Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Comparison of Short-Term Surgery Outcomes and Clinical Characteristics Between Elderly and Non-Elderly Patients with Middle Third Parasagittal and Parafalcine Meningiomas
Authors Chen Z, Lin T, Liu D, Zeng Y, Zhang X, Deng B, Guo D, Shi T, Lu M
Received 30 June 2023
Accepted for publication 24 October 2023
Published 30 October 2023 Volume 2023:19 Pages 2331—2340
DOI https://doi.org/10.2147/NDT.S428341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Richard J Porter
Zhijie Chen,* Tao Lin,* Da Liu, Yongqin Zeng, Xubiao Zhang, Bin Deng, Dongliang Guo, Tao Shi, Ming Lu
Department of Neurosurgery, Guangdong 999 Brain Hospital, Medical College of Jinan University, Guangzhou, Guangdong, 510000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ming Lu, Department of Neurosurgery, Guangdong 999 Brain Hospital, Medical College of Jinan University, No. 578, Shatai Road, Jingxi Street, Baiyun District, Guangzhou, Guangdong, 510000, People’s Republic of China, Tel +86 020 62323939-2601, Email [email protected]
Purpose: This study aims to compare the short-term surgery outcomes of the resection of meningiomas and clinical characteristics between elderly and non-elderly patients.
Patients and Methods: This retrospective study included patients who underwent a resection of middle third parasagittal and parafalcine meningiomas between January 2011 and December 2020. All lesions arise from the middle third of the parafalcine or infiltrate superior sagittal sinus (SSS). The clinical characteristics studied included neurological deficit, peritumoral brain edema (PTBE), SSS invasion, tumor size, and symptoms; perioperative complications, and short-term surgery outcomes including neurological deficit, operative blood loss, postoperative hospitalization duration, and WHO classification were compared.
Results: A total of 43 elderly patients and 63 non-elderly patients were included. Compared with non-elderly patients, elderly patients had larger lesions (P = 0.013) and presented with a larger PTBE (P = 0.019). SSS blockage was identified in 28.57% of elderly patients and 19.57% of non-elderly patients. Compared with non-elderly patients, elderly patients tended to suffer from more aggressive lesions (WHO II/III meningioma 6 vs 3, P = 0.154) and presented with longer postoperative hospital stays (17.25 ± 5.8 vs 13.50 ± 3.8, P = 0.009); conversely, while the non-elderly patients experienced more blood loss (P = 0.022) and had more perioperative reoperations (3 vs 1). No significant difference in neurological deficit was detected between the two groups (P = 0.97). After total tumor resection, patients with neurological deficits in both groups can recover during the follow-up period.
Conclusion: Among the 106 patients with middle third parasagittal and falx meningiomas in our hospital, elderly patients had larger lesions, presented with more severe PTBE, and had longer postoperative hospital stays than younger patients. Conversely, younger patients had more blood loss and serious complications than elderly patients. Postoperative neurological dysfunction in elderly patients was similar to that in middle-aged and young patients.
Keywords: meningioma, elderly, complication, neurosurgery, parasagittal meningioma
Introduction
Among all intracranial meningiomas, parasagittal meningiomas account for 16.8–30% of the cases,1,2 parafalcine meningiomas for ~8.5% of the cases,3 and falcine meningiomas for ~9% of the cases.4 Parasagittal meningiomas are distinguished based on the anterior, middle, and posterior locations along the sinus.4 Unlike anterior and posterior meningiomas, lesions along the middle of the sinus cause motor function deterioration in the early stage of the disease and are associated with a poor postoperative outcome. Regarding the effect of the physiological condition of the patient on postoperative recovery, younger patients recover faster from the operation than older patients.5 In this study, we compared the clinical characteristics and short-term surgery outcomes between elderly and non-elderly patients with middle third parasagittal and parafalcine meningiomas.
Middle third parasagittal and parafalcine meningiomas are associated with a high incidence of motor function deterioration, which occurs either postoperatively or as a symptom.4,6 Thus, preserving the venous system’s integrity and cerebral cortex during surgical resection of the meningiomas may be challenging.7 Mental health and physical well-being of the elderly are extremely important. As meningioma incidence increases with age, aging physiology, and age-related comorbidities, elderly patients are likely to encounter more postoperative complications and recover slower than non-elderly patients.5
The number of elderly individuals is increasing every year. The proportion of the world’s population aged >60 years in developed countries will increase by 38% by 2050.8 An age of >65 years is predictive of significantly higher chances of inpatient death (P < 0.001), prolonged postoperative hospitalization, higher medical expenses, and several inpatient complications.9 Elderly patients with meningiomas will seemingly require a lot of medical resources and need special attention in the future. This study was aimed at comparing the short-term surgical outcomes and clinical characteristics of middle third parasagittal and parafalcine meningiomas between elderly patients (>60 years) and non-elderly patients.
Materials and Methods
Study Design
The Ethics Committee of Guangdong 999 Brain Hospital, Medical College of Jinan University, approved this study. All patients provided written informed consent for participation in this study. The study complied with the tenets of the latest revision of the Declaration of Helsinki (2013). Data of patients with middle third parasagittal and parafalcine meningiomas who underwent craniotomy in this hospital between January 2011 and December 2020 were extracted from the hospital’s electronic records. According to the World Health Organization (WHO), individuals are considered elderly at the age of >65 years in developed countries and at the age of >60 years in developing countries. In the current study, all the subjects were citizens of China, which is a developing country. Patients aged >60 years were classified into the elderly group, whereas those aged <60 years were classified into the non-elderly group. Clinical characteristics and operative complications were compared, and subgroup analyses were performed to investigate the differences between the two groups. The tumors were classified into three categories according to their longest diameters: tiny (less than 2 cm), medium (2–5 cm), and giant (larger than 5 cm) groups.6,10,11 Operative blood loss was classified into three grades: general group (<400 mL), medium group (400–1000 mL), and massive group (>1000 mL). In line with previous literature,12 peritumoral brain edema was classified into three grades: small (<10 cm3), medium (10–50 cm3), large (>50 cm3). A three-dimensional volumetric assessment of the PTBE volume was postoperatively conducted on T2-weighted scans or CT scans.
The Simpson grade of meningioma resection was used to denote the extent of tumor resection.13 Since most patients underwent elective surgery and the duration between the day of admission to the hospital and the day of operation varied in these patients, the postoperative hospitalization duration was selected as a surgical outcome. This duration was defined as the period between the day of operation and the day of discharge or transfer to another department, such as oncology and rehabilitation. Surgery outcomes assessed at 30 days postoperatively were as follows: remaining symptoms, postoperative PTBE, perioperative complications, postoperative hospitalization duration, resolution of symptoms, new neurological symptom(s), and cumulative mortality. Patients were followed up to check for tumor recurrence for 3.5 ± 1.3 years (2–5 years).
Clinical Management
All patients underwent computed tomography (CT) scan and/or magnetic resonance imaging (MRI) within 2 weeks before surgery. CT angiography (CTA), MR venography (MRV), and digital subtraction angiography were performed if necessary. Dexamethasone and/or mannitol were preoperatively administered to patients identified as having a large PTBE on T2-weighted MRI. The indications for emergency operation included cerebral hernia, cerebral or tumor hemorrhage, and rapid tumor progression. All operations were performed by experienced surgeons. The surgical trajectory and position were determined on the basis of whether the tumor was located in the middle third parasagittal or parafalcine region. The location of the surgical bone window depended on whether the lesion was unilateral or bilateral. In patients with bilateral lesions, an interhemispheric approach was taken on the non-dominant side.
Neuronavigation system (Brainlab, Germany), Cavitron ultrasonic surgical aspirator (Integra, USA), and the Cascade Elite system for electrophysiological monitoring (Cadwell, USA) were used during microsurgical tumor resection. The lesions were diagnosed according to 2021 WHO Classification of Tumors of the Central Nervous System.14
Patient Selection
We included patients (1) with lesions located at middle third parasagittal and parafalcine on the MRI; (2) who underwent craniotomy at our hospital; and (3) who were diagnosed as having meningioma according to pathological results. Relapse patients were excluded.
Data Collection
Clinical characteristics included age, gender, comorbidity, tumor size, imaging features, and symptoms (Table 1 and Table 2). Treatment information included the operative approach, the volume of bleeding, operative complications, and the adjuvant therapy administered. All data were collected through the hospital’s electronic patient records and contained hospitalization and outpatient review records and telephonic follow-up findings. A detailed follow-up schedule was developed, and each patient was telephonically followed up by the same person according to the follow-up schedule. Six patients who changed their phone number or refused to answer the phone were lost to follow-up.
 |
Table 1 Demographic Characteristics and Preoperative Status of Our Study Cohorts |
 |
Table 2 Preoperative and Postoperative Outcomes of the Study Cohorts |
Statistical Analyses
We used SPSS 23.0 (SPSS Inc., Chicago, IL, USA) for all statistical analyses. Frequency distributions and descriptive statistics were calculated for all variables. The quantitative data of normal distribution were described as mean ± SD, and between-group differences were evaluated by two samples t-test. The quantitative data of non-normal distribution are represented as the median (range). Between-group differences were analyzed using the Mann–Whitney U-test. Chi-square and Fisher’s exact test were used to assess the qualitative data. A P value of <0.05 indicated statistical significance.
Results
We included 106 patients in this study, comprising 43 elderly patients (age: 65.15 ± 3.95 years, range: 60–76 years) and 63 non-elderly patients (age: 46.79 ± 7.94 years, range: 27–59 years). Overall and within each group, there were more female patients (65.1% in the elderly group and 71.4% in the non-elderly group) than male patients.
The most common symptom in the elderly group was hemiparesis (41.9%), followed by dizziness (34.9%), headache (25.6%), and seizures (16.3%). Patients rarely presented with speech disorder (4.7%) and visual impairment (2.3%) as chief complaints. Three elderly patients visited our clinic for a mild brain injury. In the non-elderly group, the most common symptom was headache (54%), followed by dizziness (38.1%) and hemiparesis (19%). Postoperatively, hemiparesis (41.9%) was the most frequent symptom in the elderly group, whereas in the non-elderly group, the most common symptom was hemiparesis (27%). New neurological deficits occurred in 7 non-elderly patients and 11 elderly patients (P = 0.874).
Brain MRI was performed for all patients. Classic dural tail signs were identified in 69.7% (n = 30) of elderly patients and 63.5% (n = 40) of non-elderly patients; the difference was not significant (P = 0.503). Asymptomatic brain lesions were identified in two patients of the non-elderly group (<2 cm) during a medical checkup. Furthermore, MRV revealed superior sagittal sinus (SSS) blockage in 18/80 patients (10/35 elderly patients vs 9/47 non-elderly patients). Moreover, 12 elderly patients and 17 non-elderly patients presented with SSS compressed by or involved with the lesions. SSS involvement was found in 12 (27.3%) and 13 (20.63%) patients in the elderly and non-elderly groups (P = 0.63), respectively.
In addition, the prevalence of giant meningioma was significantly higher in the elderly group [32.56% (n = 14)] than in the non-elderly group [20.63% (n = 13)] (P = 0.013). The two groups significantly differed in terms of both preoperative and postoperative PTBE (Table 2). Preoperatively, radiological examination revealed a large PTBE in 32.6% and 15.9% of patients in the elderly and non-elderly groups, respectively (P = 0.019). Postoperatively, the ratio increased to 46.5% and 23.8% in the two groups, respectively (P = 0.015). Among all patients, a large PTBE was postoperatively noted in 60% (6/10) of WHO grade II/III patients and 29.6% (29/98) of WHO grade I patients (P = 0.001, Table 3).
 |
Table 3 Comparison of Postoperative Peritumoral Edema Based on Histological Subtypes and Tumor Sizes |
The mean operative blood loss volume was significantly higher in the non-elderly group (mean: 503 ± 619 mL; range: 100–3300 mL) than in the elderly group (mean: 374 ± 322 mL; range: 100–1400 mL) (P = 0.022). Furthermore, massive blood loss occurred in seven non-elderly patients (11.1%) and three elderly patients (7%; P = 0.022). The duration of postoperative hospitalization at the Neurosurgery Department was much longer for the elderly group (mean: 17.25 ± 5.8 days) than for the non-elderly group (13.50 ± 3.8 days), and the difference was significant (P = 0.009).
The non-elderly group presented with more operative complications, such as subdural effusion (3 vs 1), incision infection (2 vs 0), and operative field bleeding (4 vs 3), than the elderly group (Table 2). Operative field bleeding and subdural hematoma were the most common operative complications in both elderly (14.0%, 6/43) and non-elderly groups (11.1%, 7/63). One 56-year-old woman whose chief complaint was repeated seizures for 3 years died on postoperative day 2. An aneurysm clip was used to stop bleeding from SSS rupture in a 65-year-old man with 1800 mL of operative blood loss during the resection of a giant meningioma involving SSS.
Pathologically, WHO grade I, II, and III meningiomas affected both groups, with no pathological differences identified (P = 0.154 for both grade I and grade II/III meningiomas, Table 4). The success rate of Simpson grade I/II resection in the elderly and non-elderly groups was 88.3% (n = 38) and 90.47% (n = 57), respectively (P = 0.787). A residual tumor (Simpson grade III/IV) was found in five (11.63%) and six (9.52%) patients in the elderly and non-elderly groups, respectively (p = 0.787).
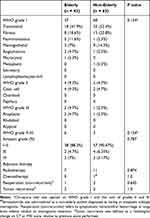 |
Table 4 Surgery Outcome and Further Treatment of the Study Cohorts |
Discussion
Middle third parasagittal and parafalcine meningiomas with SSS compression or invasion cause motor function deterioration in the early disease stage and result in a poor postoperative outcome. The incidence of meningioma increases with age and peaks between the sixth and seventh decades of life.15 Currently, few studies have directly compared the clinical characteristics and short-term surgery outcomes between elderly and non-elderly patients with middle third parasagittal and parafalcine meningiomas. Our findings revealed that the elderly group had with more comorbidities than the non-elderly group, including hypertension, diabetes mellitus, liver disease, renal disease, pulmonary disease, and cardiovascular disease; however, the rate of operative complications was similar in the two groups (P = 0.97). Simultaneous tumors occurred in five patients (all women) in the non-elderly group. A 48-year-old woman had a medical history of two concurrent tumors (a fibroid and an osteoma of the tibia) and a 45-year-old woman had concurrent glioblastoma (WHO grade IV) and fibrous meningioma (WHO grade I). In the remaining three women, uterine fibroids (n = 1), renal clear cell carcinoma (n = 1), and thyroid carcinoma (n = 1) were noted. Multiple primary brain tumors with different histological types occur in the same patient are extremely rare, and only a few such cases have been reported thus far.16,17 Tunthanathip et al reported six cases with simultaneous occurrence of multiple primary brain tumors, predominantly in elderly patients.17 In this study, one patient (a 55-year-old woman) presented with meningioma and glioblastoma.
Giant meningiomas (>5 cm) were more likely to occur in elderly patients (32.56%; 14/43) than in non-elderly patients (20.63%; 13/63; P = 0.013). Sanai and McDermott reported that giant meningiomas usually arise in an area of maximal brain compliance.18 Cerebral atrophy increases brain compliance in elderly patients, and the symptoms do not appear until the lesion compresses the central cortex. Nayak considered that meningiomas in elderly patients are usually asymptomatic and have a slow growth rate,19 and therefore, meningiomas in these patients are detected only when they become giant and cause symptoms. Furthermore, Chinese elderly tend to avoid doctor consultations before their health evidently worsens as they fear discovering additional health problems. Some studies have also shown that in many elderly patients, diagnostics and therapy are not done when the lesion is still tiny because the patients are afraid of finding more health problems and the high expenses associated with them; this leads to continued enlargement and advancement of the lesion.5,20
Lesion size and histological subtype may be the key factors of PTBE. In a previous study that investigated 42 patients aged >65 years with primary intracranial meningioma of all locations, a PTBE was preoperatively found in 20/42 patients.21 Our subgroup analyses revealed that giant intracranial meningiomas invaded vital neurovascular structures (brain tissues and peritumoral blood vessels) and caused a large PTBE in both groups. There were 29 and 50 patients in the elderly and non-elderly groups who presented with medium/tiny meningiomas, respectively. Among them, one and six patients showed a large PTBE, respectively. Among the 14 and 13 patients with giant meningiomas in the elderly and non-elderly groups, respectively, 9 patients in each group showed a large PTBE. Considering both elderly and non-elderly patients together, patients with a giant meningioma had a higher incidence rate of a large PTBE (18/27) than patients with a medium/tiny meningioma (17/79). To draw a comparison, in a study of giant intracranial meningioma, Narayan et al found that 10 patients (12.5%) among a total of 80 patients had a large PTBE, most of whom had skull base meningiomas (n = 57) and convexity meningiomas (n = 17), and only 6 (7.5%) patients had falcine meningioma.11 Based on this difference, we deduced that middle third parasagittal and parafalcine giant meningiomas are associated with a higher frequency of a large PTBE than meningiomas present at any other location.
Herein, we also compare postoperative PTBE based on histological subtypes. We discovered that PTBE was significantly larger in patients with grade II/III meningiomas than in those with grade I meningiomas. Elderly patients showed increasingly larger PTBE with advancing histological grades (P = 0.001). This is in agreement with the findings of Ressel et al’s study of 240 patients with intracranial meningiomas comprising 111 elderly patients aged >60 years.22 However, in their study, 29/111 (26.1%) elderly patients had a grade II/III meningioma at any intracranial site. Conversely, in the current study, only 6/43 (13.95%) elderly patients with middle third parasagittal and parafalcine meningiomas were diagnosed as having grade II/III meningiomas.
Rapid advances in neurosurgical techniques and perioperative care have made meningioma treatments safer and reduced tumor resection-related mortalities. However, parasagittal and falcine meningioma resections remain challenging for neurosurgeons worldwide. Thus far, several different neurosurgical techniques have been applied for parasagittal and falcine meningioma resection.6,23–28 Karthigeyan et al used a modified unilateral approach to resect middle third giant bilateral falcine meningiomas.6 To minimize the chances of any surgery-induced damage, they chose to create a surgical window on the side of the non-dominant hemisphere. Furthermore, instead of directly working over the major draining veins and the brain functional area, the tumors were excised via an oblique anterior or posterior trajectory. We have previously used this approach to resect deep and tiny falcine meningiomas. Neuronavigation has been widely applied in neurosurgery.29,30 After a comprehensive analysis of 517 cases of meningiomas, Bir et al30 emphasized on the use of neuronavigation in the operative management of meningiomas. Neuronavigation can decrease blood loss and postoperative hospitalization duration and improve recurrence-free survival and performance status. In addition, neuronavigation is best used for small (<2 cm) and deep parafalcine meningiomas; for larger tumors, it would be rather time-consuming to seek out the tumor and may cause substantial damage to the brain in the process.
In the current study, 7 elderly and 11 non-elderly patients underwent stereotactic radiotherapy (SRT) as an adjuvant therapy. Subtotal resection followed by radiosurgery is a reasonable approach for these cases, particularly when injury to critical venous structures is likely.31 Accumulated evidence has supported that radiotherapy is safe and effective in WHO grade 2 and grade 3 meningiomas.32 Literature data focused on radiosurgery outcomes for parasagittal and parafalcine meningiomas are very limited. A review study revealed that radiosurgery could be more widely applied for parafalcine and parasagittal meningiomas, particularly for small-to-medium meningiomas or subtotal resected meningiomas.31 Ding et al reported their results for parasagittal and parafalcine meningiomas (median treatment volume, 3.7 cm3) after RS treatment, and the tumor control rates were 85% at 3 years and 70% at 5 years for WHO grade I meningiomas.33
Operative blood loss is a critical determinant of surgical efficacy. In the current study, operative blood loss was much higher in the non-elderly group than in the elderly group; this could be attributed to cerebral atrophy in the elderly, which allowed for a wider surgical field and damage to fewer vessels. Furthermore, lesser blood loss in the elderly may have been aided by stricter indications and comprehensive preoperative preparation. Notably, SSS involvement was the most common reason for massive blood loss. Among the nine non-elderly patients with >800 mL of operative blood loss, seven (77.8%) had SSS involvement, whereas among the elderly, 2/5 (40%) patients with massive blood loss (1000 mL and 1800 mL, respectively) patients showed SSS involvement. A significant risk of cortical venous infarction is introduced during the closure of the middle and posterior thirds of the SSS. In this scenario, surgeons have to choose either to leave residual meningioma and await sinus occlusion by the tumor and the development of collateral flow or to attempt a total meningioma removal, thus restoring the venous outflow.34 In some studies,35,36 patch grafts and venous bypass were recommended to reconstruct the damaged veins; however, aneurysm clips were not recommended as they are susceptible to secondary thromboses.35,36 In cases with delayed vertex epidural hematoma occurring due to damaged SSS, surgical intervention should be conducted after coagulation and fibrinolysis are stable.37 In the current study as well, if SSS was damaged intraoperatively, venous reconstruction was performed using patch grafts or bypass. Notably, in a 65-year-old man with 1800 mL of operative blood loss during the resection of a giant meningioma involving the third middle SSS, an aneurysm clip was used on the sinus wall to reconstruct the ruptured SSS during the operation. Subtotal resection is a viable alternative to extremely risky operations wherein total resection could lead to numerous severe complications.38
The postoperative hospitalization duration significantly differed between the two groups. Despite markedly higher blood loss, non-elderly patients recovered faster than elderly patients. This may be explained by aging physiology and the presence of comorbidities prolonging the recovery of elderly patients.5 Elderly patients showed a tendency for fewer operative complications than non-elderly patients; however, the difference was not statistically significant (20.9% vs 22.2%, P = 0.874). In the present study, severe and fatal complications occurred more in non-elderly patients than in elderly patients. This is substantiated by the fact that four non-elderly patients underwent a craniotomy operation to remove a hematoma or an abscess, whereas only one elderly patient presented with this complication. Neurological deficit is a vital factor in evaluating the surgery outcome of middle third parasagittal and falcine meningioma patients. It was reported that a 41-year-old woman who underwent surgery for a parasagittal meningioma developed a bilateral central retinal artery occlusion.39 In the current study, at the 1-year follow-up, 18 (41.9%) elderly patients and 17 (27%) non-elderly patients experienced hemiparesis; the difference was not a statistically significant difference (P = 0.11). Nakamura et al compared the postoperative complications after the resection of cerebellopontine angle meningiomas between 21 elderly (aged >70 years) and 65 non-elderly patients and found no significant differences.40 Roser et al41 compared 43 elderly (aged >70 years) patients with skull base meningiomas with 89 controls and reported that surgical morbidity after skull base meningioma resection was not associated with the age of the patient. However, several studies have reported different results in this regard.5,42,43 This discrepancy may be explained by the surgery candidate selection criteria being more stringent in some studies and less stringent in others.44 Furthermore, the heavy operative blood loss in the non-elderly group noted in the current study may increase the rate of complications. Taken together, it is inferred that elderly patients are more likely to develop minor postoperative complications than non-elderly patients, and none of these complications are life-threatening events, which is consistent with Poon et al.5
Limitations
Firstly, this study’s retrospective design keeps us from determining the degree of resident involvement in surgery. Furthermore, this was a single-center study, and the number of patients was not sufficient to reach a convincing conclusion.
Conclusion
In the current study, we found that the prevalence of larger lesions among middle third parasagittal and parafalcine meningioma patients was more common in elderly patients than in non-elderly patients. The elderly group was associated with a larger PTBE and longer postoperative hospitalization than the non-elderly group. Conversely, the non-elderly group experienced more blood loss and had a higher reoperation rate than the elderly group. There were no significant between-group differences in neurological deficits, complications, and recurrence rates. Taken together, although elderly patients were more likely to develop minor postoperative complications than non-elderly patients, none of these complications were life-threatening events. Elderly patients need timely imaging checks to receive surgical treatment if their physical condition permits. Patient selection remains key to good surgery outcomes for middle third parasagittal and parafalcine meningiomas.
Funding
This work was supported by the Medical Scientific Research Foundation of Guangdong Province, China [grant no: B2023418].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Elzarief AA, Ibrahim MF. Long-term follow-up of motor function deterioration following microsurgical resection of middle third parasagittal and falx meningioma. Egypt J Neurol Psychiatr Neurosurg. 2018;54(1):9. doi:10.1186/s41983-018-0013-3
2. Munich SA, Eddelman D, Byrne RW. Retrospective review of a venous sparing approach to resection of parasagittal meningiomas. J Clin Neurosci. 2019;64:194–200. doi:10.1016/j.jocn.2019.02.013
3. Chung SB, Kim CY, Park CK, Kim DG, J HW. Falx meningiomas: surgical results and lessons learned from 68 cases. J Korean Neurosurg Soc. 2007;42(4):276–280. doi:10.3340/jkns.2007.42.4.276
4. Biroli A, Chiocchetta M, Gerosa M, Talacchi A. Surgical treatment of parasagittal and falcine meningiomas of the posterior third. Acta Neurochir. 2012;154(11):1987–1995. doi:10.1007/s00701-012-1454-6
5. Poon MT, Fung LH, Pu JK, Leung GK. Outcome comparison between younger and older patients undergoing intracranial meningioma resections. J Neurooncol. 2013;114(2):219–227. doi:10.1007/s11060-013-1173-8
6. Karthigeyan M, Rajasekhar R, Salunke P, Singh A. Modified unilateral approach for mid-third giant bifalcine meningiomas: resection using an oblique surgical trajectory and falx window. Acta Neurochir. 2019;161(2):327–332. doi:10.1007/s00701-018-3770-y
7. Eichberg DG, Casabella AM, Menaker SA, Shah AH, Komotar RJ. Parasagittal and parafalcine meningiomas: integral strategy for optimizing safety and retrospective review of a single surgeon series. Br J Neurosurg. 2020;34(5):559–564. doi:10.1080/02688697.2019.1635988
8. World population ageing 2017: highlights. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf.
9. Grossman R, Mukherjee D, Chang DC, et al. Preoperative Charlson comorbidity score predicts postoperative outcomes among older intracranial meningioma patients. World Neurosurg. 2011;75(2):279–285. doi:10.1016/j.wneu.2010.09.003
10. Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, P AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg. 2010;113(5):1036–1042. doi:10.3171/2010.3.JNS091966
11. Narayan V, Bir SC, Mohammed N, Savardekar AR, Patra DP, Nanda A. Surgical management of giant intracranial meningioma: operative nuances, challenges, and outcome. World Neurosurg. 2018;110:e32–e41. doi:10.1016/j.wneu.2017.09.184
12. Schwartz C, Rautalin I, Niemelä M, Korja M. Symptomatic peritumoral edema is associated with surgical outcome: a consecutive series of 72 supratentorial meningioma patients ≥ 80 years of age. J Neurooncol. 2020;148(1):109–116. doi:10.1007/s11060-020-03501-z
13. Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(1):22–39. doi:10.1136/jnnp.20.1.22
14. Louis DN, Perry A, Wesseling PA-O, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. 2021;23(8):1231–1251. doi:10.1093/neuonc/noab106
15. Schul DB, Wolf S, Krammer MJ, Landscheidt JF, Tomasino A, Lumenta CB. Meningioma surgery in the elderly: outcome and validation of 2 proposed grading score systems. Neurosurgery. 2012;70(3):555–565. doi:10.1227/NEU.0b013e318233a99a
16. Goyal A, Singh AK, Sinha S, Tatke M, Singh D, G V. Simultaneous occurrence of meningioma and glioma in brain: report of two cases. J Clin Neurosci. 2003;10(2):252–254. doi:10.1016/S0967-5868(02)00345-4
17. Tunthanathip T, Kanjanapradit K, Ratanalert S, Phuenpathom N, Oearsakul T, Kaewborisutsakul A. Multiple, primary brain tumors with diverse origins and different localizations: case series and review of the literature. J Neurosci Rural Pract. 2019;9(4):593–607.
18. Sanai N, McDermott MW. A modified far-lateral approach for large or giant meningiomas of the posterior fossa. J Neurosurg. 2010;112(5):907–912. doi:10.3171/2009.6.JNS09120
19. Nayak L, Iwamoto FM. Primary brain tumors in the elderly. Curr Neurol Neurosci Rep. 2010;10(4):252–258. doi:10.1007/s11910-010-0110-x
20. Bir SC, Konar S, Maiti TK, Guthikonda B, Nanda A. Surgical outcomes and predictors of recurrence in elderly patients with meningiomas. World Neurosurg. 2016;90:251–261. doi:10.1016/j.wneu.2016.02.066
21. Galhom AE, Madawi AA, Ellabban MM. Surgical outcomes and predictors of complication in elderly patients with meningiomas. Egypt J Neurol Psychiatr Neurosurg. 2018;54(1):3. doi:10.1186/s41983-018-0005-3
22. Ressel A, Fichte S, Brodhun M, Rosahl SK, Gerlach R. WHO grade of intracranial meningiomas differs with respect to patient’s age, location, tumor size and peritumoral edema. J Neurooncol. 2019;145(2):277–286. doi:10.1007/s11060-019-03293-x
23. Asthagiri AR, Pouratian N, Fau - Sherman J, et al. Advances in brain tumor surgery. Neurol Clin. 2007;25(4):
24. Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130(2):269–282. doi:10.1007/s11060-016-2110-4
25. Kostron H, Bauer R. Management of recurrent malignant glioma--neurosurgical strategies. Wien Med Wochenschr. 2011;161(1–2):20–21. doi:10.1007/s10354-010-0861-7
26. Li C, Zhu H, Zong X, et al. History, current situation, and future development of endoscopic neurosurgery in China. World Neurosurg. 2018;110:270–275. doi:10.1016/j.wneu.2017.11.103
27. Osman H, Georges J, Elsahy D, Hattab EM, Yocom S, Cohen-Gadol AA. In vivo microscopy in neurosurgical oncology. World Neurosurg. 2018;115:110–127. doi:10.1016/j.wneu.2018.03.218
28. Sagar S, Rick J, Chandra A, Yagnik G, Aghi MA-O. Functional brain mapping: overview of techniques and their application to neurosurgery. Neurosurg Rev. 2019;42(3):639–647. doi:10.1007/s10143-018-1007-4
29. Valencia Calderón C, Castro Cevallos A, Calderón Valdiviezo A, et al. Neuronavigation in the surgical planning of callosotomy. Neurocirugia. 2016;27(4):186–193. doi:10.1016/j.neucir.2015.06.003
30. Bir SC, Konar SK, Maiti TK, Thakur JD, Guthikonda B, Nanda A. Utility of neuronavigation in intracranial meningioma resection: a single-center retrospective study. World Neurosurg. 2016;90:546–555.e541. doi:10.1016/j.wneu.2015.12.101
31. Pinzi V, Fariselli L, Marchetti M, Scorsetti M, Navarria P. Stereotactic radiotherapy for parasagittal and parafalcine meningiomas: patient selection and special considerations. Cancer Manag Res. 2019;11:10051–10060. doi:10.2147/CMAR.S187371
32. Chen WC, Perlow HK, Choudhury A, et al. Radiotherapy for meningiomas. J Neurooncol. 2022;160(2):505–515. doi:10.1007/s11060-022-04171-9
33. Ding D, Xu Z, McNeill I, Yen C, Sheehan JP. Radiosurgery for parasagittal and parafalcine meningiomas. J Neurosurg. 2013;119(4):871–877. doi:10.3171/2013.6.JNS13110
34. Caroli E, Orlando ER, Mastronardi L, Ferrante L. Meningiomas infiltrating the superior sagittal sinus: surgical considerations of 328 cases. Neurosurg Rev. 2006;29(3):236–241. doi:10.1007/s10143-006-0020-1
35. Sekhar LN, Tzortzidis FN, Bejjani GK, S DA. Saphenous vein graft bypass of the sigmoid sinus and jugular bulb during the removal of glomus jugulare tumors. Report of two cases. J Neurosurg. 1997;86(6):1036–1041. doi:10.3171/jns.1997.86.6.1036
36. Sindou MP, Alvernia JE. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105(4):514–525. doi:10.3171/jns.2006.105.4.514
37. Kotani S, Murakami N, Doi T, Ogawa T, Hashimoto N. Acute epidural vertex hematoma with good hemostasis using delayed surgery after monitoring of coagulation and fibrinolytic parameters: a case report. Surg Neurol Int. 2023;14:73. doi:10.25259/SNI_1010_2022
38. Colli BO, Carlotti CG, Assirati JA, Dos Santos MB, Neder L, Dos Santos AC. Parasagittal meningiomas: follow-up review. Surg Neurol. 2006;66(3):S20–27. doi:10.1016/j.surneu.2006.08.023
39. Lizana J, Reinoso CMD, Aliaga N, Marani W, Montemurro N. Bilateral central retinal artery occlusion: an exceptional complication after frontal parasagittal meningioma resection. Surg Neurol Int. 2021;12:397. doi:10.25259/SNI_571_2021
40. Nakamura M, Roser F, Dormiani M, Vorkapic P, Samii M. Surgical treatment of cerebellopontine angle meningiomas in elderly patients. Acta Neurochir. 2005;147(6):603–609. doi:10.1007/s00701-005-0517-3
41. Roser F, Ebner FH, Ritz R, Samii M, Tatagiba MS, Nakamura M. Management of skull based meningiomas in the elderly patient. J Clin Neurosci. 2007;14(3):224–228. doi:10.1016/j.jocn.2005.12.004
42. Slot KA-O, Peters JVM, Vandertop WP, Verbaan D, Peerdeman SM. Meningioma surgery in younger and older adults: patient profile and surgical outcomes. Eur Geriatr Med. 2018;9(1):95–101. doi:10.1007/s41999-017-0015-1
43. Ekşi M, Canbolat Ç, Akbaş A, et al. Elderly patients with intracranial meningioma: surgical considerations in 228 patients with a comprehensive analysis of the literature. World Neurosurg. 2019;132:e350–e365. doi:10.1016/j.wneu.2019.08.150
44. Samii M, Tatagiba MC, Matthies C. Acoustic neurinoma in the elderly: factors predictive of postoperative outcome. Neurosurgery. 1992;31(4):615–620. doi:10.1227/00006123-199210000-00001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
