Back to Journals » Infection and Drug Resistance » Volume 16
Comparison of in vitro Susceptibilities of Talaromyces marneffei in Mold and Yeast Forms in Malaysia
Authors Tan XT, binti Mohd Shuhairi N, Jane Ginsapu S, Binti Shukor S, Amran F
Received 23 November 2022
Accepted for publication 9 February 2023
Published 22 March 2023 Volume 2023:16 Pages 1629—1635
DOI https://doi.org/10.2147/IDR.S398743
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xue Ting Tan, Nurliyana binti Mohd Shuhairi, Stephanie Jane Ginsapu, Surianti Binti Shukor, Fairuz Amran
Bacteriology Unit, Infectious Diseases Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Setia Alam, Selangor, Malaysia
Correspondence: Xue Ting Tan, Bacteriology unit, Infectious Diseases Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Setia Alam, Selangor, Malaysia, Tel +60 333628968, Email [email protected]
Purpose: This study was aimed to determine minimum inhibitory concentration (MIC) differences between yeast and mold forms of T. marneffei in Malaysia.
Patients and Methods: Ninety-seven clinical strains of T. marneffei were received from various Malaysian hospitals from the year 2020 until 2022. Their identities were determined using microscopic, macroscopic and molecular methods. Next, the susceptibility of yeast and mold forms of each isolate against amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole, terbinafine, caspofungin and micafungin were tested according to the broth microdilution according to the Clinical and Laboratory Standards Institute (CLSI) M38 and M27 guidelines. The geometric means of minimal inhibitory concentration (GM MIC), MIC50, and MIC90 were determined for each antifungal. Additionally, Wilcoxon signed-rank test was used to compare the significant difference of GM MICs for each antifungal, GM MIC, MIC50 and MIC90 for the combined nine antifungals against different growth forms of T. marneffei. The significance was set at p< 0.05.
Results: Micafungin had the highest GM MIC, MIC50 and MIC90 for mold form of T. marneffei. For yeast form, amphotericin B achieved the highest GM MIC and MIC50 while micafungin achieved the highest MIC90. However, the GM MIC, MIC50 and MIC90 of terbinafine and azole antifungals on T. marneffei were similar to each other, namely between 0.03 and 0.60μg/mL. The difference of GM MIC of all tested antifungals except caspofungin and micafungin was insignificant. Overall, GM MIC, MIC50 and MIC90 of the combined nine antifungals against two growth forms were insignificant.
Conclusion: The findings suggested either yeast or mold form can be used in the susceptibility testing of T. marneffei against amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole and terbinafine.
Keywords: Talaromyces marneffei, susceptibility, yeast, mold
Introduction
Talaromyces marneffei is a fungus that can cause a lethal fungal infection known as talaromycosis.1 The common clinical presentations of talaromycosis are weight loss, fever, cough, skin lesions, lymphadenopathy, hepatomegaly and diarrhoea.1 The disease is endemic in Southeast Asia in HIV-infected patients.2 The annual incidence of talaromycosis in HIV-infected patients in Malaysia was estimated at 0.22–1.1 per 100,000 cases.1,3 The mortality rate is 75% in those with delayed diagnosis and administration of antifungal therapy.4,5
Talaromyces marneffei is a dimorphic species. It grows as yeast at 37ºC but exists as a mold at 25ºC.6 The inhalation of spores of the mold form was considered the route of transmission of T. marneffei.7–9 On the other hand, the yeast form is pathogenic as it can produce proteins or toxins that can evade the immune defense of the host.10 Furthermore, the yeast form is commonly isolated from the infected tissues and observed in the intracellular infection of the macrophages.7,11–13
However, the protocol of susceptibility testing for T. marneffei has not been evaluated by any party. Moreover, the form to be used in the susceptibility test for T. marneffei remains a debate. As a result, this study was conducted to examine if a significant difference in minimum inhibitory concentrations (MIC) existed between mold and yeast forms of T. marneffei.
Materials and Methods
Isolate
The minimum number of samples calculated for this study was 97, following the calculation recommended by Ariffin.14 In the year 2020 until 2022, 97 clinical isolates of T. marneffei which isolated on potato dextrose agar (PDA) were received from various hospitals in Malaysia. Their identities were confirmed by both macroscopic, microscopic and molecular methods. The internal transcribed spacer (ITS) region of the nuclear rDNA was amplified with PCR and detected with direct DNA sequencing to determine the species.15
Susceptibility Testing
Since no existing guidelines were available for susceptibility testing of T. marneffei, the minimum inhibitory concentration (MIC) was following the broth microdilution test mentioned in CLSI M3816 and M2717 for susceptibility testing of mold and yeast, respectively. The antifungals tested were amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole, terbinafine, caspofungin and micafungin. All antifungals were purchased from Sigma-Aldrich, Missouri, United States. Each microdilution well contained 100 µL of antifungal. The final concentrations of each antifungal ranged from 0.0313 to 16.0 µg/mL.
The mold and yeast inocula of T. marneffei were obtained as mentioned by Sar et al.18 Briefly, 7-day-old slant cultures of T. marneffei mold were flooded with deionized distilled water. After the suspension had been ground, it was adjusted to 0.4×104 to 5×104 colony-forming unit (CFU)/mL. To obtain yeast inocula, the suspension was inoculated into brain heart infusion broth and incubated at 37ºC until yeast cells were grown. After broth cultures were centrifuged at 15,000 × g for 20 min, the sediment was washed three times successively with sterile deionized distilled water. Finally, the washed yeast cells were suspended in deionized distilled water and adjusted to 0.4×104 to 5×104 CFU/mL.
Following that, 100 µL of diluted inoculum suspension was added to the microdilution well. The mold mixture was then incubated at 25ºC for 96 h while the yeast mixture was incubated at 37ºC for 72 h.6
Quality Control
Each test included three reference strains; Candida parapsilosis ATCC 22019, Aspergillus flavus ATCC 204304 and A. fumigatus ATCC 204305 to ensure that the MIC obtained was within the reference range.
Data Analysis
For each antifungal test, the GM MIC, MIC50 and MIC90 were calculated. MIC50 was defined as 50% of the isolates were inhibited, whereas MIC90 is the MIC at which 90% of the isolates were inhibited. Comparisons between the GM MIC of each antifungal, GM MIC, MIC50 and MIC90 of the combined nine antifungals, against the yeast and mold forms of T. marneffei were evaluated by the Wilcoxon test using SPSS 20.0 (IBM®, Armonk, New York). P-values less than 0.05 were considered statistically significant.
Results
T. marneffei was initially isolated from blood (n=89), pleural fluid (n=2), tracheal aspirate (n=3) and skin biopsy specimen (n=3). All isolates were obtained from HIV-infected patients. The age of patients ranged from 3 to 53 years where the male (87%) is more than the female (13%). The most common clinical manifestations were fever (n=65), cough (n=66) and diarrhea (n=47).
The GM MICs for amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole, terbinafine, caspofungin and micafungin against the mold form were 1.85 μg/mL, 0.07 µg/mL, 0.04 µg/mL, 0.05 µg/mL, 0.05 µg/mL, 0.04 µg/mL, 0.10 µg/mL, 2.23 µg/mL and 4.67 µg/mL, respectively; and against the yeast form were 0.37 μg/mL, 0.06 µg/mL, 0.05 µg/mL, 0.05 µg/mL, 0.05 µg/mL, 0.04 µg/mL, 0.07 µg/mL, 0.11 µg/mL and 0.20 µg/mL, respectively (Table 1). For mold form, the GM MIC of micafungin was the highest and followed by caspofungin and amphotericin B. For yeast form, the GM MIC of amphotericin B was the highest followed by micafungin and caspofungin. The GM MICs of other antifungals were similar to each other. Furthermore, the GM MIC of micafungin and caspofungin was shown significantly different, namely 3×10−4 and 2×10−4, respectively. In general, the GM MIC results of the combined nine antifungals showed similar effectiveness against mold and yeast forms (p=0.058) (Table 2).
 |
Table 1 Minimum Inhibitory Concentrations (MIC) of 97 Clinical Isolates of Talaromyces Marneffei in Mold Form (MF) and Yeast Form (YF) to Antifungal Drugs |
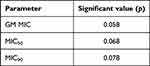 |
Table 2 Significant Difference of GM MIC, MIC50 and MIC90 of Combined Antifungals Between Mold Form (MF) and Yeast Form (YF) |
The MIC50 for amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole, terbinafine, caspofungin and micafungin against the mold form were 1 μg/mL, 0.03 µg/mL, 0.03 µg/mL, 0.03 µg/mL, 0.03 µg/mL, 0.03 µg/mL, 0.06 µg/mL, 4 µg/mL and 32 µg/mL, respectively; and against the yeast form were 0.5 µg/mL, 0.03 µg/mL, 0.03 µg/mL, 0.03 µg/mL, 0.03 µg/mL and 0.03 µg/mL, 0.03 μg/mL, 0.03 µg/mL and 0.03 µg/mL, respectively (Table 1). Similarly, the MIC50 of micafungin was the highest and followed by caspofungin and amphotericin B. For yeast form, the MIC50 of amphotericin B was the highest while readings of other antifungals were the same. There was no significant difference (p=0.068) in the MIC50 for all tested antifungals between the mold and yeast forms (Table 2).
The MIC90 for amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole, terbinafine, caspofungin and micafungin against the mold form was 8 μg/mL, 0.60 µg/mL, 0.03 µg/mL, 0.20 µg/mL, 0.13 and 0.13 µg/mL, 0.50 µg/mL, 16 µg/mL and 32 µg/mL, respectively, and against the yeast form were 8 μg/mL, 0.50 µg/mL, 0.05 µg/mL, 0.03 µg/mL, 0.13 µg/mL, 0.03 µg/mL, 0.50 µg/mL, 4 µg/mL and 32 µg/mL, respectively (Table 1). For mold form, micafungin had the highest MIC90 and followed by caspofungin and amphotericin B. On the other hand, micafungin had the highest MIC90 and followed by amphotericin B and caspofungin in the yeast form. All tested antifungals showed insignificant difference (p=0.078) in efficiency against both mold and yeast forms of T. marneffei.
Discussion
The growth form of T. marneffei to be used in susceptibility testing remains a debate. This matter is more complicated when the protocol for the susceptibility testing of T. marneffei is currently unavailable. Therefore, this study applied the protocol from available standard guidelines namely CLSI M38 and M27 to determine the existence of significant differences in MIC which resulted from mold and yeast form of T. marneffei. This is due to CLSI M38 and M27 being the references for broth dilution antifungal susceptibility testing of mold and yeasts, respectively.16,17 If the significant difference does not exist, the mold form could be used in susceptibility testing as it can grow easily in the laboratory compared to the yeast form. However, if a significant difference exists, further study has to be performed to determine which form is suitable and reflective of the treatment outcome.19
In this study, the overall GM MIC, MIC50 and MIC90 of mold form against the combined tested antifungals showed insignificant differences compared to the yeast form of T. marneffei. Specifically, the GM MIC, MIC50 and MIC90 of echinocandins and amphotericin B were the three highest records in both mold and yeast form. In comparison, the GM MIC, MIC50 and MIC90 of other antifungals were lower and their reading was almost similar.
The finding of amphotericin B in this study was similar to Sar et al from Cambodia.18 This is due to the MIC of the mold form being found as high as the MIC of the yeast form. In contrast, Sekhon et al20 reported higher MIC was observed in the yeast form of T. marneffei which was isolated from America and Europe. The MIC of >3µg/mL was found in 80% and 27% of yeast and mold forms, respectively. Furthermore, the MIC90 of amphotericin B against mold and yeast forms in this study recorded a high reading compared to other antifungals. This could be due to the production of melanin in T. marneffei.21 Melanin is important for the virulence of T. marneffei by protecting it from solar, UV or gamma radiation.22 In addition, it is also recognized as an antifungal resistance factor23 and able to make T. marneffei resistant to antifungals including amphotericin B.24
On the other hand, the majority of the studies including Sar et al18 and Sekhon et al20 reported low GM MIC of itraconazole, ≤0.5 µg/mL, against yeast and mold forms which is similar to this study. The active activities of itraconazole against T. marneffei had been reported in previous reports.6,25–27
Similar to itraconazole, the low MIC of voriconazole against both forms was also reported by other researchers such as Liu et al,9 Lau et al6 and Singh and Devi.25 Liu et al28 reported the GM MIC of voriconazole against the yeast form of the isolate was ≤0.05 µg/mL, whereas Singh and Devi25 reported the GM MIC of voriconazole against the mold form of the isolate was 0.125µg/mL.
Similar to the present study, the low MIC of posaconazole, <0.1 µg/mL, against both yeast and mold forms of T. marneffei were also found in Lau et al.6 In addition, the MIC50 and MIC90 of the mold form were 0.016 µg/mL and 0.031 µg/mL, whereas the MIC50 and MIC90 of the yeast form were equal, namely 0.002µg/mL.
On the other hand, Supparatpinyo et al26 reported a low GM MIC of ketoconazole, namely 0.027µg/mL against the yeast form of T. marneffei. This finding was parallel with the finding in this study.
Furthermore, terbinafine is a member of the allylamine class of antifungals.29 It possesses fungicidal activity to yeast and filamentous fungi.30 Unlike other classes of antifungals, it can block the fungal enzyme, namely squalene epoxidase, which is a component of the manufacturing route for fungus cell walls, hence preventing the formation of ergosterol.31 The GM MIC of mold and yeast forms of T. marneffei in Liu et al28 and Mcginnis et al32 respectively was parallel with the finding of this study.
The overall finding of echinocandin in this study was comparatively high compared with other antifungals when tested against both forms of T. marneffei. This phenomenon is consistent with the finding of Fang et al33 and Lei et al.34 The present findings supported the claim that echinocandins might have little to no effect on T. marneffei yeast as proposed by Fang et al.33
The variations in the pattern of susceptibility can be due to the unique mechanisms of action of each class of antifungal. For an instance, the polyene class of amphotericin B can disrupt fungal cell membranes via ergosterol binding, pore formation and leakage of cellular ions and eventually lead to fungal cell death.35 On the other hand, azoles prevent the C14α demethylation of lanosterol in fungi, which in turn stops the formation of ergosterol in the fungal cell membrane.36 Furthermore, echinocandins work by inhibiting the production of β-(1,3)-
The antifungals were selected according to the preferred therapy for penicilliosis in the Malaysian national antimicrobial guidelines and previous publications.38 To our knowledge, this is the first study comparing the susceptibility pattern of clinical yeast and mold forms of T. marneffei in Malaysia. Apart from that, this study also presented the first insight into the susceptibility of isavuconazole against both growth forms of this pathogen. However, this study suffers a limitation where the results were unable to be interpreted as susceptible or resistant as there are no official breakpoints for T. marneffei according to the CLSI method.
Conclusion
In conclusion, the GM MIC of each antifungal except caspofungin and micafungin were found not significantly different between the two different growth forms. Therefore, we conclude that either yeast or mold form could be used in the susceptibility testing of T. marneffei against amphotericin B, itraconazole, voriconazole, posaconazole, ketoconazole, isavuconazole and terbinafine. However, further studies are necessary to evaluate these findings with the clinical outcomes.
Ethics Approval
An ethical review was conducted and approved by the Medical Research and Ethics Committee, Ministry of Health of Malaysia, Malaysia (NMRR-20-207-53067).
Acknowledgments
The authors would like to thank the Director-General of Health, Malaysia, for his permission to publish this article. The authors would also like to express their gratitude to the Director of the Institute for Medical Research for supporting this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mohd Nizam T. Epidemiological and clinical features of talaromycosis (Penicilliosis) Marneffei among human immunodeficiency virus-infected patients in Malaysia. MedHealth. 2018;13(2):103–113. doi:10.17576/MH.2018.1302.10
2. Xu X, Ran X, Pradhan S, Lei S, Ran Y. Dermoscopic manifestations of Talaromyces (Penicillium) marneffei infection in an AIDS patient. Indian J Dermatol Venereol Leprol. 2019;85(3):348. doi:10.4103/IJDVL.IJDVL_118_17
3. Velayuthan RD, Samudi C, Singh HKL, Ng KP, Shankar EM, Denning DW. Estimation of the burden of serious human fungal infections in Malaysia. J Fungi. 2018;4(1):38. doi:10.3390/JOF4010038
4. Chen J, Zhang R, Shen Y, et al. Clinical characteristics and prognosis of penicilliosis among human immunodeficiency virus-infected patients in eastern China. Am J Trop Med Hyg. 2017;96(6):1350–1354. doi:10.4269/AJTMH.16-0521
5. HKMJ. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Available from: https://www.hkmj.org/abstracts/v14n2/103.htm.
6. Lau SKP, Lo GCS, Lam CSK, et al. In Vitro Activity of Posaconazole against Talaromyces marneffei by broth microdilution and Etest methods and comparison to itraconazole, voriconazole, and anidulafungin. Antimicrob Agents Chemother. 2017;61(3). doi:10.1128/AAC.01480-16
7. Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi:10.1128/CMR.19.1.95-110.2006
8. Baradkar V, Kumar S, Kulkarni SD. Penicillium marneffei: the pathogen at our doorstep. Indian J Dermatol Venereol Leprol. 2009;75(6):619–620. doi:10.1258/jrsm.97.8.394
9. Liu Y, Huang X, Yi X, He Y, Mylonakis E, Xi L. Detection of Talaromyces marneffei from fresh tissue of an inhalational murine pulmonary model using nested PCR. PLoS One. 2016;11(2):e0149634. doi:10.1371/JOURNAL.PONE.0149634
10. Klein BS, Tebbets B. Dimorphism and virulence in fungi. Curr Opin Microbiol. 2007;10(4):314–319. doi:10.1016/J.MIB.2007.04.002
11. Yuen WC, Chan YF, Loke SL, Seto WH, Poon GP, Wong KK. Chronic lymphadenopathy caused by Penicillium marneffei: a condition mimicking tuberculous lymphadenopathy. Br J Surg. 1986;73(12):1007–1008. doi:10.1002/BJS.1800731224
12. Pautler KB, Padhye AA, Ajello L. Imported penicilliosis marneffei in the United States: report of a second human infection. Sabouraudia. 1984;22(5):433–438. doi:10.1080/00362178485380691
13. Deng Z, Connor DH. Progressive disseminated penicilliosis caused by Penicillium marneffei. Report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. Am J Clin Pathol. 1985;84(3):323–327. doi:10.1093/AJCP/84.3.323
14. Arifin WN. Introduction to sample size calculation. Educ Med J. 2013;5(2). doi:10.5959/eimj.v5i2.130
15. Kiss L. Limits of nuclear ribosomal DNA internal transcribed spacer (ITS) sequences as species barcodes for Fungi. Proc Natl Acad Sci U S A. 2012;109(27):E1811. doi:10.1073/PNAS.1207143109
16. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Clinical and Laboratory Standards Institute; 2017.
17. Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clinical and Laboratory Standards Institute; 2017.
18. Sar B, Boy S, Keo C, et al. In vitro antifungal-drug susceptibilities of mycelial and yeast forms of Penicillium marneffei Isolates in Cambodia. J Clin Microbiol. 2006;44(11):4208. doi:10.1128/JCM.00902-06
19. Tan XT, Shuhairi LM, Jane S, Shukor SM, Amran F. Comparison of yeast and fungus form in vitro susceptibilities of Sporothrix schenckii in Malaysia: in vitro susceptibility of Sporothrix schencki. Southeast Asian J Trop Med Public Health. 2022;53(5):469–478.
20. Sekhon AS, Garg AK, Padhye AA, Hamir Z. In vitro susceptibility of mycelial and yeast forms of Penicillium marneffei to amphotericin B, fluconazole, 5-fluorocytosine and itraconazole. Eur J Epidemiol. 1993;9(5):553–558. doi:10.1007/BF00209535
21. Melanization of pathogenic fungi, particularly Aspergillus fumigatus and Penicillium marneffei - Aspergillus and Aspergillosis. Available from: https://www.aspergillus.org.uk/conference_abstracts/melanization-of-pathogenic-fungi-particularly-aspergillusfumigatus-and-penicillium-marneffei/.
22. Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003;5(4):203–223. doi:10.1046/J.1462-5814.2003.00268.X
23. Youngchim S, Youngchim S. Talaromyces marneffei infection: virulence factors and rapid diagnostics. Infect Diss Ann. 2022. doi:10.5772/INTECHOPEN.108592
24. Kaewmalakul J, Nosanchuk JD, Vanittanakom N, Youngchim S. Melanization and morphological effects on antifungal susceptibility of Penicillium marneffei. Antonie Van Leeuwenhoek. 2014;106(5):1011. doi:10.1007/S10482-014-0270-9
25. Singh RB, Devi KR. A comparative study on antifungal susceptibility of Penicillium marneffei (Talaromyces marneffei) and nonmarneffei Penicillium species. J Med Soc. 2018;32(1):22. doi:10.4103/JMS.JMS_38_17
26. Supparatpinyo K, Nelson KE, Merz WG, et al. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993;37(11):2407–2411. doi:10.1128/AAC.37.11.2407
27. Ouyang Y, Cai S, Liang H, Cao C. Administration of voriconazole in disseminated Talaromyces (Penicillium) marneffei infection: a retrospective study. Mycopathologia. 2017;182(5–6):569–575. doi:10.1007/S11046-016-0107-3
28. Liu D, Liang L, Chen J. In vitro antifungal drug susceptibilities of Penicillium marneffei from China. J Infect Chemother. 2013;19(4):776–778. doi:10.1007/S10156-012-0511-7
29. Petranyi G, Ryder NS, Stütz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984;224(4654):1239–1241. doi:10.1126/SCIENCE.6547247
30. Petranyi G, Meingassner JG, Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother. 1987;31(9):1365–1368. doi:10.1128/AAC.31.9.1365
31. Ryder NS. Effect of allylamine antimycotic agents on fungal sterol biosynthesis measured by sterol side-chain methylation. J Gen Microbiol. 1985;131(7):1595–1602. doi:10.1099/00221287-131-7-1595
32. Mcginnis MR, Nordoff NG, Ryder NS, Nunn GB. In Vitro Comparison of Terbinafine and Itraconazole against Penicillium marneffei. Antimicrob Agents Chemother. 2000;44(5):1407–1408. doi:10.1128/AAC.44.5.1407-1408.2000
33. Fang L, Liu M, Huang C, et al. MALDI-TOF MS-based clustering and antifungal susceptibility tests of talaromyces marneffei isolates from Fujian and Guangxi (China). Infect Drug Resist. 2022;15:3449–3457. doi:10.2147/IDR.S364439
34. Lei HL, Li LH, Chen WS, et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur J Clin Microbiol Infect Dis. 2018;37(6):1099–1102. doi:10.1007/S10096-018-3222-X
35. Ashbee HR, Gilleece MH. Pharmacogenomics of antifungal agents. In: Handbook of Pharmacogenomics and Stratified Medicine. Academic Press; 2014:879–900. doi:10.1016/B978-0-12-386882-4.00038-4
36. Xiao L, Madison V, Chau AS, Loebenberg D, Palermo RE, McNicholas PM. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob Agents Chemother. 2004;48(2):568–574. doi:10.1128/AAC.48.2.568-574.2004
37. Szyma Nski M, Chmielewska S, Czy_ Zewska U, Malinowska M, Tylicki A. Echinocandins-structure, mechanism of action and use in antifungal therapy. J Enzyme Inhib Med Chem. 2022;37(1):876–894. doi:10.1080/14756366.2022.2050224
38. Ministry of Health Malaysia. National antimicrobial guidelines. Officially Launched on 26th; 2019. Available from: www.pharmacy.gov.my.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
