp Pharmacol. 2017;237:265–280. doi:10.1007/164_2016_67
5. Sorino C, Negri S, Spanevello A, Visca D, Scichilone N. Inhalation therapy devices for the treatment of obstructive lung diseases: the history of inhalers towards the ideal inhaler. Eur J Intern Med. 2020;75:15–18. doi:10.1016/j.ejim.2020.02.023
6. Longest W, Spence B, Hindle M. Devices for improved delivery of nebulized pharmaceutical aerosols to the lungs. J Aerosol Med Pulm Drug Deliv. 2019;32(5):317–339. doi:10.1089/jamp.2018.1508
7. Barjaktarevic IZ, Milstone AP. Nebulized therapies in COPD: past, present, and the future. Int J Chron Obstruct Pulmon Dis. 2020;15:1665–1677. doi:10.2147/COPD.S252435
8. Ari A, Dang T, Al Enazi FH, et al. Effect of heat moisture exchanger on aerosol drug delivery and airway resistance in simulated ventilator-dependent adults using jet and mesh nebulizers. J Aerosol Med Pulm Drug Deliv. 2018;31(1):42–48. doi:10.1089/jamp.2016.1347
9. Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American college of chest physicians/American college of asthma, allergy, and immunology. Chest. 2005;127(1):335–371. doi:10.1378/chest.127.1.335
10. Dhand R. Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J Aerosol Med Pulm Drug Deliv. 2008;21(1):45–60. doi:10.1089/jamp.2007.0663
11. Bosco AP, Rhem RG, Dolovich MB. In vitro estimations of in vivo jet nebulizer efficiency using actual and simulated tidal breathing patterns. J Aerosol Med. 2005;18(4):427–438. doi:10.1089/jam.2005.18.427
12. Martin AR, Finlay WH. Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2015;12(6):889–900. doi:10.1517/17425247.2015.995087
13. Goralski JL, Davis SD. Breathing easier: addressing the challenges of aerosolizing medications to infants and preschoolers. Respir Med. 2014;108(8):1069–1074. doi:10.1016/j.rmed.2014.06.004
14. McCarthy SD, González HE, Higgins BD. Future trends in nebulized therapies for pulmonary disease. J Pers Med. 2020;10(2):37. doi:10.3390/jpm10020037
15. Sweeney L, McCloskey AP, Higgins G, Ramsey JM, Cryan SA, MacLoughlin R. Effective nebulization of interferon-γ using a novel vibrating mesh. Respir Res. 2019;20(1):66. doi:10.1186/s12931-019-1030-1
16. Liu CY, Ko HK, Fink JB, et al. Size distribution of colistin delivery by different type nebulizers and concentrations during mechanical ventilation. Pharmaceutics. 2019;11(9):459. doi:10.3390/pharmaceutics11090459
17. Chang KH, Moon SH, Yoo SK, Park BJ, Nam KC. Aerosol delivery of dornase alfa generated by jet and mesh nebulizers. Pharmaceutics. 2020;12(8):721. doi:10.3390/pharmaceutics12080721
18. Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55(7):845–851.
19. Ghazanfari T, Elhissi AM, Ding Z, Taylor KM. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int J Pharm. 2007;339(1–2):103–111. doi:10.1016/j.ijpharm.2007.02.035
20. Zhang G, David A, Wiedmann TS. Performance of the vibrating membrane aerosol generation device: aeroneb Micropump Nebulizer. J Aerosol Med. 2007;20(4):408–416. doi:10.1089/jam.2007.0622
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester (UK): John Wiley & Sons; 2019. Available from: http://www.cochrane-handbook.org.
22. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:160. doi:10.1136/bmj.n160
23. Arunsurat I, Rittayamai N, Chuaychoo B, et al. Bronchodilator efficacy of high-flow nasal cannula in COPD: vibrating mesh nebulizer versus jet nebulizer [published online ahead of print, 2023 Aug 22]. Respir Care. 2023. doi:10.4187/respcare.11139
24. Cushen B, Alsaid A, Greene G, Costello RW. Response to bronchodilators administered via different nebulizers in patients with COPD exacerbation. Respir Care. 2023;68(11):1532–1539. doi:10.4187/respcare.10132
25. Madney YM, Harb HS, Porée T, Eckes M, Boules ME, Abdelrahim MEA. Preliminary bronchodilator dose effect on aerosol-delivery through different nebulizers in noninvasively ventilated COPD patients [published online ahead of print, 2022 Mar 2]. Exp Lung Res. 2022;1–9. doi:10.1080/01902148.2022.2047243
26. Avdeev SN, Nuralieva GS, Soe AK, Gainitdinova VV, Fink JB. Comparison of vibrating mesh and jet nebulizers during noninvasive ventilation in acute exacerbation of chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv. 2021;34(6):358–365. doi:10.1089/jamp.2020.1665
27. Madney YM, Fathy M, Elberry AA, Rabea H, Abdelrahim ME. Aerosol delivery through an adult high-flow nasal cannula circuit using low-flow oxygen. Respir Care. 2019;64(4):453–461. doi:10.4187/respcare.06345
28. Galindo-Filho VC, Alcoforado L, Rattes C, et al. A mesh nebulizer is more effective than jet nebulizer to nebulize bronchodilators during non-invasive ventilation of subjects with COPD: a randomized controlled trial with radiolabeled aerosols. Respir Med. 2019;153:60–67. doi:10.1016/j.rmed.2019.05.016
29. Reminiac F, Vecellio L, Bodet-Contentin L, et al. Nasal high-flow bronchodilator nebulization: a randomized cross-over study. Ann Intensive Care. 2018;8(1):128. doi:10.1186/s13613-018-0473-8
30. Saeed H, Mohsen M, Salah Eldin A, et al. Effects of fill volume and humidification on aerosol delivery during single-limb noninvasive ventilation. Respir Care. 2018;63(11):1370–1378. doi:10.4187/respcare.06022
31. ElHansy MHE, Boules ME, El Essawy AFM, et al. Inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during invasive mechanical ventilation. Pulm Pharmacol Ther. 2017;45:159–163. doi:10.1016/j.pupt.2017.06.004
32. Hassan A, Salah Eldin R, Abdelrahman MM, Abdelrahim ME. In-vitro/in-vivo comparison of inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during non-invasive ventilation. Exp Lung Res. 2017;43(1):19–28. doi:10.1080/01902148.2017.1282993
33. Matuszak M, Ochowiak M, Włodarczak S, Krupińska A, Doligalski M. State-of-the-art review of the application and development of various methods of aerosol therapy. Int J Pharm. 2022;614:121432. doi:10.1016/j.ijpharm.2021.121432
34. Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25(3):140–147. doi:10.1089/jamp.2011.0916
35. Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Med Devices. 2015;8:131–139. doi:10.2147/MDER.S48888
36. Hatley RH, Byrne SM. Variability in delivered dose and respirable delivered dose from nebulizers: are current regulatory testing guidelines sufficient to produce meaningful information? Med Devices. 2017;10:17–28. doi:10.2147/MDER.S125104
37. Bennett G, Joyce M, Sweeney L, MacLoughlin R. In vitro study of the effect of breathing pattern on aerosol delivery during high-flow nasal therapy. Pulm Ther. 2019;5(1):43–54. doi:10.1007/s41030-019-0086-x
38. Dugernier J, Ehrmann S, Sottiaux T, et al. Aerosol delivery during invasive mechanical ventilation: a systematic review. Crit Care. 2017;21(1):264. doi:10.1186/s13054-017-1844-5
39. Gowda AA, Cuccia AD, Smaldone GC. Reliability of vibrating mesh technology. Respir Care. 2017;62(1):65–69. doi:10.4187/respcare.04702
40. Dunne RB, Shortt S. Comparison of bronchodilator administration with vibrating mesh nebulizer and standard jet nebulizer in the emergency department. Am J Emerg Med. 2018;36(4):641–646. doi:10.1016/j.ajem.2017.10.067
41. Pini L, Ziletti GC, Ciarfaglia M, Giordani J, Tantucci C. Acute effect of bronchodilator on intrathoracic airway wall compliance in COPD patients. Lung. 2022;200(4):473–480. doi:10.1007/s00408-022-00556-9
42. Alobaidi NY, Almeshari MA, Stockley JA, Stockley RA, Sapey E. The prevalence of bronchodilator responsiveness of the small airway (using mid-maximal expiratory flow) in COPD - A retrospective study. BMC Pulm Med. 2022;22(1):493. doi:10.1186/s12890-022-02235-0
43. Schermer T, Heijdra Y, Zadel S, et al. Flow and volume responses after routine salbutamol reversibility testing in mild to very severe COPD. Respir Med. 2007;101(6):1355–1362. doi:10.1016/j.rmed.2006.09.024
44. MacDonald MI, Polkinghorne KR, MacDonald CJ, et al. Elevated blood lactate in COPD exacerbations associates with adverse clinical outcomes and signals excessive treatment with β2 -agonists. Respirology. 2023;28(9):860–868. doi:10.1111/resp.14534
45. Tobin AE, Pellizzer AM, Santamaria JD. Mechanisms by which systemic salbutamol increases ventilation. Respirology. 2006;11(2):182–187. doi:10.1111/j.1440-1843.2006.00832.x
46. Boules ME, Laz NI, Elberry AA, Hussein RRS, Abdelrahim MEA. Effect of pressures and type of ventilation on aerosol delivery to chronic obstructive pulmonary disease patients. Beni Suef Univ J Basic Appl Sci. 2022;11(1):57. doi:10.1186/s43088-022-00234-y
47. Chatmongkolchart S, Schettino GP, Dillman C, Kacmarek RM, Hess DR. In vitro evaluation of aerosol bronchodilator delivery during noninvasive positive pressure ventilation: effect of ventilator settings and nebulizer position. Crit Care Med. 2002;30(11):2515–2519. doi:10.1097/00003246-200211000-00018
48. Madney YM, Laz NI, Elberry AA, Rabea H, Abdelrahim MEA. Aerosol delivery aspects within a high-flow therapy system in COPD patients. ERJ Open Res. 2021;7(1):00422–2020. doi:10.1183/23120541.00422-2020
49. Lin HL, Chen CS, Fink JB, et al. In vitro evaluation of a vibrating-mesh nebulizer repeatedly use over 28 days. Pharmaceutics. 2020;12(10):971. doi:10.3390/pharmaceutics12100971
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
