Back to Journals » Cancer Management and Research » Volume 12
Comparison of Radiation-Induced Secondary Malignancy Risk Between Sequential and Simultaneous Integrated Boost for the Treatment of Nasopharyngeal Carcinoma: Intensity-Modulated Radiotherapy versus Volumetric-Modulated Arc Therapy
Authors Haciislamoglu E , Cinar Y, Eren M, Canyilmaz E, Gurcan F , Serdar L , Yoney A
Received 6 January 2020
Accepted for publication 19 March 2020
Published 8 April 2020 Volume 2020:12 Pages 2513—2521
DOI https://doi.org/10.2147/CMAR.S244901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Emel Haciislamoglu,1 Yunus Cinar,2 Mehmet Eren,2 Emine Canyilmaz,1 Fatih Gurcan,3 Lasif Serdar,4 Adnan Yoney1
1Department of Radiation Oncology, Faculty of Medicine, Karadeniz Technical University, Trabzon, Turkey; 2Department of Radiation Oncology, Faculty of Medicine, Recep Tayyip Erdogan University, Rize, Turkey; 3Department of Computer Engineering, Faculty of Engineering, Karadeniz Technical University, Trabzon, Turkey; 4Department of Radiation Oncology, Kanuni Research and Education Hospital, Trabzon, Turkey
Correspondence: Emel Haciislamoglu
Department of Radiation Oncology, Faculty of Medicine, Karadeniz Technical University, Trabzon, Turkey
Tel +90 462 377 56 01
Fax +90 462 325 22 70
Email [email protected]
Purpose: This study aimed to compare the secondary cancer risk (SCR) between the sequential boost (SEQ) technique and simultaneous integrated boost (SIB) technique in intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) in patients with nasopharyngeal carcinoma (NPC) using the concepts of organ equivalent dose (OED) and excess absolute risk (EAR).
Patients and Methods: IMRT-SEQ, VMAT-SEQ, IMRT-SIB, and VMAT-SIB plans were created with identical objective functions for five patients with early-stage NPC. Three different planning tumor volumes (PTVs; PTV1, PTV2, and PTV3) were delineated for each patient, and the prescribed doses were 50 Gy, 60 Gy, and 70 Gy (2 Gy/fraction), respectively, for the SEQ technique and 52.8 Gy, 59.4 Gy, and 69.3 Gy (33 fractions), respectively, for the SIB technique.
Results: All plans were clinically acceptable. There was no difference in most OED-based SCRs between IMRT and VMAT when the same fractionation scheme was used. Compared with the SEQ technique, the SIB technique in IMRT and VMAT was associated with the lowest OEDs for the oral cavity, pharynx, parotids, and submandibular glands, resulting in SCR reduction. SCR for the parotids was much lower than that for the other assessed organs when the SIB technique was used.
Conclusion: Our findings suggest that OED-based SCRs are lower with the SIB technique than with the SEQ technique in IMRT and VMAT in most organs for which SCR was calculated; furthermore, SCR for the parotids is much lower than that for other organs when the SIB technique is used in patients with NPC.
Keywords: excess absolute risk, intensity-modulated radiotherapy, organ equivalent dose, secondary cancer risk, volumetric-modulated arc therapy
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy in most parts of the world. According to the latest statistics, approximately 80% of patients with NPC are observed in Asia, particularly in Southeast Asia and South China. A previous report mentioned that the estimated incidence rate of NPC in China was 60.6 per 100,000 individuals and the associated mortality rate was 34.1 per 100,000 individuals.1
Radiotherapy (RT) is an essential component of curative-intent treatment for NPC, and stage I disease is treated with RT alone.2,3 The anatomical locations and proximities of numerous organs at risk (OARs) make RT for NPC very demanding. Different RT modalities, such as intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT), have been used widely to improve the local control rate of NPC.4 IMRT can provide better parotid sparing and improved quality of life compared with three-dimensional conformal radiotherapy (3D-CRT) in patients with early-stage NPC.5,6 IMRT can be applied using either a sequential (SEQ) boost7,8 or simultaneous integrated boost (SIB) technique.9,10 Normally, SEQ uses a conventional dose of 1.8–2 Gy/fraction throughout the course of RT, whereas SIB provides an opportunity to simultaneously treat both the primary and secondary targets at different doses.11
Although IMRT and VMAT have been shown to improve dose conformity and reduce doses to OARs, low-dose volumes in non-target tissues have been found to be greater with IMRT or VMAT than with 3D-CRT. Radiation exposure of a large volume of non-target tissue might have a negative impact in terms of secondary cancer risk (SCR).12,13 The exact mechanism of radiation-induced second malignancy is unknown. However, currently, it is a growing concern in oncology because of the high number of cancer survivors, and efforts are being made to prevent or decrease the incidence of radiation-induced second malignancy. Several models exist for the theoretical determination of the risk associated with RT. These models have various degrees of complexity, and they could be used for extrapolating the epidemiological knowledge derived from conventional treatment techniques to new RT techniques and for comparing the risks associated with different treatment techniques.
To our knowledge, only one previous report has compared radiation-induced SCR between IMRT-SIB and VMAT-SIB in patients with NPC14 and no previous report has compared the SEQ and SIB techniques. The present study aimed to compare SCR, which is considered as a late toxicity, between the SEQ and SIB techniques in IMRT and VMAT in patients with NPC using the concepts of organ equivalent dose (OED) and excess absolute risk (EAR) for dose–response modeling.
Materials and Methods
Patient Characteristics
Computed tomography (CT) scans of five patients with NPC who had undergone RT were retrospectively selected for this study. The median age of the patients was 45 years (range, 35–61 years). According to the American Joint Committee on Cancer staging system, the clinical stage distribution of the patients was stage I–II.
Ethics Statement
This study was approved by the Karadeniz Technical University, Faculty of Medicine, Farabi Hospital Ethics/Institutional Review Board (Number:2019/264, Date:16.09.2019); due to the secondary use of existing data, the patient informed consent was waived by the institutional review board. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the Helsinki declaration.
Delineation of Target Volumes and Organs at Risk
The patients underwent CT (3-mm slice thickness) in the supine position. Gross tumor volume (GTV) was defined as the visualization of any gross tumor on CT images or other images (magnetic resonance imaging and positron emission tomography). Clinical target volume (CTV) was defined as GTV plus areas considered at risk for containing microscopic disease delineated by a physician. CTV3 was defined as GTV plus a 5-mm margin around GTV. This margin can be reduced to as low as 1 mm for tumors in close proximity to critical structures. For CTV2, all potential routes of spread for primary and nodal GTVs were delineated by a radiation oncologist. For CTV1, all levels of the neck, except for level I, were defined as low-risk subclinical regions, with a prescription dose of 50 Gy or 52.8 Gy. A 3-mm margin was added to all CTVs to create respective planning target volumes (PTVs; PTV1, PTV2, and PTV3). The mean PTV1, PTV2, and PTV3 volumes of 5 patients were 602 ± 76 cm3 (range 530–682), 177 ± 7 cm3 (range 169–184), and 123 ± 27 cm3 (range 88–147), respectively. PTVs were trimmed to 3 mm from the skin surface (Figure 1).
OARs were delineated according to the Radiation Therapy Oncology Group 0225 protocol.15 The delineated OARs included the brain stem, spinal cord, oral cavity, pharynx, parotids, submandibular glands, mandible, optic nerves, optic chiasm, lens, mandible, pituitary gland, and soft tissue (total exposed volume minus PTV1).
Treatment Planning
For each patient, IMRT-SEQ, VMAT-SEQ, IMRT-SIB, and VMAT-SIB plans were created with the same goals and objectives. The Eclipse treatment planning system (version 10, Varian Medical Systems, Palo Alto, CA, USA) was used for treatment planning, utilizing 6 MV photon beams.
The SEQ technique had the following three plans: 2 Gy × 25 fractions (50 Gy) to the low-risk PTV (PTV1), followed by two different sequential boosts (2 Gy × 5 fractions; 60 Gy and 70 Gy) to the medium- and high-risk PTVs (PTV2 and PTV3). On the other hand, the SIB technique involved the treatment of the low-, medium-, and high-risk PTVs with doses of 52.8 Gy, 59.4 Gy, and 69.3 Gy, respectively, in 33 fractions with plan normalization to cover at least 95% of PTV1 with 95% of the prescribed dose.
SCR was calculated at the brain stem, spinal cord, oral cavity, pharynx, parotids, submandibular glands, mandible, and soft tissue. Dose constraints were used to create acceptable dose limits for the brain stem, spinal cord, and parotids among the organs for which SCR was calculated; the dose constraint process was excluded for other organs, such as the oral cavity, pharynx, submandibular glands, mandible, and soft tissue. The plans were iteratively optimized to obtain optimal coverage of PTVs and sparing of OARs. The dose constraints for the OARs are presented in Table 1.
 |
Table 1 Dose Constraints of the Organs at Risk (OARs) |
The IMRT plans involved nine (PTV1), seven (PTV2), and five (PTV3) coplanar fields with equally spaced gantry angles for SEQ and nine coplanar fields with equally spaced gantry angles for SIB. The VMAT plans involved two full coplanar arcs.
Calculation of Secondary Cancer Risk Estimates
It is known that for doses below 2 Gy, the dose–response relationship is linear.12 However, for higher doses and inhomogeneous dose distributions, the dose–response relationship is not linear12,16 and other dose–response functions are required to describe the relation. To facilitate the estimation of SCR for irradiated organs, Schneider et al introduced the concept of OED, according to which any two dose distributions in an organ are equivalent if they cause the same radiation-induced cancer incidence.16
Different models for OED calculation are available according to the different assumptions of cell behavior after dose exposure.17,18 Schneider’s full mechanistic dose–response model was used in this study. The full mechanistic model accounts for killing and fractionation effects.18,19 OEDs for the brain stem, spinal cord, oral cavity, pharynx, parotids, and submandibular glands were calculated using a full mechanistic dose–response model based on differential dose–volume histograms (dDVHs), according to the following formula:
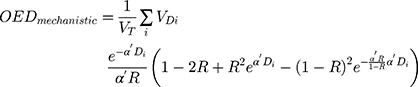
where 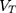 is the total organ volume and
is the total organ volume and 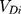 is the volume of the organ that is exposed to dose
is the volume of the organ that is exposed to dose 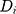 . The sum involves all the bins of the dose–volume histogram. Additionally, the parameter R describes repopulation and the repair ability between the delivered dose fractions. The parameter
. The sum involves all the bins of the dose–volume histogram. Additionally, the parameter R describes repopulation and the repair ability between the delivered dose fractions. The parameter 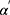 is calculated as follows:
is calculated as follows:
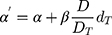
where α and β are parameters from the linear quadratic model of cell killing, describing the linear and quadratic dose response of the tissue to radiation.
OEDs for the mandible and soft tissue were calculated using a specific mechanistic sarcoma model based on intermediate repopulation (R = 0.5) according to the following formula:
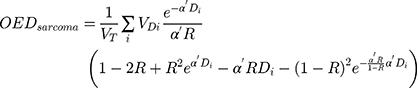
The risk of developing secondary solid cancer after RT is usually represented by EAR. The EAR for the development of solid cancer describes the absolute difference in cancer rates between persons exposed to a dose d and those not exposed to a dose beyond the natural dose exposition per 10,000 persons per year.18 EAR can be calculated as follows:18

where 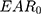 is the initial slope of the dose–response curve at a low dose. The function
is the initial slope of the dose–response curve at a low dose. The function 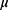 takes into account the age of the population examined based on the patient’s age at the time of irradiation (agex) and the attained age of the patient in years (agea). It can be calculated as follows:
takes into account the age of the population examined based on the patient’s age at the time of irradiation (agex) and the attained age of the patient in years (agea). It can be calculated as follows:
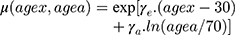
where 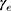 and
and 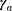 are age-modifying factors (
are age-modifying factors (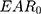 was originally calculated for persons exposed at the age of 30 years and attaining the age of 70 years). All EARs in this study were calculated with age modification for patients irradiated at the age of 45 years (agex) and attaining the age of 70 years (agea).
was originally calculated for persons exposed at the age of 30 years and attaining the age of 70 years). All EARs in this study were calculated with age modification for patients irradiated at the age of 45 years (agex) and attaining the age of 70 years (agea).
The site-specific parameters were derived from a combined fit to data from atomic bomb survivors and patients treated with RT for Hodgkin disease, assuming an α/β
value of 3 Gy.18 The difference in the baseline risks for developing cancer without exposure to radiation between the Japanese and Western populations was included. The parameters used for the OED and EAR calculations are presented in Table 2.
 |
Table 2 Risk Parameters for All Tissues |
Statistical Analysis
The two-tailed Wilcoxon signed-rank test was used to compare differences between the DVH parameters of the IMRT and VMAT plans (each pair of patient-specific DVH values was compared). All statistical analyses were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA). Significance level was set at p = 0.05.
Results
The mean volume of the PTV1, PTV2, and PTV3 were 624.96 ± 112.17 cm3, 177.58 ± 6.96 cm3, and 123.44 ± 27.49 cm3, respectively. In all five cases, all plans were clinically acceptable, with at least 95% of the PTVs receiving 95% of the prescribed dose.
The mean parotid volume was 47 ± 13.28 cm3. For IMRT-SEQ, VMAT-SEQ, IMRT-SIB, and VMAT-SIB, the mean doses (Dmean) for the right parotid were 25.80 ± 1.83 Gy, 23.23 ± 0.90 Gy, 25.15 ± 1.03 Gy, and 22.39 ± 1.23 Gy, respectively, and those for the left parotid were 26.70 ± 1.44 Gy, 23.43 ± 1.49 Gy, 26.47 ± 1.19 Gy, and 23.31 ± 0.74 Gy, respectively. The dosimetric data for the parotids and soft tissue are summarized in Table 3. The Dmean of the parotids was significantly higher with IMRT than with VMAT for both the SEQ and SIB techniques. The V30 of the parotids and the soft tissue doses (V1, V3, and V5) were not significantly different (Table 3).
 |
Table 3 Comparison of the Parotid and Soft Tissue Dose–Volume Metrics as a Function of Plan Modality |
The OED and EAR values for all OARs according to the SEQ and SIB techniques in IMRT and VMAT are shown in Table 4. The relative difference between SCRs associated with the SEQ and SIB techniques was greatest for the parotids compared with the findings for all other organs in both IMRT and VMAT, with a reduction of approximately 40% (Table 4). On comparing only the SEQ and SIB techniques, IMRT and VMAT showed similar SCRs (except for the mandible [SEQ] and parotids [SIB]). SIB in both IMRT and VMAT resulted in the lowest OEDs for the oral cavity, pharynx, parotids, and submandibular glands, and was thus associated with SCR reduction compared with SEQ; conversely, SEQ in both IMRT and VMAT significantly reduced the OED and EAR of the soft tissue compared with SIB.
 |
Table 4 OED and EAR Values for All OARs According to the SEQ and SIB Techniques in IMRT and VMAT |
Figure 2 shows the OED and EAR values for all OARs stratified according to the techniques. As shown in Figure 2E, the EAR-SEQ:EAR-SIB ratio was the greatest for the parotids compared with the findings for all other OARs, indicating that parotids have a higher SCR with the SEQ technique than with the SIB technique.
Discussion
Owing to technological advancements, new RT techniques, such as IMRT, have been developed with the intention of not only improving tumor coverage but also sparing OARs compared with traditional two-dimensional RT.5,20-22 Furthermore, VMAT has been shown to be superior to IMRT with regard to improving dose homogeneity and sparing critical organs at multiple tumor sites.23–25
IMRT and VMAT can be applied using either the SEQ or SIB technique. The SIB technique allows simultaneous delivery of different doses to different target volumes within a single treatment fraction, enabling the shortening of treatment duration and enhancing the biological equivalent dose. Most clinical studies involving NPC used the SIB technique9,10,22,26 and rarely performed comparisons with the other technique.7,8,27 To our knowledge, only one previous study has compared the radiation-induced SCR between IMRT-SIB and VMAT-SIB in patients with NPC14 and no previous report has compared the SEQ and SIB techniques in terms of SCR. Therefore, the findings of the present study comparing the SEQ and SIB techniques in IMRT and VMAT are important.
Dosimetric studies comparing IMRT-SEQ and IMRT-SIB revealed that both techniques provided the same target coverage; however, IMRT-SIB showed better parotid sparing, whereas IMRT-SEQ lowered the maximum doses to the spinal cord and brain stem.28,29 In the present study, all plans showed equally good PTV coverage. Our dosimetric data indicated that parotid sparing was slightly better with IMRT-SIB and VMAT-SIB than with IMRT-SEQ and VMAT-SEQ in terms of the Dmean and V30; however, there were no significant differences. According to our results, the Dmean to the parotids was significantly lower in VMAT than in IMRT for both SEQ and SIB.
In this study, most OED-based SCRs, including those in the oral cavity, pharynx, parotids, and submandibular glands, were significantly lower with the IMRT-SIB and VMAT-SIB plans than with the IMRT-SEQ and VMAT-SEQ plans. However, the most striking result in our study was regarding SCR for the parotids. The relative difference in SCR between the SEQ and SIB techniques was the greatest for the parotids in both IMRT and VMAT, with an approximately 40% reduction with the SIB technique, although there were no significant differences with regard to the Dmean and V30 of the parotids. In this study, SCR was calculated on the basis of the radiation dose received by OARs. To calculate the risks of nonhomogeneous doses to the organs, the concept of OED was used for directly considering the dose–response relationship for the organs. Our results showed that although there was no difference between the plans in terms of Dmean, there could be a difference in terms of SCR associated with Dmax and nonhomogeneous dose distribution in the organs. In NPC cases, the parotids might touch the treatment field (especially with regard to PTV1), and we should pay particular attention to the organs near a treated region considering the secondary malignancy potential.
In a study by Lee et al differences in SCR between IMRT-SIB and VMAT-SIB were assessed with regard to NPC. The authors found that the OED-based SCR was slightly higher for the oral cavity and mandible when VMAT-SIB was used.14 According to their results, there was no significant difference in terms of SCR to other organs, including the brain stem, parotids, pharynx, submandibular glands, lungs, spinal cord, and healthy tissue. The present study found no differences between IMRT-SIB and VMAT-SIB for the oral cavity and mandible and found that SCR was slightly higher for the parotids when VMAT-SIB was used. A direct comparison of our data with data from other groups is not straightforward because of the possible differences in GTV delineation, treatment margins, irradiation volume, and adopted methods.
VMAT was equivalent or superior to IMRT in terms of PTV coverage and OAR sparing. However, higher SCR should be taken into consideration; it might be caused by the distribution of low-dose radiation to non-target healthy tissue.14,30 According to our results, there were no significant differences between IMRT and VMAT regarding V1, V3, and V5 values with both the SEQ and SIB techniques and regarding SCR with the same techniques for the soft tissue. However, SCR for the soft tissue was significantly lower with the SEQ technique than with the SIB technique in both IMRT and VMAT.
There are a number of limitations in our work. We performed this analysis of the SCR in only five patients. Other studies involving similar cancer risk assessments also used a small number of patients (typically two to three cases per study). The reason for the relatively small sample size in this type of studies is that the primary interest is the investigation of the differences between planning techniques rather than the factors associated with inter-patient variability.31,32 There are also other uncertainties in radiation-induced secondary cancer models and parameters.
In epidemiological studies, radiation-induced malignancy might be influenced by factors such as radiation dose and age at initial exposure.33 In this study, the median age of the patients was 45 years (range, 35–61 years). We selected patients who were relatively young at the time of treatment to take into account the age dependence of SCR. All SCRs were calculated with age modification for patients irradiated at the age of 45 years (because the median age of the patients is 45 years) and attaining the age of 70 years to obtain exact SCRs. SCR is a non-negligible late complication encountered by young patients, especially long-term survivors of NPC.
In this study, most OED-based SCRs were significantly lower with the SIB technique than with the SEQ technique. However, SIB might carry a high risk of regional recurrence because of the low dose per fraction at the elective nodal region and might cause late adverse toxicities because of the high dose per fraction near GTV. For further clarification, randomized clinical trials comparing the treatment outcomes between these two techniques are needed.
Conclusion
IMRT and VMAT are the standards of care for NPC and can be applied using either the SEQ or SIB technique. Although IMRT and VMAT have become common treatment modalities for NPC, there is concern regarding SCR associated with their use. Our findings suggest that OED-based SCRs are usually lower with the SIB technique than with the SEQ technique in IMRT and VMAT and that SCR for the parotids is dramatically lower than that for other organs when the SIB technique is used in patients with NPC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
2. Chan AT, Grégoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS–ESMO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:83–85. doi:10.1093/annonc/mds266
3. Lee Y, Ho CY. Headache as the sole symptom of nasopharyngeal carcinoma and its clinical implications. Sci World J. 2012;1–5.
4. Lee TF, Chao PJ, Ting HM, et al. Comparative analysis of SmartArc-based dual arc volumetric-modulated arc radiotherapy (VMAT) versus intensity-modulated radiotherapy (IMRT) for nasopharyngeal carcinoma. J Appl Clin Med Phys. 2011;12(4):158–174. doi:10.1120/jacmp.v12i4.3587
5. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981–991. doi:10.1016/j.ijrobp.2006.06.013
6. AbdElWahab SA, Mohammed DA, Gaballah AM, Abdallah MM. Three-dimensional conformal versus intensity modulated radiation therapy in treatment of nasopharyngeal carcinoma. Egypt J Hosp Med. 2018;71:3492–3499.
7. Lertbutsayanukul C, Khorprasert C, Shotelersuk K, et al. Intensity-modulated radiation therapy in head-and-neck cancer, first report in Thailand. J Med Assoc Thai. 2006;89(12):2068–2076.
8. Songthong A, Chakkabat C, Kannarunimit D, Lertbutsayanukul C. Efficacy of intensity-modulated radiotherapy with concurrent carboplatin in nasopharyngeal carcinoma. Radiol Oncol. 2015;49(2):155–162. doi:10.2478/raon-2014-0044
9. Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group Phase II trial 0225. J Clin Oncol. 2009;27:3684–3690. doi:10.1200/JCO.2008.19.9109
10. Wang R, Wu F, Lu H, et al. Definitive intensity-modulated radiation therapy for nasopharyngeal carcinoma: long-term outcome of a multicenter prospective study. J Cancer Res Clin Oncol. 2013;139(1):139–145. doi:10.1007/s00432-012-1313-0
11. Butler EB, Teh BS, Grant WH
12. Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56(1):83–88. doi:10.1016/S0360-3016(03)00073-7
13. Kry SF, Salehpour M, Followill DS, et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62(4):1195–1203. doi:10.1016/j.ijrobp.2005.03.053
14. Lee HF, Lan JH, Chao PJ, et al. Radiation-induced secondary malignancies for nasopharyngeal carcinoma: a pilot study of patients treated via IMRT or VMAT. Cancer Manag Res. 2018;10:131–141. doi:10.2147/CMAR.S145713
15. Lee NY, Harris J, Garden A, et al. Phase II multi-institutional study of IMRT chemotherapy for nasopharyngeal carcinoma (RTOG 0225): preliminary results. Int J Radiat Oncol Biol Phys. 2007;69:13–14. doi:10.1016/j.ijrobp.2007.07.025
16. Schneider U, Zwahlen D, Ross D, Kaser-Hotz B. Estimation of radiation-induced cancer from three-dimensional dose distributions: concept of organ equivalent dose. Int J Radiat Oncol Biol Phys. 2005;61(5):1510–1515. doi:10.1016/j.ijrobp.2004.12.040
17. Schneider U, Walsh L. Cancer risk estimates from the combined Japanese a-bomb and hodgkin cohorts for doses relevant to radiotherapy. Radiat Environ Biophys. 2008;47(2):253–263. doi:10.1007/s00411-007-0151-y
18. Schneider U, Sumila M, Robotka J. Site-specific dose-response relationships for cancer induction from the combined Japanese a-bomb and hodgkin cohorts for doses relevant to radiotherapy. Theor Biol Med. 2011;8(1):27. doi:10.1186/1742-4682-8-27
19. Schneider U. Mechanistic model of radiation-induced cancer after fractionated radiotherapy using the linear-quadratic formula. Med Phys. 2009;36:1138–1143. doi:10.1118/1.3089792
20. Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys. 2003;56(1):145–157. doi:10.1016/S0360-3016(03)00075-0
21. Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–4879. doi:10.1200/JCO.2007.11.5501
22. Peng G, Wang T, Yang KY, et al. A prospective randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi:10.1016/j.radonc.2012.08.013
23. Palma D, Vollans E, James K, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):996–1001. doi:10.1016/j.ijrobp.2008.02.047
24. Jin X, Yi J, Zhou Y, et al. Comparison of whole-field simultaneous integrated boost VMAT and IMRT in the treatment of nasopharyngeal cancer. Med Dosim. 2013;38(4):418–423. doi:10.1016/j.meddos.2013.05.004
25. Wu Z, Xie C, Hu M, et al. Dosimetric benefits of IMRT and VMAT in the treatment of middle thoracic esophageal cancer: is the conformal radiotherapy still an alternative option? J Appl Clin Med Phys. 2014;15(3):93–101. doi:10.1120/jacmp.v15i3.4641
26. Wong FC, Ng AW, Lee VH, et al. Whole-field simultaneous integrated-boost intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010;76(1):138–145. doi:10.1016/j.ijrobp.2009.01.084
27. Tao H, Wei Y, Huang W, et al. Comparison of long-term survival and toxicity of simultaneous integrated boost vs conventional fractionation with intensity-modulated radiotherapy for the treatment of nasopharyngeal carcinoma. Onco Targets Ther. 2016;9:1865–1873.
28. Dogan N, King S, Emami B, et al. Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. Int J Radiat Oncol Biol Phys. 2003;57:1480–1491. doi:10.1016/S0360-3016(03)01569-4
29. Chen SW, Yang SN, Liang JA, et al. Comparative dosimetric study of two strategies of intensity-modulated radiotherapy in nasopharyngeal cancer. Med Dosim. 2005;30(4):219–227. doi:10.1016/j.meddos.2005.07.001
30. Wolff D, Stieler F, Welzel G, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol. 2009;93(2):226–233. doi:10.1016/j.radonc.2009.08.011
31. Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):616–622. doi:10.1016/j.ijrobp.2009.01.001
32. Murray LJ, Thompson CM, Lilley J, et al. Radiation-induced second primary cancer risks from modern external beam radiotherapy for early prostate cancer: impact of stereotactic ablative radiotherapy (SABR), volumetric modulated arc therapy (VMAT) and flattening filter free (FFF) radiotherapy. Phys Med Biol. 2015;60:1237–1257. doi:10.1088/0031-9155/60/3/1237
33. Wei Z, Xie Y, Xu J, et al. Radiation-induced sarcoma of head and neck: 50 years of experience at a single institution in an endemic area of nasopharyngeal carcinoma in China. Med Oncol. 2012;29(2):670–676. doi:10.1007/s12032-011-9828-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.