Back to Journals » International Journal of Women's Health » Volume 15
Comparison of Low-Energy Radiofrequency Thermal Vaginal Therapy with Sham Treatment for Stress Urinary Incontinence in Postmenopausal Women: A Randomized Controlled Trial
Authors Chinthakanan O, Saraluck A , Kijmanawat A, Aimjirakul K, Wattanayingcharoenchai R, Manonai J
Received 14 September 2023
Accepted for publication 8 November 2023
Published 15 November 2023 Volume 2023:15 Pages 1779—1790
DOI https://doi.org/10.2147/IJWH.S431233
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Orawee Chinthakanan, Apisith Saraluck, Athasit Kijmanawat, Komkrit Aimjirakul, Rujira Wattanayingcharoenchai, Jittima Manonai
Department of Obstetrics & Gynaecology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Apisith Saraluck, Department of Obstetrics & Gynaecology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, 10400, Thailand, Tel +66-2-2012167, Fax +66-2-2011416, Email [email protected]
Introduction and Hypothesis: Low-energy radiofrequency (RF) thermal vaginal therapy for vaginal laxity and the genitourinary syndrome of menopause denatures collagen fibrils in the endopelvic fascia; fiber tightening during healing may stabilize the urethra and bladder neck, thereby resolving female stress urinary incontinence (SUI), especially in postmenopausal women. This study compared RF vaginal therapy with sham treatment for mild to moderate SUI.
Methods: This double-blinded, randomized controlled trial, conducted at a tertiary center from September 2018 to April 2021, recruited postmenopausal women with mild to moderate degree of SUI who had never undergone surgery, energy-based therapy, or vaginal estrogen treatment. The intervention group received vaginal RF laser treatment; the sham group did not. The primary outcome was the 1-hour pad-weight test (PWT) result. Secondary outcomes were Incontinence Quality of Life (I-QOL), Urogenital Distress Inventory (UDI-6), Incontinence Impact Questionnaire (IIQ-7), Patient Global Impression of Improvement (PGI-I), percentage of improvement among all participants, and adverse events. Data were analyzed using STATA 17.0.
Results: Forty-nine participants randomized to RF (n = 23) and sham (n = 26) groups were eligible for analysis. PWT decreased during follow-up in the RF group but remained stable in the sham group; PWT did not significantly differ between groups. The 1-year post-treatment success rate was higher in the RF group (69.6%) than in the sham group (38.5%). At 1 year post-treatment, there were no statistically significant differences in any secondary outcomes.
Conclusion: Low-energy RF vaginal therapy is an alternative treatment for mild to moderate SUI in postmenopausal women without serious adverse events. Larger randomized controlled trials should be conducted.
Plain Language Summary: Why was the study doneLow-energy radiofrequency vaginal therapy for vaginal laxity and the genitourinary syndrome of menopause may be beneficial for postmenopausal women with stress urinary incontinence.
What did the researchers do and findIn this randomised controlled study conducted at a tertiary-care hospital, This study compared RF vaginal therapy with sham treatment for postmenopausal female SUI with mild to moderate degree.One-hour pad weight test decreased during follow-up in the RF group but remained stable in the sham group.The 1-year post-treatment success rate was higher in the RF group (69.6%) than in the sham group (38.5%).
What do these results meanHis study was constituted the first randomized double-blind clinical trial to examine the efficacy of vaginal RF laser therapy, compared with sham treatment, in postmenopausal women with SUI. Additionally, this study generated data that can provide insights regarding evaluate subjective and objective outcomes within 1 year after treatment.
Keywords: radiofrequency, laser, stress urinary incontinence, postmenopausal women, randomized controlled trial
Introduction
Female stress urinary incontinence (SUI), defined as involuntary leakage with effort, exertion, or sneezing/coughing, is the most prevalent type of incontinence in women (approximately 86% of incontinence cases).1 SUI can be caused by bladder neck anatomical defects, urethral hypermobility, and/or neuromuscular defects (ie, intrinsic sphincter deficiency). Although it is not life-threatening, it can negatively affect quality of life (QOL), self-esteem, lifestyle, and relationships; it has the potential to cause social embarrassment and isolation.2 Therefore, the goals of SUI management include curing or improving SUI, along with relief from psychological and social suffering. Surgical and non-surgical management methods may be used during treatment. Surgical treatment options include a mid-urethral sling and colposuspension.Non-invasive treatments for SUI include lifestyle modifications, pelvic floor muscle training, vaginal pessaries, electrostimulation, medical devices, injectable bulking agents, local estrogen therapy, radiofrequency (RF), and the novel approach of laser treatment. Pelvic floor muscle training reportedly cures SUI in nearly 60% of cases.3 The treatment of SUI with energy-based devices is an increasingly popular topic. Physician intervention is frequently driven by high demand from patients who desire non-invasive, high-tech treatment. To our knowledge, there are insufficient data to support this approach. Recent research suggests that laser therapy (eg, fractional CO2 lasers and Er:YAG lasers) may be beneficial as elective treatment for SUI.4 However, no standard guidelines or recommendations for SUI treatment have been conclusively established because of persistent controversy and the need for additional evidence. Additionally, some medical recommendations for the treatment of SUI in postmenopausal women include vaginal estrogen, which has effects on menopausal genitourinary syndrome and the urethral and vaginal mucosa.5 RF vaginal laser therapy is designed to denature collagen fibrils in the fascia; the beneficial shrinkage and tightening of these tissues during the healing process has led to widespread use in the treatment of vaginal laxity, the genitourinary syndrome of menopause, and female sexual dysfunction.6 Through this healing process, RF vaginal laser therapy has the potential to recreate the hammock mechanism (ie, contraction and elevation of paravaginal connective tissue), which should stabilize the urethra and bladder neck, leading to continence restoration. A histological analysis of low-dose non-ablative RF treatments revealed that the extracellular tissue matrix shrinks and tightens, reducing tissue compliance without scarring or stricture formation.7 Previously, a study of the effects of non-ablative, monopolar transcutaneous temperature controlled radiofrequency (TTCRF) technology on postmenopausal women with genuine stress urinary incontinence (SUI) found significant improvement in both objective and subjective symptoms.8 Furthermore, another method of using nonsurgical, transurethral radiofrequency energy tissue micro-remodeling to determine the effect of menopause and hormone replacement therapy found that RF micro-remodeling resulted in 81% of subjects greater I-QOL score improvement at 12 months compared to 49% of sham subjects.9 Thus, RF methods are suitable for organs with a surface or lumen, as illustrated by their use in the treatment of SUI. Low-energy RF thermal vaginal therapy utilizes a tip that is moved in a circumferential manner around the opening; it delivers monopolar RF energy pulses that stimulate collagen restoration at the cellular level, promoting vaginal tightening and rejuvenation.7 To our knowledge, no studies have investigated the efficacy of low-energy RF thermal vaginal therapy for SUI in postmenopausal women, nor have any studies compared this therapy with sham or other treatments. There is insufficient evidence to determine whether the intervention improves disease-specific QOL or causes adverse events (mild or serious). This study evaluated the efficacy of transvaginal RF with the Viveve® System as treatment for postmenopausal SUI.
Materials and Methods
Study Design and Setting
This randomized controlled trial was conducted at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, which is a tertiary referral center. Participants were divided into two groups: low-energy RF thermal vaginal therapy (Viveve System) and sham. The study design and procedures were approved by the Ethics Committee of Ramathibodi Hospital, Mahidol University Bangkok, Thailand (MURA2019/1144). This clinical trial was registered in the Thai Clinical Trial Registry (TCTR20181115004).
Sample Size Estimation
Sample size will be calculated based on a previous study of laser vaginal treatment for female stress urinary incontinence using 1-hour pad test as a primary outcome.10 Sample size estimations is calculated based on risk ratio difference from the previous studies.Assuming the mean 1-hour pad weight test was 3.52 ± 1.89 g for RF group at 12 months and mean difference was 1, the sample size should be 29 each group to provide 80% power at significance level of 95%. The total will be 32 per group (total N=64).
Study Participants
Participants were recruited from the Female Pelvic Medicine and Reconstructive Surgery Clinic via hospital recruitment. Potential participants were asked to meet with a research project assistant; they received extensive information about the study and an enrollment form. After participants provided written informed consent to take part in the study, they completed screening questionnaires.Subsequently, they underwent physical examinations including a pelvic exam, POP-Q, stress test, 1-hour pad-weight test (PWT), post-void residual urine, and urine analysis. The PWT was performed in accordance with standard recommendations, using a bladder volume of > 200 mL (measured via BladderScan or ultrasound). Urine loss was measured as the increase in perineal pad weight during a 60-minute standardized activity period. The test was initiated by ingestion of 500 mL non-carbonated water.1 Participants were instructed not to void before or during the test; they performed maneuvers and exercises during the 1‐hour period while wearing a pre‐weighed absorbent perineal pad. After the test, the pad weight was determined using a standardized measurement tool.
Inclusion and Exclusion Criteria
This study included postmenopausal women aged > 50 years with a diagnosis of mild to moderate SUI and last menstrual cycle > 1 year prior to enrollment. All participants had a positive Q-tip test result, no history of transvaginal laser or RF treatment, no previous anti-incontinence procedures, and no vaginal estrogen use within the previous 3 months. Exclusion criteria were anterior compartment pelvic organ prolapse ≥ stage II, any suspicion of urgency symptoms or urge urinary incontinence, urinary retention, obesity (body mass index > 35 kg/m2), severe SUI (ie, > 2 pads per day or a PWT of > 50 mL/hour), photosensitivity, use of HRT, contraindications for estrogen therapy, undiagnosed abnormal vaginal bleeding, and use of corticosteroids or immunosuppressants or any medications that can affect lower urinary tracts symptoms such as antimuscarinic drugs. Patients who showed urgency incontinence during the history, questionnaires, or pad test in addition to the SUI component were excluded from the trial. All participants underwent urinalysis to confirm the absence of urinary tract infection.
Randomization and Blinding
Each participant was randomly allocated to a treatment group. The randomization sequence was generated using a block randomization technique with varying block sizes of 4 to 8 and a treatment ratio of 1:1. Sequence generation was performed under the supervision of a senior statistician from the Department of Clinical Epidemiology and Biostatistics at Ramathibodi Hospital, using STATA version 17.0; this automated process was conducted without investigator involvement. Allocation was stratified according to center and site of enrollment. After enrollment through the randomization process, each participant received one treatment. In the sham group, the probe was applied and the RF equipment was activated in a manner similar to the procedure in the treatment group; however, no energy was delivered during the sham procedure. The sham probe was created by the RF equipment manufacturer; it mimicked the sensations and sounds of the treatment probe. The investigators and participants were blinded. Additional urogynecologists served as blinded evaluators to verify the results. Furthermore, group allocation information was concealed from the analysts involved in data management. An independent statistician created a set of sealed code-break envelopes, which were only unsealed if optimal clinical management of a participant required knowledge of the randomized treatment. Code-break envelopes were not routinely opened for study participants who had completed treatment. The independent statistician maintained a backup copy of the confidential randomization list.
Procedure Description
Participants in the low-energy RF thermal vaginal therapy (Viveve System) group received the following treatment for SUI: 220 pulses of RF, divided into 110 pulses using a 5-cm tip and 110 pulses using an 8-cm tip. Pulses were located at the distal margin of the treatment tip (2 cm, 3 cm, 4 cm, and 5 cm beyond the hymenal ring), with 100, 25, and 5 pulses on the bilateral side of the urethra in each quadrant. Our procedure is a double-lined trial.Prior to performing the procedure, the matchine is set up and adjusted by a member of the research staff who is knowledgeable and skilled in machine set up. Investigators are unable to see the energy setting display, and the probe is made of a thick, comforting material.The sound of matchine and any signal was set to be identical for the treatment group and the control group. The researcher will be informed by research assistance when the pulses reach the value with the same sound between two groups.For the patients, we only included those who had never undergone a thermal therapy procedure, so they did not know whether they were receiving real energy or a placebo. Both groups underwent the procedure using the same quadrant energy release probe with the same rhythm and voice of matchine, so that the total duration of the procedure was the same for each patient. The duration of 1 case is approximately 45 minutes. Due to the minimally invasive nature of this office-based procedure, no anesthesia is required during or after treatment. All participants receive the intervention for one session only, with clinical outcomes monitored as per protocol.
Outcome Measures
The primary outcome was the PWT result. Subjective and objective outcomes were measured at 1, 3, 6, and 12 months after treatment. Adverse events were recorded throughout the study period. All patients were asked to complete the Thai version of the Incontinence Quality of Life Questionnaire (I-QOL),11 the Thai version of the Urogenital Distress Inventory (UDI-6),12 the Thai version of the Incontinence Impact Questionnaire (IIQ-7),12 and the Thai version of the Patient Global Impression of Improvement (PGI-I) at 1, 3, 6, and 12 months after treatment. Additionally, participant-rated significant improvement in SUI symptoms was regarded as subjective overall improvement, assessed by the percentage change in clinical SUI at 1 year after treatment. Clinical symptoms were considered significantly improved if participants reported > 50% improvement in PWT at 1 year after treatment.13
Data Analysis
STATA version 17.0 was used for statistical analysis. Demographic data were analyzed by comparisons of the two groups. For categorical data, frequencies and percentages were reported; the chi-squared test or Fisher’s exact test were used for comparisons between groups. For continuous data, means, medians, standard deviations, and modes were reported; Student’s t-test or quantile regression was used for statistical analysis. Mixed linear regression analysis was used to compare outcome measures between intervention groups.
Results
In total, 68 participants were assessed for eligibility; four were excluded based on exclusion criteria. Therefore, 64 participants were randomly divided into two groups: 32 were assigned to the RF intervention and 32 were assigned to the sham treatment. Participants were followed-up at 1, 3, 6, and 12 months after treatment. Because the follow-up period occurred during the coronavirus disease 2019 (COVID-19) pandemic in Thailand, nine participants in the RF group and six participants in the sham group were lost to follow-up; the final analysis included 23 participants in the RF group and 26 participants in the sham group (Figure 1). Participant characteristics at the start of the study are shown in Table 1. All demographic characteristics (eg, age, body mass index, smoking and alcohol statuses, gravidity, and previous vaginal delivery) did not significantly differ between groups. Baseline SUI measurements (ie, 1-hour PWT, I-QOL score, IIQ-7 score, and UDI-6 score) did not significantly differ between the two groups (Table 1).
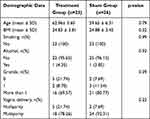 |
Table 1 Characteristics of RF Treatment and Sham Control Groups at Pre-Treatment |
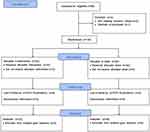 |
Figure 1 CONSORT flowchart of patients’ recruitment and study flow. |
The volume of urine loss was analyzed using the 1-hour PWT as the primary outcome. In the RF group, the mean PWT (95% confidence interval) decreased from 9.67 g (5.03–14.30) before treatment to 6.37 g (2.29–10.46) at 1 month, 6.25 g (2.04–10.47) at 3 months, 7.02 g (2.81–11.24) at 6 months, and 3.58 g (−0.69 to 7.86) at 1 year after treatment. In the sham group, the mean PWT (95% confidence interval) was 8.14 g (4.71–11.57) before treatment and 7.19 g (3.34–11.04) at 1 month, 11.05 g (7.04–15.06) at 3 months, 8.69 g (4.74–12.64) at 6 months, and 8.99 g (2.29–10.46) at 1 year after treatment (Table 2). PWT tended to decrease over time in the RF group, whereas it remained stable in the sham group. The treatment group had the best PWT outcome at 1 year after treatment. Mixed linear regression analysis revealed that the treatment group had a numerically better clinical result for SUI treatment at each follow-up visit, compared with the sham group (Figure 2); however, the differences were not statistically significant. Nonetheless, at 3 months and 1 year after treatment, the results were marginally significant (p = 0.09 and 0.08, respectively) (Table 2). When success was defined as 50% reduction in PWT from baseline[11], the RF group had a higher success rate (69.6% (16/23)) than the sham group (38.5% (10/26)) at 1 year after treatment (Figure 3).
 |
Figure 2 The primary outcome of the 1-hour pad weight test (PWT) was compared between the RF and sham groups using a linear graph. |
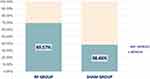 |
Figure 3 The bar chart illustrates the clinically significant improvement in SUI symptoms by decrease in PWT more than 50% at the one-year follow-up. |
Secondary outcomes were the I-QOL, IIQ-7, UDI-6, and PGI-I scores. The I-QOL scores increased in both groups, indicating better QOL among all participants. The highest score in the treatment group was observed 3 months after treatment (79.41, compared with 60.95 at baseline), whereas the highest score in the sham group was observed 1 month after treatment (79.61, compared with 65.35 at baseline). However, these scores did not significantly differ between groups (Table 3). After the intervention, IIQ-7 scores decreased in both groups, but they did not significantly differ between groups (Table 4). Similarly, UDI-6 scores decreased after treatment in both groups, indicating improvement; however, the scores did not significantly differ between groups (Table 5). Finally, there were no significant differences between groups in mean PGI-I scores, which ranged from 2 to 3 (ie, “significantly better” and “a little better”) (Table 6). All participants in both groups reported no pain or discomfort during the procedures and had no adverse effects as of their longest recent follow-up.
Discussion
There are various treatment options for symptomatic SUI in postmenopausal women. Transvaginal RF treatment is a new, innovative approach; it may be the treatment of choice for some patients. The primary findings in this study indicate that RF treatment for SUI can improve subjective and objective outcomes. Compared with the sham treatment, RF treatment tended to produce greater improvements in various outcomes, but the differences were not statistically significant. However, at 3 and 6 months after treatment, the results suggested that RF treatment slightly improved outcomes. Additionally, overall outcomes were generally more favorable in the treatment group than in the sham group. Nearly 70% of participants in the RF group reported treatment success (ie, significant reduction of PWT at 1 year after treatment, compared with baseline), whereas only 38% of participants in the sham group reported treatment success. With respect to secondary outcomes (based on patient satisfaction and well-being after treatment), the I-QOL, UDI-6, and IIQ-7 scores showed QOL improvement after RF treatment. Despite the lack of a statistically significant difference, the RF group tended to have a better outcome. We speculate that RF treatment is more effective than sham treatment, but the present findings represent preliminary data; a larger randomized controlled trial may clarify this efficacy. This study investigated both subjective and objective measurement because PWT was investigated in the context of our clinic, where participants were asked to perform measurement leakage in objective measurement. However, this may not reflect an improvement in feelings during daily life activities. However, even though PWT is one of the good tools for reflection on the objective measurement of the severity of SUI and treatment follow-up, urodynamics can also be helpful for the investigation of leak point pressure and urethral pressure, which could be beneficial for the research of novel methods.14 Only one previous study investigated SUI treatment with the method used in the present study: a non-ablative cryogen-cooled monopolar RF laser (Viveve).13 The results of that study indicated that RF laser treatment improved SUI symptoms with a ~50% reduction in pad weight from baseline for approximately half of the participants at 12 months after treatment, along with improvements in SUI-related patient-reported outcomes. Those data are consistent with findings in the RF group in the present study, whereby overall outcomes were favorable and pad weight decreased by > 50% during the 1-year follow-up period. Additionally, in the previous study, the patient-reported SUI events decreased and subjective outcomes improved.13 However, the previous study did not compare laser and sham treatment methods; therefore, it is unclear whether a placebo effect arose from pelvic floor muscle exercises or pelvic muscle contractions/spasms, particularly because the majority of participants in that study were premenopausal women with presumably better vaginal mucosa that supported more tightening and better overall outcomes. Another study investigated vaginal laxity in postmenopausal women who received low-energy dynamic quadripolar RF therapy. After treatment, participants displayed rapid improvements in self-perception of introital looseness and related symptoms (eg, urinary incontinence); however, that study only used a quick survey without objective measures or details regarding SUI.15 Although there have been some analyses of RF therapy for the treatment of urinary incontinence, only a few have focused on SUI. In one study, 70% of patients demonstrated a reduction in PWT at 1 month after RF therapy, whereas 30% demonstrated worsening; the overall improvement was statistically significant, and the satisfaction rate was 90%.16 Nevertheless, that study was conducted with the Spectra G2-TTonederm, an RF device calibrated for use on the urethral meatus (the present study used a laser that probes the vaginal lumen), and its sample size of 10 participants was insufficient to draw any conclusions. Some studies of RF treatment for SUI have used invasive techniques. There are three RF treatment approaches for SUI: laparoscopic, transvaginal, and transurethral. The mechanism of RF applicator introduction into the body varies among the approaches. A multicenter study using laparoscopic RF bipolar energy for mild to moderate genuine stress incontinence without complications demonstrated a 79% cure rate (according to urodynamic testing) and an 81% improvement in QOL.17 A bipolar generator is used for the transvaginal approach; the surgery involves dissection of the anterior vaginal wall to reveal the inferior portion of the endopelvic fascia. At 1 year after treatment, a multicenter trial of 120 women with genuine stress incontinence revealed a 73% cure rate and incontinence improvements without severe complications.18 Importantly, there have been few studies of Phase 1 transurethral RF as treatment for SUI.
Although a previous study showed that RF laser therapy can improve vaginal laxity and vulvovaginal atrophy in postmenopausal women,19 there are no published data concerning its use as treatment for SUI in postmenopausal women; it is possible that RF laser therapy could successfully control SUI. RF vaginal laser therapy functions by heat-mediated rapid modification of structural collagen. This heat activates the tissue matrix, which comprises components such as elastin and collagen. Additionally, during the healing phase, the micro-inflammatory stimulation of fibroblasts causes treated tissue to shrink, activating neoelastogenesis and neocollagenesis.8,20 This compression results in enhanced bladder neck stabilization, ultimately leading to continence restoration via reduction of urethral hypermobility. After treatment, granulation tissue develops within 7 days; this tissue is replaced through a fibroblastic response 3 weeks later. In most patients, the inflammatory response largely subsides within 45 days after treatment.21 Some reports have suggested that a collagen remodeling procedure, possibly requiring an interval of 90 days, improves treatment outcomes. During RF-tissue interactions, the heat generated in the dermis reaches a thermal dose threshold, above which collagen begins to denature (60°C) and becomes completely denatured (70–75°C). Partial collagen denaturation by RF is greatest at 67°C; this temperature is associated with optimal neocollagenesis and clinical effects in the epidermis. Temperatures between 40°C and 45°C stimulate collagen production by fibroblasts and elicit effective skin tightening.22 Furthermore, vaginal estrogen tablets are recommended as standard treatment for SUI because they improve the vaginal mucosa and urethral mucosa; in a similar manner, RF laser therapy can also improve the vaginal mucosa and urethral mucosa.5,23
In the present study, no adverse effects and complications of RF laser vaginal therapy (eg, ulceration, necrosis, and scarring) were reported at 1 year after treatment, consistent with the findings in previous studies; these results imply that vaginal RF laser treatment is safe.6 In contrast, bleeding, bladder perforation, urethral injury, infection, groin pain, and a 6-week period of sexual abstinence have been reported as complications of other surgical treatments for SUI, including mid-urethral sling and colposuspension.24 Therefore, RF therapy could serve as an alternative treatment for patients who have contraindications to surgery or prefer non-surgical management. Moreover, for patients with contraindications to vaginal estrogen therapy for SUI, RF could be particularly beneficial (eg, in postmenopausal women).
A notable strength of this study was that it constituted the first randomized double-blind clinical trial to examine the efficacy of vaginal RF laser therapy, compared with sham treatment, in postmenopausal women with SUI. Additionally, this study generated data that can provide insights regarding evaluate subjective and objective outcomes within 1 year after treatment. Nevertheless, this study had some limitations. First, RF vaginal laser therapy is not currently standard treatment. Second, the use of a vaginal probe is expensive and financial support is limited. Third, only some experimental investigations could be performed because of the small sample size, potentially hindering hypothesis testing. In particular, outcome measures with the potential to prove hypotheses concerning the mechanism of SUI (eg, urodynamic studies or ultrasound measurements of the urethral angle) were not included in this study; however, they could be incorporated into future investigations. Fourth, the period between participant recruitment and follow-up was affected by the COVID-19 pandemic; the government’s lockdown policy caused some participants to be lost to follow-up. Because the statistical significance of the present results was inconclusive, a larger randomized controlled trial should be conducted in the future.
Conclusion
Low-energy RF vaginal therapy can improve mild to moderate SUI treatment outcomes in postmenopausal women without serious adverse events. However, this study did not reveal a statistically significant difference between low-energy RF thermal vaginal therapy and sham treatment in terms of SUI improvement in postmenopausal women. A larger randomized controlled trial should be conducted to clarify differences between the groups.
Data Sharing Statement
Data can be made available on reasonable request to the corresponding author.
Consent Statement
The participant provided written informed consent.
Acknowledgments
We thank Assoc. Prof. Sasivimol Rattanasiri, Mr. Chanatpon Aonnuam, and Ms. Sasiporn Sitthisorn for assistance with statistical analyses. We thank Mrs. Sirirat Sarit-apirak, Mrs. Jittrat Dechdee, Ms. Nuttanan Panthong, Ms. Peeranuch Mangmeesri, and Ms. Rasika Phornprapha for research assistance and management, including preparation of research documents and data. We also thank Ryan Chastain-Gross, Ph.D., from Edanz (https://www.edanz.com/ac) for editing a draft of this manuscript.
This work was presented as a short oral podium presentation at the 2023 IUGA Annual Meeting, The Hague, Netherlands, 24 June 2023.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
lThis was a physician-initiated study. Viveve Medical sponsored the instruments. This study also received funding from the Faculty of Medicine, Mahidol University. All fellows and lecturers in the department participated in this study.
Disclosure
O.Chinthakanan reports industry funding from Viveve Medical for instrument only during the trial period. All authors declare no other conflicts of interest in this work.
References
1. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi:10.1007/s00192-009-0976-9
2. Ghoniem G, Stanford E, Kenton K, et al. Evaluation and outcome measures in the treatment of female urinary stress incontinence: International Urogynecological Association (IUGA) guidelines for research and clinical practice. Int Urogynecol J. 2008;19(1):5–33. doi:10.1007/s00192-007-0495-5
3. Dumoulin C, Cacciari LP, Hay-Smith EJC. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2018;10(10):Cd005654. PMID: 30288727; PMCID: PMC6516955. doi:10.1002/14651858.CD005654.pub4
4. Ranjbar A, Mehrnoush V, Darsareh F, et al. Vaginal laser therapy for stress urinary incontinence: a systematic review of prospective randomized clinical trials. J Menopausal Med. 2022;28(3):103–111. PMID: 36647273; PMCID: PMC9843031. doi:10.6118/jmm.22017
5. Nambiar AK, Arlandis S, Bø K, et al. European Association of urology guidelines on the diagnosis and management of female non-neurogenic lower urinary tract symptoms. Part 1: diagnostics, overactive bladder, stress urinary incontinence, and mixed urinary incontinence. Eur Urol. 2022;82(1):49–59. PMID: 35216856. doi:10.1016/j.eururo.2022.01.045
6. González-Gutiérrez MD, López-Garrido Á, Cortés-Pérez I, Obrero-Gaitán E, León-Morillas F, Ibáñez-Vera AJ. Effects of non-invasive radiofrequency diathermy in pelvic floor disorders: a systematic review. Medicina. 2022;58(3):437. PMID. doi:10.3390/medicina58030437
7. Vos JA, Livengood RH, Jessop M, Coad JE, editors. Non-Ablative Hyperthermic Mesenchymal Regeneration: A Proposed Mechanism of Action Based on the Vivev Model. Energy-Based Treatment of Tissue and Assessment VI. Society of Photo-Optical Instrumentation Engineers (SPIE). March 01, 2011 doi:10.1117/12.876859.
8. Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous Temperature Controlled Radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149–159. PMID: 27608749.
9. Lenihan JP. Comparison of the quality of life after nonsurgical radiofrequency energy tissue micro-remodeling in premenopausal and postmenopausal women with moderate-to-severe stress urinary incontinence. Am J Obstet Gynecol. 2005;192(6):1995–8; discussion 9–2001. PMID: 15970873. doi:10.1016/j.ajog.2005.02.041
10. González Isaza P, Jaguszewska K, Cardona JL, Lukaszuk M. Long-term effect of thermoablative fractional CO(2) laser treatment as a novel approach to urinary incontinence management in women with genitourinary syndrome of menopause. Int Urogynecol J. 2018;29(2):211–215. PMID: PMC5780538. doi:10.1007/s00192-017-3352-1
11. Chaisaeng S, Santingamkun A, Opanuraks J, Ratchanon S, Bunyaratavej C. IQOL: translation & reliability for use with urinary incontinence patients in Thailand. J Med Assoc Thai. 2006;89(Suppl 3):S33–9. PMID: 17722303.
12. Weerasopone S, Santingamkul A. Validation of the Thai version of the incontinence impact questionnaire (IIQ-7) and the urogenital distress inventory (UDI-6). Chula Med J. 2016;60:389–398.
13. Allan BB, Bell S, Husarek K. A 12-month feasibility study to investigate the effectiveness of cryogen-cooled monopolar radiofrequency treatment for female stress urinary incontinence. Can Urol Assoc J. 2020;14(7):E313–e8. PMID: 32017688; PMCID: PMC7337709. doi:10.5489/cuaj.6145
14. Yande SD, Joglekar OV, Joshi M. Role of urodynamics in stress urinary incontinence: a critical appraisal. J Midlife Health. 2016;7(3):119–125. PMID: 27721639; PMCID: PMC5051231. doi:10.4103/0976-7800.191016
15. Vicariotto F, De Seta F, Faoro V, Raichi M. Dynamic quadripolar radiofrequency treatment of vaginal laxity/menopausal vulvo-vaginal atrophy: 12-month efficacy and safety. Minerva Ginecol. 2017;69(4):342–349. PMID: 28608667. doi:10.23736/s0026-4784.17.04072-2
16. Lordelo P, Vilas Boas A, Sodré D, Lemos A, Tozetto S, Brasil C. New concept for treating female stress urinary incontinence with radiofrequency. Int Braz J Urol. 2017;43(5):896–902. PMID: 28727373; Central PMCID: PMC5678521. doi:10.1590/s1677-5538.Ibju.2016.0621
17. Ross JW, Galen DI, Abbott K, et al. A prospective multisite study of radiofrequency bipolar energy for treatment of genuine stress incontinence. J Am Assoc Gynecol Laparosc. 2002;9:493–499. doi:10.1016/S1074-3804(05)60525-7
18. Dmochowski RR, Avon M, Ross J, et al. Transvaginal radio frequency treatment of the endopelvic fascia: a prospective evaluation for the treatment of genuine stress urinary incontinence. J Urol. 2003;169(3):1028–1032. PMID: 12576838. doi:10.1097/01.ju.0000048686.50716.ef
19. Juhász MLW, Korta DZ, Mesinkovska NA. Vaginal rejuvenation: a retrospective review of lasers and radiofrequency devices. Dermatol Surg. 2021;47(4):489–494. PMID: 33165070. doi:10.1097/dss.0000000000002845
20. Lordêlo P, Leal MR, Brasil CA, Santos JM, Lima MC, Sartori MG. Radiofrequency in female external genital cosmetics and sexual function: a randomized clinical trial. Int Urogynecol J. 2016;27(11):1681–1687. PMID: 27116198. doi:10.1007/s00192-016-3020-x
21. Dillon B, Dmochowski R. Radiofrequency for the treatment of stress urinary incontinence in women. Curr Urol Rep. 2009;10(5):369–374. PMID: 19709484. doi:10.1007/s11934-009-0058-z
22. Kozma B, Candiotti K, Póka R, Takács P. The effects of heat exposure on vaginal smooth muscle cells: elastin and collagen production. Gynecol Obstet Invest. 2018;83(3):247–251. doi:10.1159/000486785
23. Cody JD, Richardson K, Moehrer B, Hextall A, Glazener CM. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2009;4:Cd001405. PMID: 19821277. doi:10.1002/14651858.CD001405.pub2
24. Chinthakanan O, Miklos JR, Moore RD, Karp DR, Nogueiras GM, Davila GW. The indication and surgical treatment of 286 midurethral synthetic sling complications: a multicenter study. Surg Technol Int. 2016;29:167–171. PMID: 27780346.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





