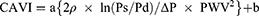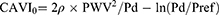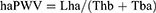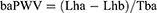Back to Journals » Vascular Health and Risk Management » Volume 18
Comparison of Predictive Ability of Arterial Stiffness Parameters Including Cardio-Ankle Vascular Index, Pulse Wave Velocity and Cardio-Ankle Vascular Index0
Authors Nagayama D , Fujishiro K , Suzuki K, Shirai K
Received 12 June 2022
Accepted for publication 6 September 2022
Published 12 September 2022 Volume 2022:18 Pages 735—745
DOI https://doi.org/10.2147/VHRM.S378292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Harry Struijker-Boudier
Daiji Nagayama,1,2 Kentaro Fujishiro,3 Kenji Suzuki,3 Kohji Shirai4
1Department of Internal Medicine, Nagayama Clinic, Tochigi, Japan; 2Center of Diabetes, Endocrinology and Metabolism, Toho University, Sakura Medical Center, Chiba, Japan; 3Research and Development Division, Japan Health Promotion Foundation, Tokyo, Japan; 4Internal Medicine, Mihama Hospital, Chiba, Japan
Correspondence: Daiji Nagayama, Nagayama Clinic, 2-12-22, Tenjin-Cho, Oyama-City, Tochigi, 323-0032, Japan, Tel +81-285-22-0219, Fax +81-285-22-0219, Email [email protected]
Abstract: Cardio-ankle vascular index (CAVI) was developed to reflect the stiffness of the arterial tree from the aortic origin to the ankle. This arterial stiffness parameter is useful for assessing the severity of cardiovascular disease (CVD) and its risk. However, compared to pulse wave velocity (PWV), the conventional gold standard of arterial stiffness parameter, there has been a concern regarding CAVI that there are fewer longitudinal studies for CVD. Furthermore, the accuracy of CAVI for atherosclerotic diseases compared to other parameters has not been well validated. This review article aims to summarize recent findings to clarify the predictive ability of CAVI in longitudinal studies. First, several large longitudinal studies have found that not only baseline CAVI but also CAVI changes during the observation period predict cardiovascular events. Second, CAVI may have superior discriminatory power for all-cause mortality and major adverse cardiovascular endpoints compared to PWV. Furthermore, one large longitudinal study found CAVI to be a stronger predictor for renal function decline compared to PWV as well as CAVI0, a variant of CAVI that mathematically excludes BP dependence. Additionally, CAVI shows the properties that allow the elucidation of specific hemodynamics in aortic valve disease or hypovolemia. In conclusion, CAVI may be a modifiable arterial stiffness parameter not only for predicting and preventing atherosclerotic diseases but also for elucidating specific hemodynamic pathophysiology.
Keywords: cardio-ankle vascular index, pulse wave velocity, CAVI0, longitudinal study, cardiovascular disease, renal function decline
Plain Language Summary
- Cardio-ankle vascular index (CAVI) is an arterial stiffness parameter in which the blood pressure dependence observed in pulse wave velocity (PWV) is eliminated.
- Many longitudinal studies revealed the independent contribution of CAVI for cardiovascular events.
- CAVI0 is a variant of CAVI that theoretically corrects even more strongly for the dependence on blood pressure.
- CAVI may predict the development of atherosclerotic diseases more effectively than PWV and CAVI0.
Preface
Arterial stiffness reflects the ageing and loss of elasticity in blood vessels, and is used as a predictor of cardiovascular disease (CVD).1 The most commonly used arterial stiffness parameter is pulse wave velocity (PWV).2 However, this parameter is inherently influenced by blood pressure (BP) at the time of measurement,3 and may underestimate the degree of vascular dysfunction due to CVD risks other than hypertension. The cardio-ankle vascular index (CAVI) has been established to address this issue. CAVI reflects the stiffness of the whole arterial tree comprising the aorta, femoral artery and tibial artery.4 The BP independence of CAVI has been confirmed both theoretically and experimentally.3–5 CAVI can therefore clarify the intrinsic impact of BP on atherogenesis,6 and has been reported to be associated positively with a number of CVD risk factors, severity of CVD and future cardiovascular (CV) events. Additionally, appropriate therapeutic interventions to reduce CAVI are expected to contribute to the prevention of future CV events.7 However, there has been a concern regarding CAVI that there are fewer longitudinal studies for CV events compared to PWV. Moreover, a warning was issued that CAVI may lead to erroneous conclusions because it does not fully correct for BP dependence. Spronck et al8 have proposed CAVI0 as a variant of CAVI that theoretically corrects even more strongly for the BP dependence. The accuracy of these aforementioned arterial stiffness parameters has not yet been fully compared. Based on these backgrounds, this review article aims to summarize recent findings about CAVI in longitudinal studies for predicting atherosclerotic diseases. In addition, the predictive ability of the three arterial stiffness parameters (CAVI, PWV and CAVI0) was compared.
CAVI as a Predictor of Cardiovascular Events
Table 1 shows large-scale longitudinal studies examining the predictive ability of CAVI in which there were more than 200 participants. In most studies, baseline CAVI was a predictor of future cardiovascular (CV) events or mortality, and was also associated with new-appearance of atrial fibrillation.21
 |  |  |
Table 1 Summary on Association of CAVI with Cardiovascular Outcomes in Prospective Studies |
Especially, some reports showed that the change in CAVI was also associated with CVD outcomes. Otsuka et al10 demonstrated that persistent impairment of arterial stiffness was an independent risk for future CV events in 211 subjects with coronary artery disease. The study revealed that the incidence of CV events for 2.9 years was lower in the group with improved CAVI at 6 months compared to those without (Figure 1). Additionally, Saiki et al20 also reported that the change in CAVI during the first year was associated with 3 point-major adverse cardiac events (MACE) in the 5-year prospective cohort trial enrolling 254 dyslipidemic patients. Furthermore, the annual change in CAVI throughout the observation period was significantly higher in subjects with CV events compared to those without (Figure 2). In other words, appropriate therapeutic interventions to reduce CAVI are expected to contribute to the prevention of future CVD events.
 |
Figure 1 Kaplan–Meier curves of event-free survival according to CAVI. 211 patients with coronary artery disease (age 65±10 years, 118 men). (A) Comparison of Kaplan–Meier curves of event-free survival between patients above the median and below the median CAVI value in the first CAVI test. (B) Comparison of Kaplan–Meier curves of event-free survival between patients with persistently impaired CAVI and improved CAVI. Reprinted with permission from Otsuka T, Fukuda S, Shimada K et al. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014;37(11):1014–1020.10 Abbreviation: CAVI, cardio-ankle vascular index. |
 |
Figure 2 Differences in the annual CAVI changes in patients with or without CV events. 254 patients with CVD risk (age 64.8 ± 9.3 years, 118 men). (A) Primary end points and (B) 3P-MACE. Mean ± SD, Mann–Whitney U-test. Annual CAVI change was defined as the annual change in CAVI until the occurrence of any CV event or the end of 5-year study period. Primary end point: composite of cardiovascular death, sudden death of unknown origin, nonfatal myocardial infarction, nonfatal stroke, transient ischemic attack, and heart failure requiring hospitalization. 3P-MACE: three-point major cardiac adverse events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke). Reprinted with permission from Saiki A, Watanabe Y, Yamaguchi T et al. CAVI-Lowering Effect of Pitavastatin May Be Involved in the Prevention of Cardiovascular Disease: Subgroup Analysis of the TOHO-LIP. J Atheroscler Thromb. 2021;28(10):1083–1094. Creative Common.20 Abbreviations: CAVI, cardio-ankle vascular index; CV, cardiovascular. |
Measurement of the Three Arterial Stiffness Parameters
The methods for measuring arterial stiffness parameters cited in this review article are described.
CAVI and CAVI0 are based on the stiffness index β and a wave equation derived from Newton’s second law. CAVI is calculated by the following formula:4
where Ps is systolic blood pressure (SBP); Pd is diastolic blood pressure (DBP); ΔP is Ps - Pd; ρ is blood density; PWV is pulse wave velocity, and a and b are constants. On the other hand, CAVI0 is calculated by the following formula:8
where Pref is reference pressure (ie, 100 mmHg).
There are several variants of PWV with different ranges of targeted vessels. Heart-ankle PWV (haPWV) and brachial-ankle PWV (baPWV) mainly cited in this article were calculated by the following formulas:9
where Lha is the arterial path length from the aortic annulus to the midpoint of the right ankle cuff estimated by the subjects height, Thb is the time interval between the commencement of the second heart sound and the dicrotic notch on the right brachial arterial pressure wave, Tba is the “foot-to-foot” time interval between brachial and posterior-tibial arterial pressure waves, respectively, and Lhb is the arterial path length from the aortic annulus to the midpoint of right brachial cuff estimated by the subjects height.
Comparison of the Predictive Ability of CAVI with Other Arterial Stiffness Parameters
This section lists longitudinal studies comparing the arterial stiffness parameters.
Comparison of CAVI and PWV
The Nagahama study,25 a five-year longitudinal study of 8850 Japanese community residents, showed that baPWV and CAVI were similarly associated with future CVD events. However, it was concluded that the association was clearer for baPWV. On the other hand, several longitudinal studies have been reported showing the superiority of CAVI.
We have previously conducted a longitudinal study comparing CAVI with haPWV, an index incorporated into the CAVI structural equation including central and peripheral arteries.19 Of the 209 hemodialysis patients, 38 died for 6 years. Resultantly, in the Cox-proportional hazards analyses, 1 SD increase in both parameters contributed almost equally to all-cause mortality [CAVI: hazards ratio (HR) 1.595, haPWV: HR 1.695]. On the other hand, receiver operating characteristic analysis showed that CAVI had better discriminatory power for all-cause mortality compared to haPWV (Figure 3).
 |
Figure 3 Discriminatory powers of arterial stiffness parameters for the prediction of all-cause mortality. Of the 209 hemodialysis patients (mean age 60 years, 129 men), 38 patients died during the 6-year period. Curves represent receiver-operating-characteristics analyses for discriminating the probability of all-cause mortality. The Youden’s J statistic was used to select the optimum cutoff point of each arterial stiffness parameter. *P < 0.05, †P < 0.001 between haPWV and CAVI. Reprinted with permission from Dove Medical Press. Murakami K, Inayama E, Itoh Y et al. The Role of Cardio-Ankle Vascular Index as a Predictor of Mortality in Patients on Maintenance Hemodialysis. Vasc Health Risk Manag. 2021;17:791–798.19 Abbreviations: AUC, area under curve; NRI, net reclassification index; IDI, integrated discrimination improvement; 95% CI, 95% confidence interval; haPWV, heart-ankle pulse wave velocity; CAVI, cardio-ankle vascular index. |
Kirigaya et al17 also compared the predictive ability of CAVI and baPWV in 387 consecutive patients with history of acute coronary syndrome (ACS) in a longitudinal study. Resultantly, MACE (CV death, recurrence of ACS, heart failure requiring hospitalization or stroke) occurred in 62 patients (16.0%) during a median follow-up of 62 months. Multivariate analysis suggested that mean CAVI was a significant predictor of MACE (HR = 1.494) and CV death (HR = 2.217), but baPWV was not.
Comparison of CAVI, PWV and CAVI0 (Part 1)
Spronck et al28 compared the three arterial stiffness parameters including haPWV, CAVI and CAVI0 in 154 outpatients during an average of 2.53 years of observation, and the predictive ability of each parameter for the composite endpoint [death (n = 21) and heart failure requiring hospitalization (n = 18)] was examined. Resultantly, after adjustment for baseline heart failure status assessed by Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score and systolic BP, only right sided haPWV (HR = 1.58) and right sided CAVI (HR = 1.44) remained predictive, whereas only CAVI0 was not a significant factor.
Comparison of CAVI, PWV and CAVI0 (Part 2)
Most renal impairment is attributable to renal atherosclerosis, and renal function can be quantified using estimated glomerular filtration rate (eGFR). The predictive ability of three arterial stiffness parameters for renal function decline was therefore validated.29 A total of 27,864 Japanese urban residents without renal impairment at baseline who participated in two to eight consecutive (mean 3.5 ± 1.7 times) annual health examinations were studied, and 6.6% of them developed renal function decline (eGFR < 60 mL/min/1.73m2).
The discriminatory power for renal function decline showed a decreasing trend of CAVI to haPWV to CAVI0 (C-statistic: 0.740 vs 0.734 vs 0.726). The cutoffs defined using Youden’s J statistic were CAVI 8.0, haPWV 7.23, and CAVI0 11.6 (Table 2A). The results of the analyses showed that CAVI was most strongly associated with renal function decline, followed by haPWV and CAVI0, in that order (Table 2B). Furthermore, the contribution of each parameter (increase above cutoff or by 1 SD) to renal function decline was assessed using Cox-proportional hazards analysis (Table 3). If the arterial stiffness parameter is not significant in Model 1 [confounders: age, sex, BMI, proteinuria, systolic BP, fasting plasma glucose and high-density lipoprotein cholesterol (HDL-C)], it implies a lack of predictive ability beyond any metabolic parameters including BP, for renal function decline. Alternatively, if the arterial stiffness parameter is significant in Model 1 but not in Model 2 (confounders: treatments for hypertension, diabetes and dyslipidemia replaced by systolic BP, FPG and HDL-C in Model 1), it implies that the parameter depends on any metabolic abnormality. In other words, if the arterial stiffness parameter is not extracted as a significant factor in both Model 1 and 2, the parameter can be considered as having no value as a new risk factor. The results confirmed that only CAVI significantly contributed to the renal function decline in both models. On the other hand, haPWV and CAVI0 were not necessarily significant contributors.
 |
Table 2 Comparison of Associations of Arterial Stiffness Parameters with Renal Function Decline |
 |
Table 3 Adjusted Hazard Ratios of Arterial Stiffness Parameters for Renal Function Decline |
The cutoff is defined by the intersection point in the frequency distribution curves of arterial stiffness parameter in each of the two groups with and without renal function decline. Since arterial stiffness is strongly age-dependent, the cutoffs vary widely across age groups. In other words, the cutoff for predicting renal function decline is higher when restricted to older people. Therefore, when the cutoff of arterial stiffness parameter estimated in this study is treated for predicting renal function decline, the interpretation should only be targeted to the middle aged general Japanese population.
In summary, haPWV, a remarkable BP dependent arterial stiffness parameter, was more predictive for renal function decline than CAVI0, which has been proposed to theoretically eliminate BP dependence. However, CAVI was most strongly associated with renal function decline compared with haPWV and CAVI0. The major difference between CAVI and CAVI0 is that CAVI employs β over a range of diastolic to systolic pressures, whereas CAVI0 employs β at only diastolic pressure. This difference in structural formula might cause the divergence of predictive ability between CAVI and CAVI0.
Comparison of the Predictive Ability for Hemodynamic Changes
Finally, the predictive ability of arterial stiffness parameters for the specific hemodynamic is discussed.
Mestanik et al30 reported that cold pressor test caused a transient increase in CAVI, but not in CAVI0. Since the cold pressor test inherently induces vascular smooth muscle contraction through sympathetic activation,31 this result therefore means that CAVI, not CAVI0, is influenced by short-term arterial smooth muscle contraction. This finding is consistent with our previous report revealing that CAVI can be reduced by α1- adrenergic receptor blocker, but not by β1 blocker.3 In other words, CAVI accurately reflects not only organic stiffness (ie, arteriosclerosis) but also functional stiffness (ie, vascular smooth muscle contraction).
Plunde et al32 reported that aortic valve replacement (AVR) for aortic valve disease increased CAVI. The prolonged pulse wave caused by preoperative aortic valve disease leads to an extended and larger arterial dilatation to comply with the systolic flow and consequently resulting in a modified measurement of arterial stiffness. Relief of obstruction by AVR therefore leads to true arterial stiffness can be measured by CAVI.33 However, carotid-femoral PWV, which essentially lacks information on the aortic valve and ascending aorta, remained unchanged after AVR in the same population.
Nagasawa et al34 used a rabbit model to assess the effect of changes in intra-aortic blood volume on arterial stiffness. When blood was removed, BP and blood flow in the common carotid artery gradually decreased. At that point, CAVI increased during blood removal, whereas haPWV conversely decreased slightly as the decreased BP. The finding suggests that CAVI, not haPWV, accurately reflects the acute response of conduit artery elasticity to changes in intra-aortic blood volume.
Conclusions
As discussed in this chapter, CAVI may predict the development of atherosclerotic diseases more effectively compared to PWV and CAVI0, and is also useful for assessing the pathophysiology of hemodynamics in relation to left ventricular function and peripheral organ blood flow. This index is a suitable arterial stiffness parameter reflecting the Windkessel effect, and may therefore open up a new horizons of vascular function research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mitchell GF, Hwang SJ, Vasan RS., et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–511. doi:10.1161/CIRCULATIONAHA.109.886655
2. Cavalcante JL, Lima JA, Redheuil A, et al. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi:10.1016/j.jacc.2010.12.017
3. Shirai K, Song M, Suzuki J, et al. Contradictory effects of β1- and α1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI) - CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49–55. doi:10.5551/jat.3582
4. Shirai K, Utino J, Otsuka K, et al. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. doi:10.5551/jat.13.101
5. Shirai K, Suzuki K, Tsuda S, et al. Comparison of cardio–ankle vascular index (CAVI) and CAVI0 in large healthy and hypertensive populations. J Atheroscler Thromb. 2019;26:603–615. doi:10.5551/jat.48314
6. Nagayama D, Watanabe Y, Saiki A, et al. Difference in positive relation between cardio-ankle vascular index (CAVI) and each of four blood pressure indices in real-world Japanese population. J Hum Hypertens. 2019;33(3):210–217. doi:10.1038/s41371-019-0167-1
7. Saiki A, Ohira M, Yamaguchi T, et al. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J Atheroscler Thromb. 2020;27(8):732–748. doi:10.5551/jat.RV17043
8. Spronck B, Avolio AP, Tan I, et al. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens. 2017;35(1):98–104. doi:10.1097/HJH.0000000000001132
9. Kubota Y, Maebuchi D, Takei M, et al. Cardio- ankle vascular index is a predictor of cardiovascular events. Artery Res. 2011;5:91–96. doi:10.1016/j.artres.2011.03.005
10. Otsuka T, Fukuda S, Shimada K, et al. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014;37(11):1014–1020. doi:10.1038/hr.2014.116
11. Laucevičius A, Ryliškytė L, Balsytė J, et al. Association of cardio-ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina. 2015;51(3):152–158. doi:10.1016/j.medici.2015.05.001
12. Satoh-Asahara N, Kotani K, Yamakage H, et al. Japan Obesity and Metabolic Syndrome Study (JOMS) Group: cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242(2):461–468. doi:10.1016/j.atherosclerosis.2015.08.003
13. Sato Y, Nagayama D, Saiki A, et al. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23:596–605. doi:10.5551/jat.31385
14. Chung SL, Yang CC, Chen CC, et al. Coronary artery calcium score compared with cardio-ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb. 2015;22:1255–1265. doi:10.5551/jat.29926
15. Gohbara M, Iwahashi N, Sano Y, et al. Clinical impact of the cardio-ankle vascular index for predicting cardiovascular events after acute coronary syndrome. Circ J. 2016;80:1420–1426. doi:10.1253/circj.CJ-15-1257
16. Hitsumoto T. Clinical Usefulness of the cardio-ankle vascular index as a predictor of primary cardiovascular events in patients with chronic kidney disease. J Clin Med Res. 2018;10:883–890. doi:10.14740/jocmr3631
17. Kirigaya J, Iwahashi N, Tahakashi H, et al. Impact of cardio-ankle vascular index on long- term outcome in patients with acute coronary syndrome. J Atheroscler Thromb. 2020;27(7):657–668. doi:10.5551/jat.51409
18. Miyoshi T, Ito H, Shirai K, et al. CAVI‐J (Prospective Multicenter Study to Evaluate Usefulness of Cardio‐Ankle Vascular Index in Japan) investigators. predictive value of the cardio-ankle vascular index for cardiovascular events in patients at cardiovascular risk. J Am Heart Assoc. 2021;10(16):e020103. doi:10.1161/JAHA.120.020103
19. Murakami K, Inayama E, Itoh Y, et al. The role of cardio-ankle vascular index as a predictor of mortality in patients on maintenance hemodialysis. Vasc Health Risk Manag. 2021;17:791–798. doi:10.2147/VHRM.S339769
20. Saiki A, Watanabe Y, Yamaguchi T, et al. CAVI-lowering effect of pitavastatin may be involved in the prevention of cardiovascular disease: subgroup analysis of the TOHO-LIP. J Atheroscler Thromb. 2021;28(10):1083–1094. doi:10.5551/jat.60343
21. Nagayama D, Fujishiro K, Nakamura K, et al. Cardio-ankle vascular index is associated with prevalence and new-appearance of atrial fibrillation in Japanese urban residents: a retrospective cross-sectional and cohort study. Vasc Health Risk Manag. 2022;18:5–15. doi:10.2147/VHRM.S351602
22. Sumin AN, Shcheglova AV, ZHidkova II, et al. Assessment of arterial stiffness by cardio-ankle vascular index for prediction of five-year cardiovascular events after coronary artery bypass surgery. Glob Heart. 2021;16(1):90. doi:10.5334/gh.1053
23. Watanabe K, Yoshihisa A, Sato Y, et al. Cardio-ankle vascular index reflects impaired exercise capacity and predicts adverse prognosis in patients with heart failure. Front Cardiovasc Med. 2021;8:631807. doi:10.3389/fcvm.2021.631807
24. Sato Y, Yoshihisa A, Ichijo Y, et al. Cardio-ankle vascular index predicts post-discharge stroke in patients with heart failure. J Atheroscler Thromb. 2021;28(7):766–775. doi:10.5551/jat.58727
25. Yasuharu T, Setoh K, Kawaguchi T, et al; Nagahama study group. Brachial-ankle pulse wave velocity and cardio-ankle vascular index are associated with future cardiovascular events in a general population: the Nagahama Study. J Clin Hypertens. 2021;23(7):1390–1398. doi:10.1111/jch.14294
26. Aiumtrakul N, Supasyndh O, Krittayaphong R, et al. Cardio-ankle vascular index with renal progression and mortality in high atherosclerosis risk: a prospective cohort study in CORE-Thailand. Clin Exp Nephrol. 2022;26(3):247–256. doi:10.1007/s10157-021-02149-x
27. Rerkasem A, Tangmunkongvorakul A, Aurpibul L, et al. Association of cardio-ankle vascular index and future major adverse cardiovascular events in older adults living with HIV. AIDS Care. 2022:1–9. doi:10.1080/09540121.2022.2029820
28. Spronck B, Obeid MJ, Paravathaneni M, et al. Predictive ability of pressure-corrected arterial stiffness indices: comparison of pulse wave velocity, cardio-ankle vascular index (CAVI), and CAVI0. Am J Hypertens. 2021:hpab168. doi:10.1093/ajh/hpab168
29. Nagayama D, Fujishiro K, Miyoshi T, et al. Predictive ability of arterial stiffness parameters for renal function decline: a retrospective cohort study comparing cardio-ankle vascular index (CAVI), pulse wave velocity and CAVI0. J Hypertens. 2022;40(7):1294–1302. doi:10.1097/HJH.0000000000003137
30. Mestanik M, Spronck B, Jurko A, et al. P135 assessment of novel blood pressure corrected cardio-ankle vascular index in response to acute blood pressure changes. Artery Res. 2019;25:S173. doi:10.2991/artres.k.191224.158
31. Lamotte G, Boes CJ, Low PA, et al. The expanding role of the cold pressor test: a brief history. Clin Auton Res. 2021;31(2):153–155. doi:10.1007/s10286-021-00796-4
32. Plunde O, Cereceda AF, Bäck M. Cardiovascular risk factors and hemodynamic measures as determinants of increased arterial stiffness following surgical aortic valve replacement. Front Cardiovasc Med. 2021;8:754371. doi:10.3389/fcvm.2021.754371
33. Plunde O, Bäck M. Arterial stiffness in aortic stenosis and the impact of aortic valve replacement. Vasc Health Risk Manag. 2022;18:117–122. doi:10.2147/VHRM.S358741
34. Nagasawa Y, Shimoda A, Shiratori H, et al. Analysis of effects of acute hypovolemia on arterial stiffness in rabbits monitored with cardio-ankle vascular index. J Pharmacol Sci. 2022;148(3):331–336. doi:10.1016/j.jphs.2022.01.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.